Ch.8 Third Month to Birth, the Fetus and Plenta
1/38
Earn XP
Description and Tags
it's the whole chapter
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
39 Terms
DEVELOPMENT OF THE FETUS
The period from the beginning of the ninth week to birth is known as the fetal period. It is characterized by maturation of tissues and organs and rapid growth of the body. The length of the fetus is usually indicated as the crown-rump length (CRL) (sitting height) or as the crown-heel length (CHL), the measurement from the vertex of the skull to the heel (standing height). These measurements, expressed in centimeters, are correlated with the age of the fetus in weeks or months (Table 8.1). Growth in length is particularly striking during the third, fourth, and fifth months, whereas an increase in weight is most striking during the last 2 months of gestation. In general, the length of pregnancy is considered to be 280 days or 40 weeks after the onset of the last normal menstrual period (LNMP) or, more accurately, 266 days or 38 weeks after fertilization. For the purposes of the following discussion, age is calculated from the time of fertilization and is expressed in weeks or calendar months.
Monthly Changes
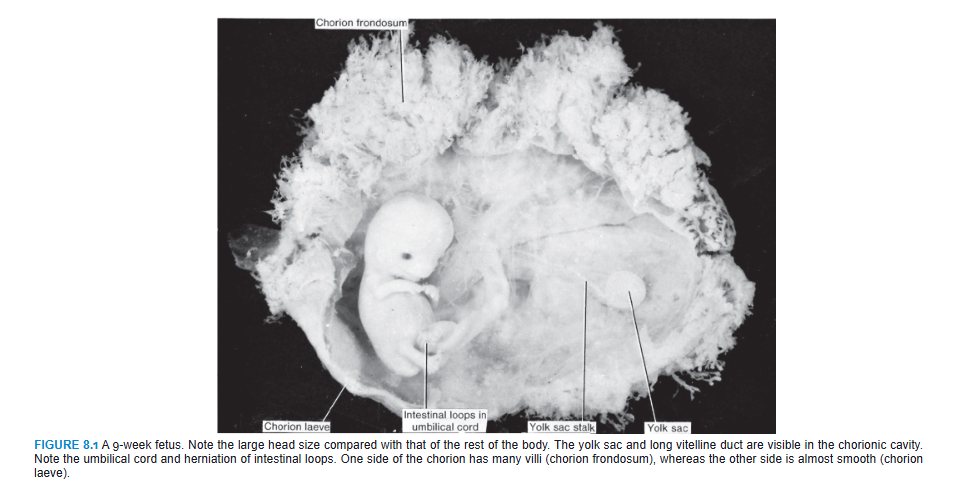
One of the most striking changes taking place during fetal life is the relative slowdown in growth of the head compared with the rest of the body. At the beginning of the third month, the head constitutes approximately half of the CRL (Figs. 8.1 and 8.2). By the beginning of the fifth month, the size of the head is about one-third of the CHL, and at birth, it is approximately one-quarter of the CHL (Fig. 8.2). Hence, over time, growth of the body accelerates but that of the head slows down.
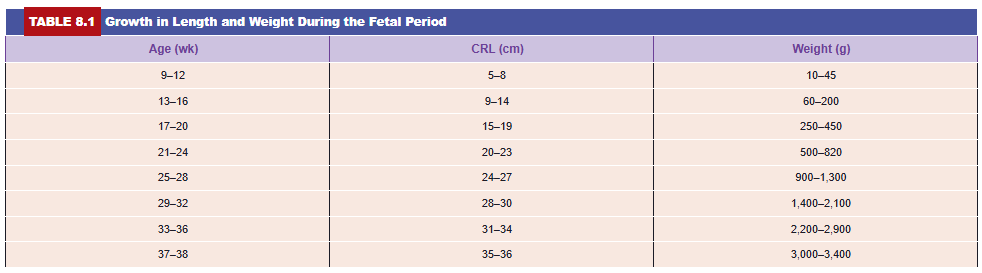
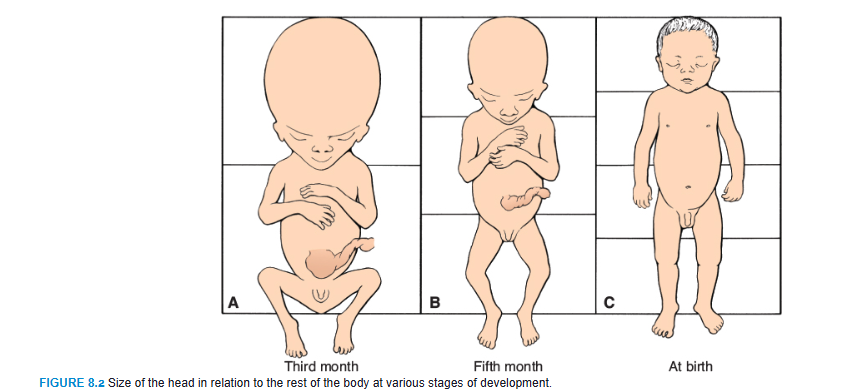
During the third month, the face becomes more human looking (Figs. 8.3 and 8.4). The eyes, initially directed laterally, move to the ventral aspect of the face, and the ears come to lie close to their definitive position at the side of the head (Fig. 8.3). The limbs reach their relative length in comparison with the rest of the body, although the lower limbs are still a little shorter and less well developed than the upper extremities. Primary ossification centers are present in the long bones and skull by the 12th week. Also, by the 12th week, external genitalia develop to such a degree that the sex of the fetus can be determined by external examination (ultrasound). During the 6th week, intestinal loops cause a large swelling (herniation) in the umbilical cord, but by the 12th week, the loops have withdrawn into the abdominal cavity. At the end of the third month, reflex activity can be evoked in aborted fetuses, indicating muscular activity.
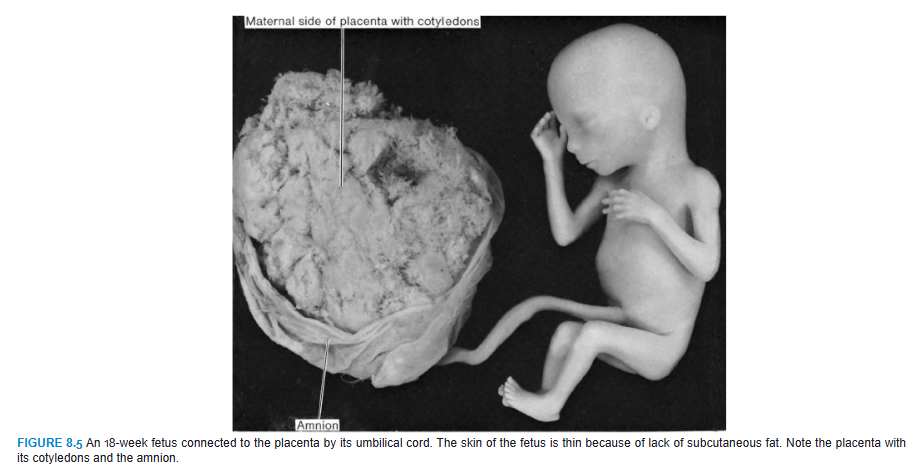
During the fourth and fifth months, the fetus lengthens rapidly (Fig. 8.5 and Table 8.1), and at the end of the first half of intrauterine life, its CRL is approximately 15 cm, about half the total length of the newborn. The weight of the fetus increases little during this period and by the end of the fifth month is still <500 g. The fetus is covered with fine hair, called lanugo hair; eyebrows and head hair are also visible. During the fifth month, the mother can feel movements of the fetus.
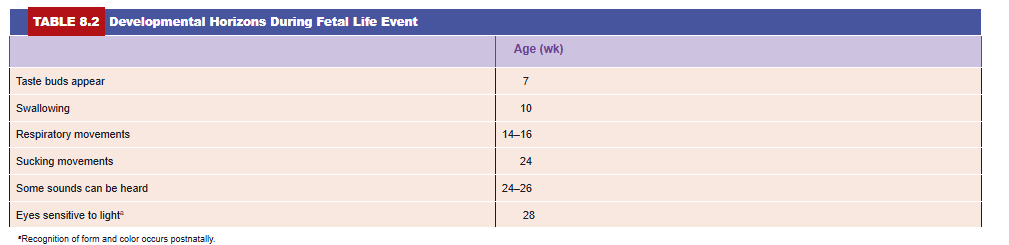
During the second half of intrauterine life, weight increases considerably, particularly during the last 2.5 months, when 50% of the full-term weight (approximately 3,200 g) is added. During the sixth month, the skin of the fetus is reddish and has a wrinkled appearance because of the lack of underlying connective tissue. A fetus born early in the sixth month has great difficulty surviving. Although several organ systems are able to function, the respiratory system and the central nervous system have not differentiated sufficiently, and coordination between the two systems is not yet well established. By 6.5 to 7 months, the fetus has a CRL of about 25 cm and weighs approximately 1,100 g. If born at this time, the infant has a 90% chance of surviving. Some developmental events occurring during the first 7 months are indicated in Table 8.2.
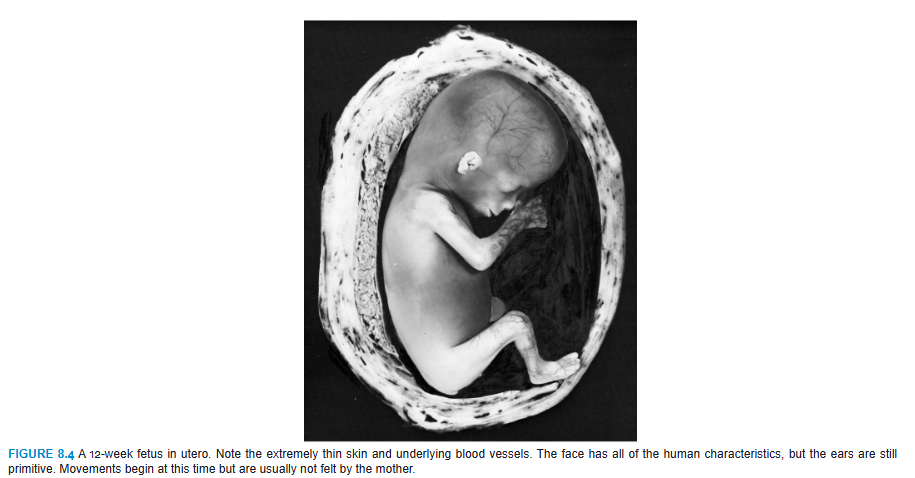
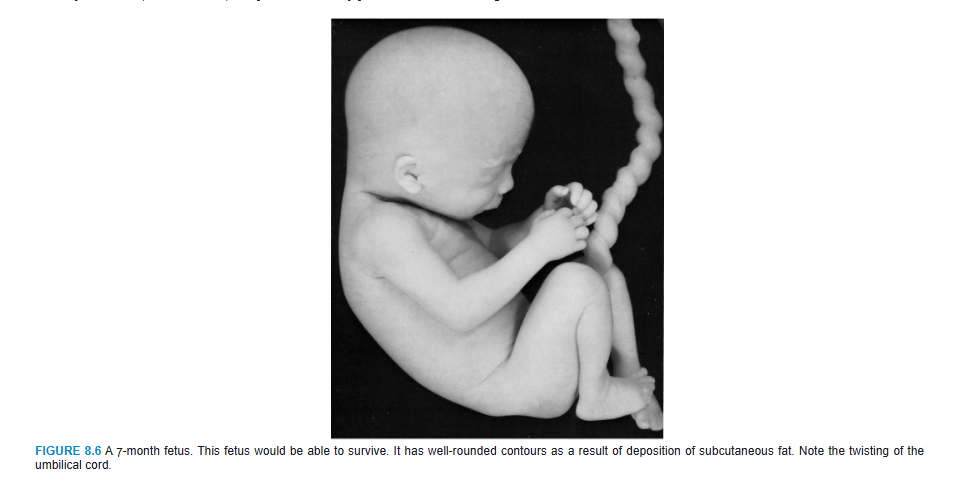
During the last 2 months, the fetus obtains well-rounded contours as the result of deposition of subcutaneous fat (Fig. 8.6). By the end of intrauterine life, the skin is covered by a whitish, fatty substance (vernix caseosa) composed of secretory products from sebaceous glands.
At the end of the ninth month, the skull has the largest circumference of all parts of the body, an important fact with regard to its passage through the birth canal. At the time of birth, the weight of a normal fetus is 3,000 to 3,400 g, its CRL is about 36 cm, and its CHL is about 50 cm. Sexual characteristics are pronounced, and the testes should be in the scrotum.
Time of Birth
The date of birth is most accurately indicated as 266 days, or 38 weeks, after fertilization. The oocyte is usually fertilized within 12 hours of ovulation; however, sperm deposited in the reproductive tract up to 6 days prior to ovulation can survive to fertilize oocytes. Thus, most pregnancies occur when sexual intercourse occurs within a 6-day period that ends on the day of ovulation. A pregnant woman usually will see her obstetrician when she has missed two successive menstrual bleeds. By that time, her recollection about coitus is usually vague, and it is readily understandable that the day of fertilization is difficult to determine.
The obstetrician calculates the date of birth as 280 days or 40 weeks from the first day of the LNMP. In women with regular 28-day menstrual periods, the method is fairly accurate, but when cycles are irregular, there may be substantial miscalculations. An additional complication occurs when the woman has some bleeding about 14 days after fertilization as a result of erosive activity by the implanting blastocyst (see Chapter 4, “Day 13,” p. 54). Hence, the day of delivery is not always easy to determine. Most fetuses are born within 10 to 14 days of the calculated delivery date. If they are born much earlier, they are categorized as premature; if born later, they are considered postmature.
Occasionally, the age of an embryo or small fetus must be determined. A reasonable estimate can be formulated by combining data on the onset of the LNMP with fetal length, weight, and other morphologic characteristics typical for a given month of development. A valuable tool for assisting in this determination is ultrasound, which can provide an accurate (1 to 2 days) measurement of CRL during the 7th to 14th weeks. Measurements commonly used in the 16th to 30th weeks are biparietal diameter (BPD), head and abdominal circumference, and femur length. An accurate determination of fetal size and age is important for managing pregnancy, especially if the mother has a small pelvis or if the fetus has a birth defect.
Low Birth Weight
There is considerable variation in fetal length and weight, and sometimes, these values do not correspond with the calculated age of the fetus in months or weeks. Most factors influencing length and weight are genetically determined, but environmental factors also play an important role.
The average size of a newborn is 2,500 to 4,000 g (6 to 9 lb) with a length of 51 cm (20 in). The term low birth weight (LBW) refers to a weight <2,500 g, regardless of gestational age. Many infants weigh <2,500 g because they are preterm (born before 37 weeks of gestation). In contrast, the terms intrauterine growth restriction (IUGR) and small for gestational age (SGA) take into account gestational age.
IUGR is a term applied to infants who do not attain their optimal intrauterine growth. These infants are pathologically small and at risk for poor outcomes. Infants who are SGA have a birth weight that is below the 10th percentile for their gestational age. These babies may be pathologically small (they may have IUGR) or they may be constitutionally small (healthy but smaller in size). The challenge is to differentiate the two conditions so that healthy but small babies are not subjected to high-risk protocols used for babies with IUGR.
Approximately 1 in 10 babies have IUGR and therefore have an increased risk of neurologic problems, congenital malformations, meconium aspiration, hypoglycemia, hypocalcemia, and respiratory distress syndrome (RDS). There are also long-term effects on these infants: In other words, what happens in the womb does not stay in the womb and adverse fetal exposures may predispose individuals to health problems as they get older (Barker hypothesis). For example, babies with IUGR have been shown to have a greater chance of developing a metabolic disorder later in life, such as obesity, hypertension, hypercholesterolemia, cardiovascular disease, and type 2 diabetes.
Causative factors for IUGR include chromosomal abnormalities; teratogens; congenital infections (rubella, cytomegalovirus, toxoplasmosis, and syphilis); poor maternal health (hypertension and renal and cardiac disease); the mother’s nutritional status and socioeconomic level; her use of cigarettes, alcohol, and other drugs; placental insufficiency; and multiple births (e.g., twins, triplets).
The major growth-promoting factor during development before and after birth is insulin-like growth factor 1 (IGF-1), which has mitogenic and anabolic effects. Fetal tissues express IGF-1, and serum levels are correlated with fetal growth. Mutations in the IGF-1 gene result in IUGR, and this growth retardation continues after birth. In contrast to the prenatal period, postnatal growth depends on growth hormone (GH). This hormone binds to its receptor (growth hormone receptor [GHR]), activating a signal transduction pathway and resulting in synthesis and secretion of IGF-1. Mutations in the GHR result in Laron dwarfism, which is characterized by marked short stature, and sometimes blue sclera. These individuals show little or no IUGR because IGF-1 production does not depend on GH during fetal development.
![<p>Low Birth Weight</p><p>There is considerable variation in fetal length and weight, and sometimes, these values do not correspond with the calculated age of the fetus in months or weeks. Most factors influencing length and weight are genetically determined, but environmental factors also play an important role.</p><p>The average size of a newborn is 2,500 to 4,000 g (6 to 9 lb) with a length of 51 cm (20 in). The term low birth weight (LBW) refers to a weight <2,500 g, regardless of gestational age. Many infants weigh <2,500 g because they are preterm (born before 37 weeks of gestation). In contrast, the terms intrauterine growth restriction (IUGR) and small for gestational age (SGA) take into account gestational age.</p><p>IUGR is a term applied to infants who do not attain their optimal intrauterine growth. These infants are pathologically small and at risk for poor outcomes. Infants who are SGA have a birth weight that is below the 10th percentile for their gestational age. These babies may be pathologically small (they may have IUGR) or they may be constitutionally small (healthy but smaller in size). The challenge is to differentiate the two conditions so that healthy but small babies are not subjected to high-risk protocols used for babies with IUGR.</p><p>Approximately 1 in 10 babies have IUGR and therefore have an increased risk of neurologic problems, congenital malformations, meconium aspiration, hypoglycemia, hypocalcemia, and respiratory distress syndrome (RDS). There are also long-term effects on these infants: In other words, what happens in the womb does not stay in the womb and adverse fetal exposures may predispose individuals to health problems as they get older (Barker hypothesis). For example, babies with IUGR have been shown to have a greater chance of developing a metabolic disorder later in life, such as obesity, hypertension, hypercholesterolemia, cardiovascular disease, and type 2 diabetes.</p><p>Causative factors for IUGR include chromosomal abnormalities; teratogens; congenital infections (rubella, cytomegalovirus, toxoplasmosis, and syphilis); poor maternal health (hypertension and renal and cardiac disease); the mother’s nutritional status and socioeconomic level; her use of cigarettes, alcohol, and other drugs; placental insufficiency; and multiple births (e.g., twins, triplets).</p><p>The major growth-promoting factor during development before and after birth is insulin-like growth factor 1 (IGF-1), which has mitogenic and anabolic effects. Fetal tissues express IGF-1, and serum levels are correlated with fetal growth. Mutations in the IGF-1 gene result in IUGR, and this growth retardation continues after birth. In contrast to the prenatal period, postnatal growth depends on growth hormone (GH). This hormone binds to its receptor (growth hormone receptor [GHR]), activating a signal transduction pathway and resulting in synthesis and secretion of IGF-1. Mutations in the GHR result in Laron dwarfism, which is characterized by marked short stature, and sometimes blue sclera. These individuals show little or no IUGR because IGF-1 production does not depend on GH during fetal development.</p>](https://knowt-user-attachments.s3.amazonaws.com/71e75622-b4d2-4f96-9bdb-e28b5ee8cdec.png)
FETAL MEMBRANES AND PLACENTA
The placenta is the organ that facilitates nutrient and gas exchange between the maternal and fetal compartments. As the fetus begins the ninth week of development, its demands for nutritional and other factors increase, causing major changes in the placenta. Foremost among these is an increase in surface area between maternal and fetal components to facilitate exchange. The disposition of fetal membranes is also altered as production of amniotic fluid increases.
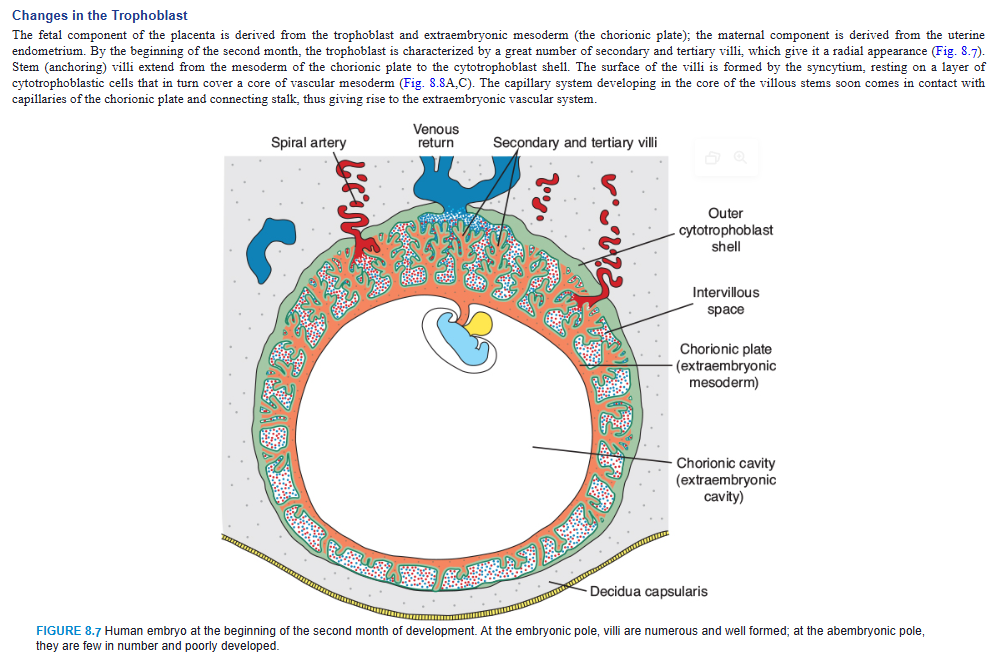
Changes in the Trophoblast
The fetal component of the placenta is derived from the trophoblast and extraembryonic mesoderm (the chorionic plate); the maternal component is derived from the uterine endometrium. By the beginning of the second month, the trophoblast is characterized by a great number of secondary and tertiary villi, which give it a radial appearance (Fig. 8.7). Stem (anchoring) villi extend from the mesoderm of the chorionic plate to the cytotrophoblast shell. The surface of the villi is formed by the syncytium, resting on a layer of cytotrophoblastic cells that in turn cover a core of vascular mesoderm (Fig. 8.8A,C). The capillary system developing in the core of the villous stems soon comes in contact with capillaries of the chorionic plate and connecting stalk, thus giving rise to the extraembryonic vascular system.
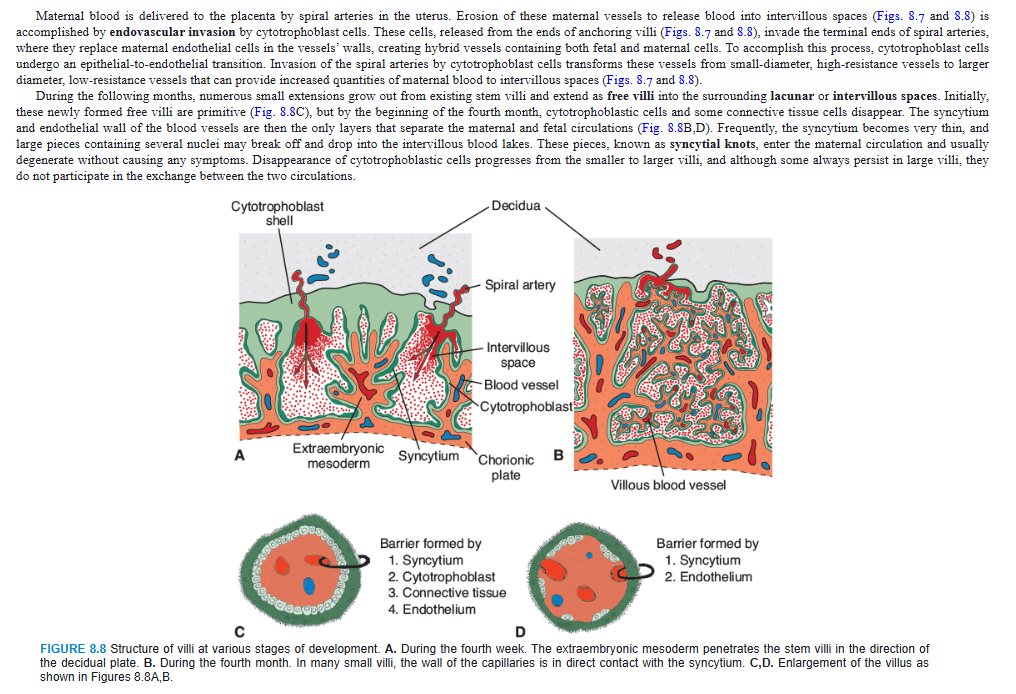
Maternal blood is delivered to the placenta by spiral arteries in the uterus. Erosion of these maternal vessels to release blood into intervillous spaces (Figs. 8.7 and 8.8) is accomplished by endovascular invasion by cytotrophoblast cells. These cells, released from the ends of anchoring villi (Figs. 8.7 and 8.8), invade the terminal ends of spiral arteries, where they replace maternal endothelial cells in the vessels’ walls, creating hybrid vessels containing both fetal and maternal cells. To accomplish this process, cytotrophoblast cells undergo an epithelial-to-endothelial transition. Invasion of the spiral arteries by cytotrophoblast cells transforms these vessels from small-diameter, high-resistance vessels to larger diameter, low-resistance vessels that can provide increased quantities of maternal blood to intervillous spaces (Figs. 8.7 and 8.8).
During the following months, numerous small extensions grow out from existing stem villi and extend as free villi into the surrounding lacunar or intervillous spaces. Initially, these newly formed free villi are primitive (Fig. 8.8C), but by the beginning of the fourth month, cytotrophoblastic cells and some connective tissue cells disappear. The syncytium and endothelial wall of the blood vessels are then the only layers that separate the maternal and fetal circulations (Fig. 8.8B,D). Frequently, the syncytium becomes very thin, and large pieces containing several nuclei may break off and drop into the intervillous blood lakes. These pieces, known as syncytial knots, enter the maternal circulation and usually degenerate without causing any symptoms. Disappearance of cytotrophoblastic cells progresses from the smaller to larger villi, and although some always persist in large villi, they do not participate in the exchange between the two circulations.
Clinical Correlates
Preeclampsia
Preeclampsia is a condition characterized by maternal hypertension and proteinuria due to reduced organ perfusion and occurs in approximately 5% of pregnancies. The condition may progress to eclampsia, which is characterized by seizures. Preeclampsia begins suddenly anytime from approximately 20 weeks’ gestation to term and may result in fetal growth retardation, fetal death, or maternal death. In fact, preeclampsia is a leading cause of maternal mortality in the United States and is completely reversible by delivery of the baby. However, delivery too early puts the infant at risk for complications related to preterm birth. Despite many years of research, the cause of preeclampsia is unknown. The condition appears to be a trophoblastic disorder related to failed or incomplete differentiation of cytotrophoblast cells, many of which do not undergo their normal epithelial-to-endothelial transformation. As a result, invasion of maternal blood vessels by these cells is rudimentary. How these cellular abnormalities lead to hypertension and other problems is not clear. Risk factors for preeclampsia include preeclampsia in a previous pregnancy, nulliparity (first pregnancy), obesity, family history of preeclampsia, multiple gestation (twins or more), and medical conditions such as hypertension and diabetes. Preeclampsia also commonly occurs in women with hydatidiform moles (see Chapter 4, p. 58), in which case it occurs early in pregnancy.
CHORION FRONDOSUM AND DECIDUA BASALIS
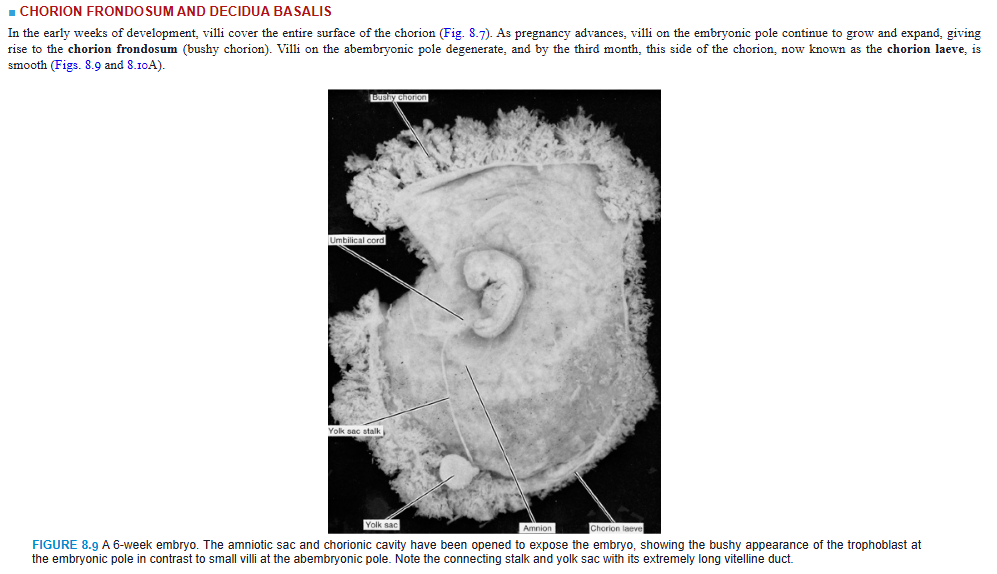
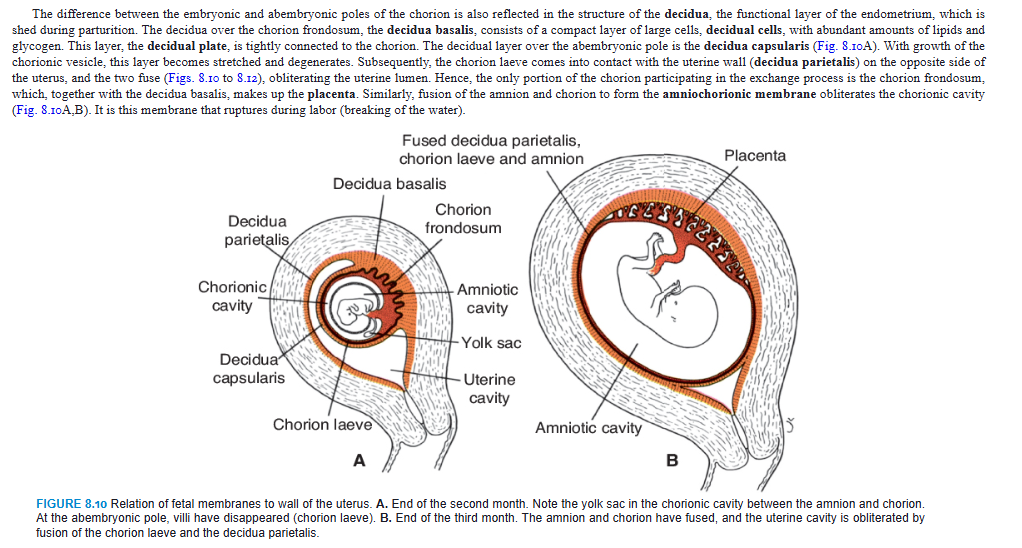
In the early weeks of development, villi cover the entire surface of the chorion (Fig. 8.7). As pregnancy advances, villi on the embryonic pole continue to grow and expand, giving rise to the chorion frondosum (bushy chorion). Villi on the abembryonic pole degenerate, and by the third month, this side of the chorion, now known as the chorion laeve, is smooth (Figs. 8.9 and 8.10A).
The difference between the embryonic and abembryonic poles of the chorion is also reflected in the structure of the decidua, the functional layer of the endometrium, which is shed during parturition. The decidua over the chorion frondosum, the decidua basalis, consists of a compact layer of large cells, decidual cells, with abundant amounts of lipids and glycogen. This layer, the decidual plate, is tightly connected to the chorion. The decidual layer over the abembryonic pole is the decidua capsularis (Fig. 8.10A). With growth of the chorionic vesicle, this layer becomes stretched and degenerates. Subsequently, the chorion laeve comes into contact with the uterine wall (decidua parietalis) on the opposite side of the uterus, and the two fuse (Figs. 8.10 to 8.12), obliterating the uterine lumen. Hence, the only portion of the chorion participating in the exchange process is the chorion frondosum, which, together with the decidua basalis, makes up the placenta. Similarly, fusion of the amnion and chorion to form the amniochorionic membrane obliterates the chorionic cavity (Fig. 8.10A,B). It is this membrane that ruptures during labor (breaking of the water).
STRUCTURE OF THE PLACENTA
By the beginning of the fourth month, the placenta has two components: (1) a fetal portion, formed by the chorion frondosum, and (2) a maternal portion, formed by the decidua basalis (Fig. 8.10B). On the fetal side, the placenta is bordered by the chorionic plate (Fig. 8.13); on its maternal side, it is bordered by the decidua basalis, of which the decidual plate is most intimately incorporated into the placenta. In the junctional zone, trophoblast and decidual cells intermingle. This zone, characterized by decidual and syncytial giant cells, is rich in amorphous extracellular material. By this time, most cytotrophoblast cells have degenerated. Between the chorionic and decidual plates are the intervillous spaces, which are filled with maternal blood. They are derived from lacunae in the syncytiotrophoblast and are lined with syncytium of fetal origin. The villous trees grow into the intervillous blood lakes (Figs. 8.8 and 8.13).
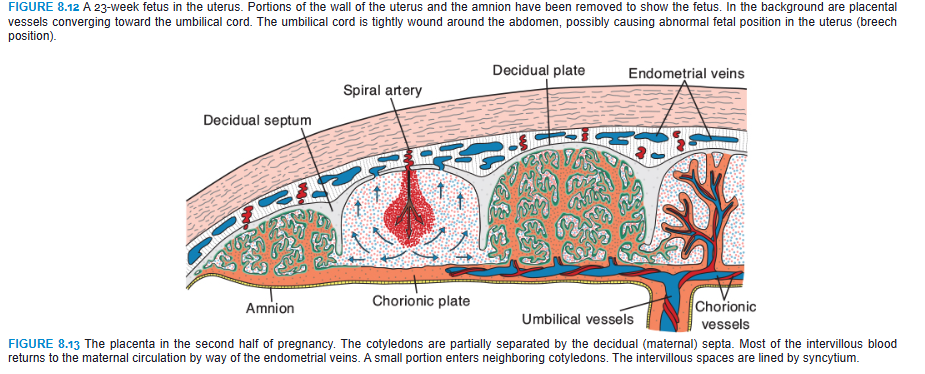
During the fourth and fifth months, the decidua forms a number of decidual septa, which project into intervillous spaces but do not reach the chorionic plate (Fig. 8.13). These septa have a core of maternal tissue, but their surface is covered by a layer of syncytial cells so that at all times, a syncytial layer separates maternal blood in intervillous lakes from fetal tissue of the villi. As a result of this septum formation, the placenta is divided into a number of compartments, or cotyledons (Fig. 8.14). Because the decidual septa do not reach the chorionic plate, contact between intervillous spaces in the various cotyledons is maintained.
As a result of the continuous growth of the fetus and expansion of the uterus, the placenta also enlarges. Its increase in surface area roughly parallels that of the expanding uterus, and throughout pregnancy, it covers approximately 15% to 30% of the internal surface of the uterus. The increase in thickness of the placenta results from arborization of existing villi and is not caused by further penetration into maternal tissues.
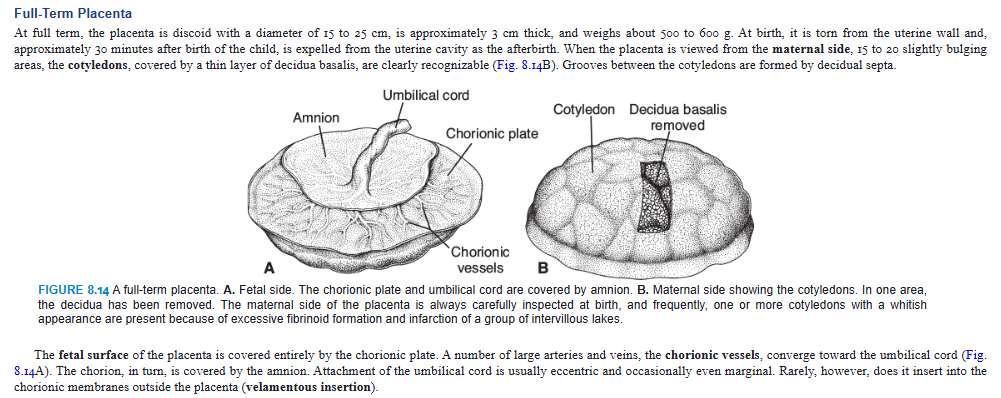
Full-Term Placenta
At full term, the placenta is discoid with a diameter of 15 to 25 cm, is approximately 3 cm thick, and weighs about 500 to 600 g. At birth, it is torn from the uterine wall and, approximately 30 minutes after birth of the child, is expelled from the uterine cavity as the afterbirth. When the placenta is viewed from the maternal side, 15 to 20 slightly bulging areas, the cotyledons, covered by a thin layer of decidua basalis, are clearly recognizable (Fig. 8.14B). Grooves between the cotyledons are formed by decidual septa.
The fetal surface of the placenta is covered entirely by the chorionic plate. A number of large arteries and veins, the chorionic vessels, converge toward the umbilical cord (Fig. 8.14A). The chorion, in turn, is covered by the amnion. Attachment of the umbilical cord is usually eccentric and occasionally even marginal. Rarely, however, does it insert into the chorionic membranes outside the placenta (velamentous insertion).
Circulation of the Placenta
Cotyledons receive their blood through 80 to 100 spiral arteries that pierce the decidual plate and enter the intervillous spaces at more or less regular intervals (Fig. 8.13). Pressure in these arteries forces the blood deep into the intervillous spaces and bathes the numerous small villi of the villous tree in oxygenated blood. As the pressure decreases, blood flows back from the chorionic plate toward the decidua, where it enters the endometrial veins (Fig. 8.13). Hence, blood from the intervillous lakes drains back into the maternal circulation through the endometrial veins.
Collectively, the intervillous spaces of a mature placenta contain approximately 150 mL of blood, which is replenished about three or four times per minute. This blood moves along the chorionic villi, which have a surface area of 4 to 14 m2. Placental exchange does not take place in all villi, however, only in those that have fetal vessels in intimate contact with the covering syncytial membrane. In these villi, the syncytium often has a brush border consisting of numerous microvilli, which greatly increases the surface area and, consequently, the exchange rate between maternal and fetal circulations (Fig. 8.8D). The placental membrane, which separates maternal and fetal blood, is initially composed of four layers: (1) the endothelial lining of fetal vessels, (2) the connective tissue in the villus core, (3) the cytotrophoblastic layer, and (4) the syncytium (Fig. 8.8C). From the fourth month onward, the placental membrane thins because the endothelial lining of the vessels comes in intimate contact with the syncytial membrane, greatly increasing the rate of exchange (Fig. 8.8D). Sometimes called the placental barrier, the placental membrane is not a true barrier, as many substances pass through it freely. Because the maternal blood in the intervillous spaces is separated from the fetal blood by a chorionic derivative, the human placenta is considered to be of the hemochorial type. Normally, there is no mixing of maternal and fetal blood. However, small numbers of fetal blood cells occasionally escape across microscopic defects in the placental membrane.
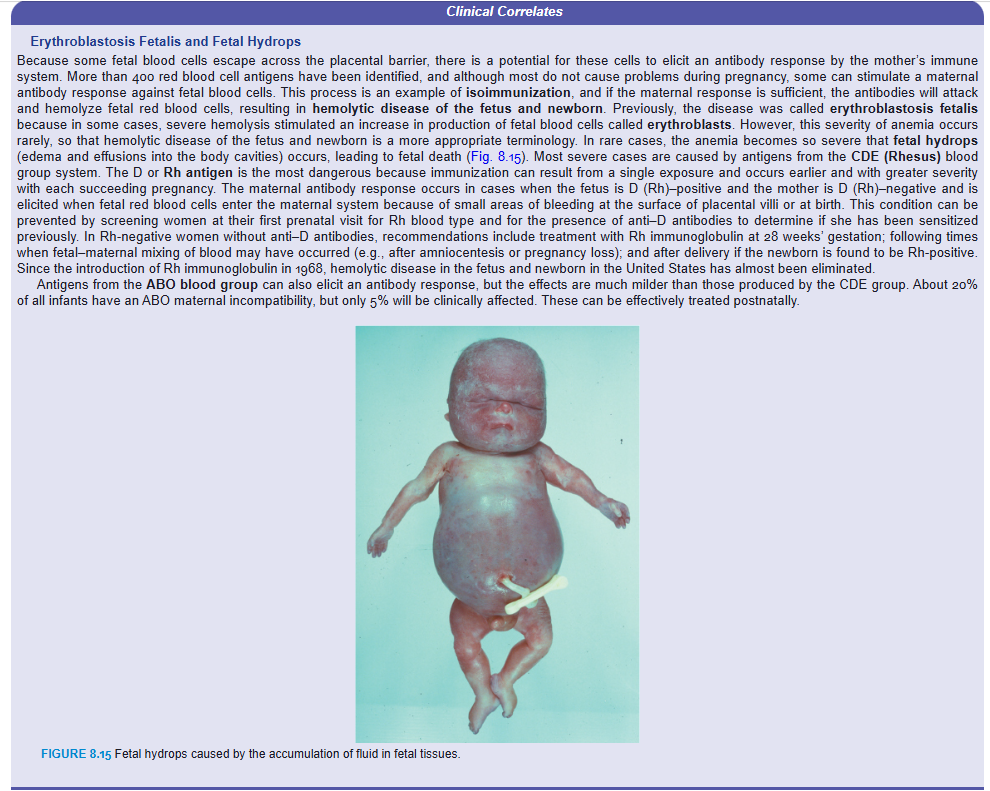
Erythroblastosis Fetalis and Fetal Hydrops
Because some fetal blood cells escape across the placental barrier, there is a potential for these cells to elicit an antibody response by the mother’s immune system. More than 400 red blood cell antigens have been identified, and although most do not cause problems during pregnancy, some can stimulate a maternal antibody response against fetal blood cells. This process is an example of isoimmunization, and if the maternal response is sufficient, the antibodies will attack and hemolyze fetal red blood cells, resulting in hemolytic disease of the fetus and newborn. Previously, the disease was called erythroblastosis fetalis because in some cases, severe hemolysis stimulated an increase in production of fetal blood cells called erythroblasts. However, this severity of anemia occurs rarely, so that hemolytic disease of the fetus and newborn is a more appropriate terminology. In rare cases, the anemia becomes so severe that fetal hydrops (edema and effusions into the body cavities) occurs, leading to fetal death (Fig. 8.15). Most severe cases are caused by antigens from the CDE (Rhesus) blood group system. The D or Rh antigen is the most dangerous because immunization can result from a single exposure and occurs earlier and with greater severity with each succeeding pregnancy. The maternal antibody response occurs in cases when the fetus is D (Rh)–positive and the mother is D (Rh)–negative and is elicited when fetal red blood cells enter the maternal system because of small areas of bleeding at the surface of placental villi or at birth. This condition can be prevented by screening women at their first prenatal visit for Rh blood type and for the presence of anti–D antibodies to determine if she has been sensitized previously. In Rh-negative women without anti–D antibodies, recommendations include treatment with Rh immunoglobulin at 28 weeks’ gestation; following times when fetal–maternal mixing of blood may have occurred (e.g., after amniocentesis or pregnancy loss); and after delivery if the newborn is found to be Rh-positive. Since the introduction of Rh immunoglobulin in 1968, hemolytic disease in the fetus and newborn in the United States has almost been eliminated.
Antigens from the ABO blood group can also elicit an antibody response, but the effects are much milder than those produced by the CDE group. About 20% of all infants have an ABO maternal incompatibility, but only 5% will be clinically affected. These can be effectively treated postnatally
Function of the Placenta
Main functions of the placenta are (1) exchange of metabolic and gaseous products between maternal and fetal bloodstreams and (2) production of hormones.
Exchange of Gases
Exchange of gases—such as oxygen, carbon dioxide, and carbon monoxide—is accomplished by simple diffusion. At term, the fetus extracts 20 to 30 mL of oxygen per minute from the maternal circulation, and even a short-term interruption of the oxygen supply is fatal to the fetus. Placental blood flow is critical to oxygen supply, since the amount of oxygen reaching the fetus primarily depends on the amount of blood delivered, not upon rates of diffusion.
Exchange of Nutrients and Electrolytes
Exchange of nutrients and electrolytes, such as amino acids, free fatty acids, carbohydrates, and vitamins, is rapid and increases as pregnancy advances.
Transmission of Maternal Antibodies
Immunologic competence begins to develop late in the first trimester, by which time the fetus makes all of the components of complement. Immunoglobulins consist almost entirely of maternal immunoglobulin G (IgG), which begins to be transported from mother to fetus at approximately 14 weeks. In this manner, the fetus gains passive immunity against various infectious diseases. Newborns begin to produce their own IgG, but adult levels are not attained until the age of 3 years.
Hormone Production
By the end of the fourth month, the placenta produces progesterone in sufficient amounts to maintain pregnancy if the corpus luteum is removed or fails to function properly. In all probability, all hormones are synthesized in the syncytial trophoblast. In addition to progesterone, the placenta produces increasing amounts of estrogenic hormones, predominantly estriol, until just before the end of pregnancy, when a maximum level is reached. These high levels of estrogens stimulate uterine growth and development of the mammary glands.
During the first 2 months of pregnancy, the syncytiotrophoblast also produces human chorionic gonadotropin (hCG), which maintains the corpus luteum. This hormone is excreted by the mother in the urine, and in the early stages of gestation, its presence is used as an indicator of pregnancy. Another hormone produced by the placenta is somatomammotropin (formerly placental lactogen). It is a growth hormone–like substance that gives the fetus priority on maternal blood glucose and makes the mother somewhat diabetogenic. It also promotes breast development for milk production
AMNION AND UMBILICAL CORD
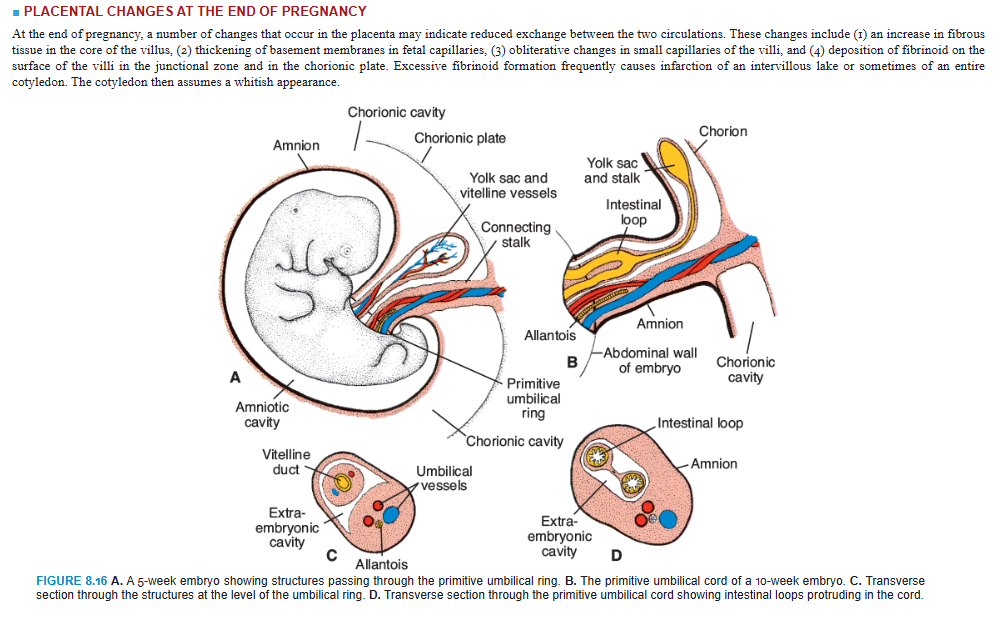
The oval line of reflection between the amnion and embryonic ectoderm (amnio-ectodermal junction) is the primitive umbilical ring. At the fifth week of development, the following structures pass through the ring (Fig. 8.16A,C): (1) the connecting stalk, containing the allantois and the umbilical vessels, consisting of two arteries and one vein; (2) the yolk stalk (vitelline duct), accompanied by the vitelline vessels; and (3) the canal connecting the intraembryonic and extraembryonic cavities (Fig. 8.16C). The yolk sac proper occupies a space in the chorionic cavity, that is, the space between the amnion and chorionic plate (Fig. 8.16B).
During further development, the amniotic cavity enlarges rapidly at the expense of the chorionic cavity, and the amnion begins to envelop the connecting and yolk sac stalks, crowding them together and giving rise to the primitive umbilical cord (Fig. 8.16B). Distally, the cord contains the yolk sac stalk and umbilical vessels. More proximally, it contains some intestinal loops and the remnant of the allantois (Fig. 8.16B,D). The yolk sac, found in the chorionic cavity, is connected to the umbilical cord by its stalk. At the end of the third month, the amnion has expanded so that it comes in contact with the chorion, obliterating the chorionic cavity (Fig. 8.10B). The yolk sac then usually shrinks and is gradually obliterated.
The abdominal cavity is temporarily too small for the rapidly developing intestinal loops, and some of them are pushed into the extraembryonic space in the umbilical cord. These extruding intestinal loops form a physiologic umbilical hernia (see Chapter 15). At approximately the end of the third month, the loops are withdrawn into the body of the embryo, and the cavity in the cord is obliterated. When the allantois and the vitelline duct and its vessels are also obliterated, all that remains in the cord are the umbilical vessels surrounded by Wharton jelly. This tissue, which is rich in proteoglycans, functions as a protective layer for the blood vessels. The walls of the arteries are muscular and contain many elastic fibers, which contribute to a rapid constriction and contraction of the umbilical vessels after the cord is tied off.
PLACENTAL CHANGES AT THE END OF PREGNANCY
At the end of pregnancy, a number of changes that occur in the placenta may indicate reduced exchange between the two circulations. These changes include (1) an increase in fibrous tissue in the core of the villus, (2) thickening of basement membranes in fetal capillaries, (3) obliterative changes in small capillaries of the villi, and (4) deposition of fibrinoid on the surface of the villi in the junctional zone and in the chorionic plate. Excessive fibrinoid formation frequently causes infarction of an intervillous lake or sometimes of an entire cotyledon. The cotyledon then assumes a whitish appearance.
AMNIOTIC FLUID
The amniotic cavity is filled with a clear, watery fluid that is produced in part by amniotic cells but is derived primarily from maternal blood. The amount of fluid increases from approximately 30 mL at 10 weeks of gestation to 450 mL at 20 weeks to 800 to 1,000 mL at 37 weeks. During the early months of pregnancy, the embryo is suspended by its umbilical cord in this fluid, which serves as a protective cushion. The fluid (1) absorbs jolts, (2) prevents adherence of the embryo to the amnion, and (3) allows for fetal movements. The volume of amniotic fluid is replaced every 3 hours. From the beginning of the fifth month, the fetus swallows its own amniotic fluid, and it is estimated that it drinks about 400 mL a day, about half of the total amount. Fetal urine is added daily to the amniotic fluid in the fifth month, but this urine is mostly water because the placenta is functioning as an exchange for metabolic wastes. During childbirth, the amniochorionic membrane forms a hydrostatic wedge that helps to dilate the cervical canal.
Clinical Correlates
Umbilical Cord Abnormalities
At birth, the umbilical cord is approximately 1 to 2 cm in diameter and 50 to 60 cm long. It is tortuous, causing false knots. Length of a cord reflects the amount of intrauterine movement of the fetus, and shortened cords have been observed in fetal movement disorders and with intrauterine constraint. An extremely long cord may encircle the neck of the fetus, usually without increased risk, whereas a short one may cause difficulties during delivery by pulling the placenta from its attachment in the uterus.
Normally, there are two arteries and one vein in the umbilical cord. In 1 in 200 newborns, however, only a single umbilical artery is present, and these babies have approximately 20% chance of having cardiac and other vascular defects. The missing artery either fails to form (agenesis) or degenerates early in development.
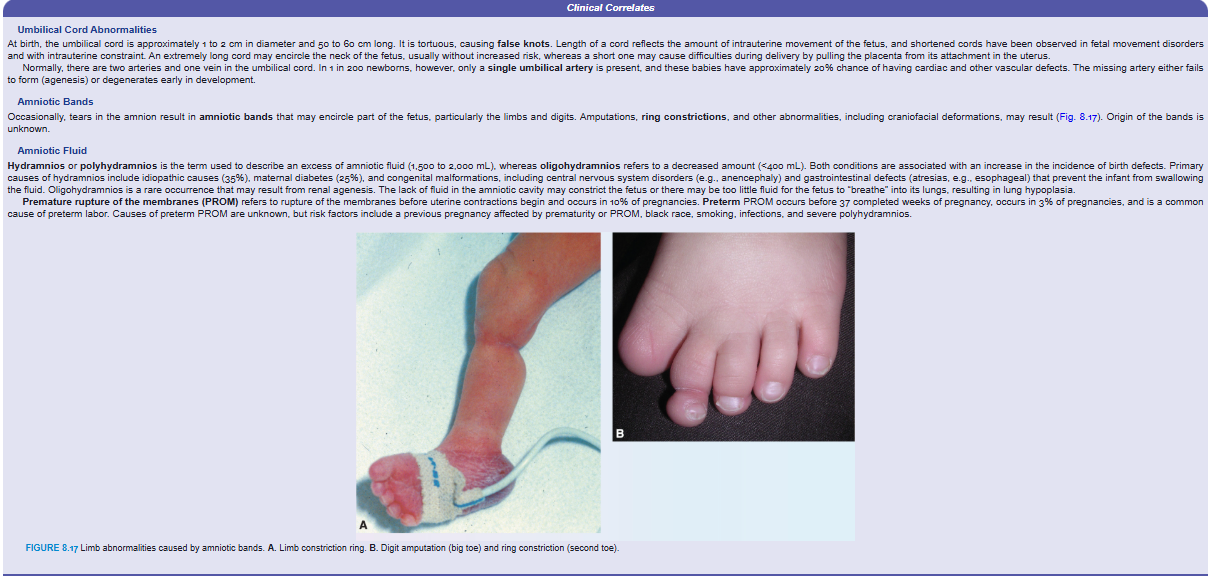
Amniotic Bands
Occasionally, tears in the amnion result in amniotic bands that may encircle part of the fetus, particularly the limbs and digits. Amputations, ring constrictions, and other abnormalities, including craniofacial deformations, may result (Fig. 8.17). Origin of the bands is unknown.
Amniotic Fluid
Hydramnios or polyhydramnios is the term used to describe an excess of amniotic fluid (1,500 to 2,000 mL), whereas oligohydramnios refers to a decreased amount (<400 mL). Both conditions are associated with an increase in the incidence of birth defects. Primary causes of hydramnios include idiopathic causes (35%), maternal diabetes (25%), and congenital malformations, including central nervous system disorders (e.g., anencephaly) and gastrointestinal defects (atresias, e.g., esophageal) that prevent the infant from swallowing the fluid. Oligohydramnios is a rare occurrence that may result from renal agenesis. The lack of fluid in the amniotic cavity may constrict the fetus or there may be too little fluid for the fetus to “breathe” into its lungs, resulting in lung hypoplasia.
Premature rupture of the membranes (PROM) refers to rupture of the membranes before uterine contractions begin and occurs in 10% of pregnancies. Preterm PROM occurs before 37 completed weeks of pregnancy, occurs in 3% of pregnancies, and is a common cause of preterm labor. Causes of preterm PROM are unknown, but risk factors include a previous pregnancy affected by prematurity or PROM, black race, smoking, infections, and severe polyhydramnios.
FETAL MEMBRANES IN TWINS
The frequency of multiple gestation (e.g., twins, triplets) has increased substantially in recent years and now accounts for over 3% of all live births in the United States. Over the last several years the rate of twin births has increased to 3.2%. The reasons for this increase are twofold: the increasing age of mothers at the time of their infants’ birth and the increasing use of fertility treatments, including assisted reproductive technologies (ARTs).
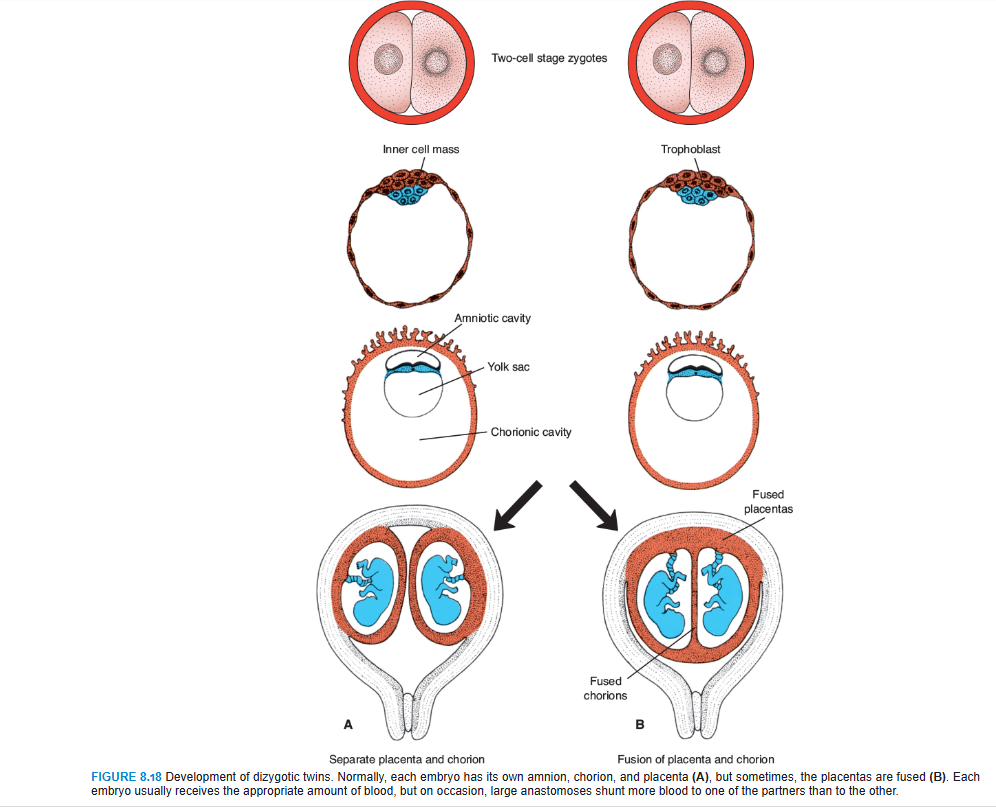
Dizygotic Twins
Approximately 90% of twins are dizygotic, or fraternal, and their incidence increases with maternal age (doubling at age 35 years) and with fertility procedures, including ART. They result from simultaneous shedding of two oocytes and fertilization by different spermatozoa. Because the two zygotes have different genetic constitutions, the twins have no more resemblance than any other brothers or sisters. They may or may not be of different sex. The zygotes implant individually in the uterus, and usually each develops its own placenta, amnion, and chorionic sac (Fig. 8.18A). Sometimes, however, the two placentas are so close together that they fuse. Similarly, the walls of the chorionic sacs may also come into close apposition and fuse (Fig. 8.18B). Occasionally, each dizygotic twin possesses red blood cells of two different types (erythrocyte mosaicism), indicating that fusion of the two placentas was so intimate that red cells were exchanged.
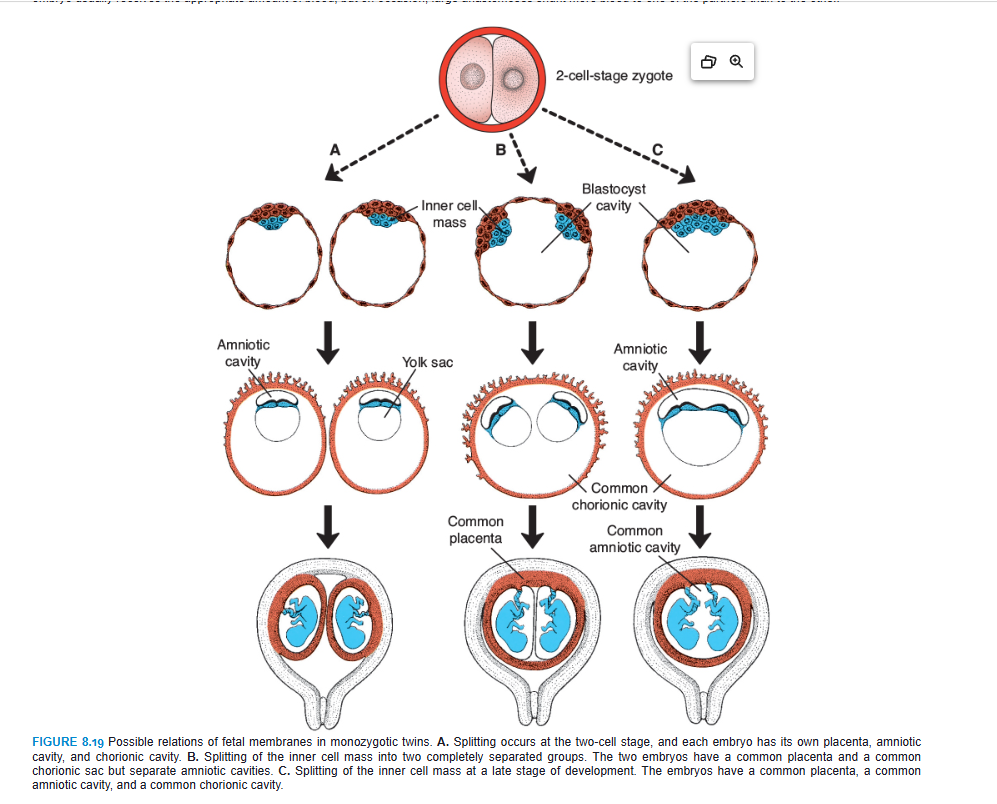
Monozygotic Twins
The second type of twins, which develops from a single fertilized ovum, is monozygotic, or identical, twins. The rate for monozygotic twins is 3 to 4 per 1,000. They result from splitting of the zygote at various stages of development. The earliest separation is believed to occur at the two-cell stage, in which case two separate zygotes develop. The blastocysts implant separately, and each embryo has its own placenta and chorionic sac (Fig. 8.19A). Although the arrangement of the membranes of these twins resembles that of dizygotic twins, the two can be recognized as partners of a monozygotic pair by their strong resemblance in blood groups, fingerprints, sex, and external appearance, such as eye and hair color.
Splitting of the zygote usually occurs at the early blastocyst stage. The inner cell mass splits into two separate groups of cells within the same blastocyst cavity (Fig. 8.19B). The two embryos have a common placenta and a common chorionic cavity but separate amniotic cavities (Fig. 8.19B). In rare cases, the separation occurs at the bilaminar germ disc stage, just before the appearance of the primitive streak (Fig. 8.19C). This method of splitting results in formation of two partners with a single placenta and a common chorionic and amniotic sac. Although the twins have a common placenta, blood supply is usually well balanced.
Although triplets are rare (about 1 per 7,600 pregnancies), birth of quadruplets, quintuplets, and so forth is rarer. In recent years, multiple births have occurred more frequently in mothers given gonadotropins (fertility drugs) for ovulatory failure.
PARTURITION (BIRTH)
For the first 34 to 38 weeks of gestation, the uterine myometrium does not respond to signals for parturition (birth). During the last 2 to 4 weeks of pregnancy, however, this tissue undergoes a transitional phase in preparation for the onset of labor. Ultimately, this phase ends with a thickening of the myometrium in the upper region of the uterus and a softening and thinning of the lower region and cervix.
Labor itself is divided into three stages: (1) effacement (thinning and shortening) and dilatation of the cervix (this stage ends when the cervix is fully dilated), (2) delivery of the fetus, and (3) delivery of the placenta and fetal membranes. Stage 1 is produced by uterine contractions that force the amniotic sac against the cervical canal like a wedge, or if the membranes have ruptured, then pressure will be exerted by the presenting part of the fetus, usually the head. Stage 2 is also assisted by uterine contractions, but the most important force is provided by increased intra-abdominal pressure from contraction of abdominal muscles. Stage 3 requires uterine contractions and is aided by increasing intra-abdominal pressure.
As the uterus contracts, the upper part retracts, creating a smaller and smaller lumen, while the lower part expands, thereby producing direction to the force. Contractions usually begin about 10 minutes apart; then, during the second stage of labor, they may occur <1 minute apart and last from 30 to 90 seconds. Their occurrence in pulses is essential to fetal survival, as they are of sufficient force to compromise uteroplacental blood flow to the fetus.
Clinical Correlates
Abnormalities Associated with Twins
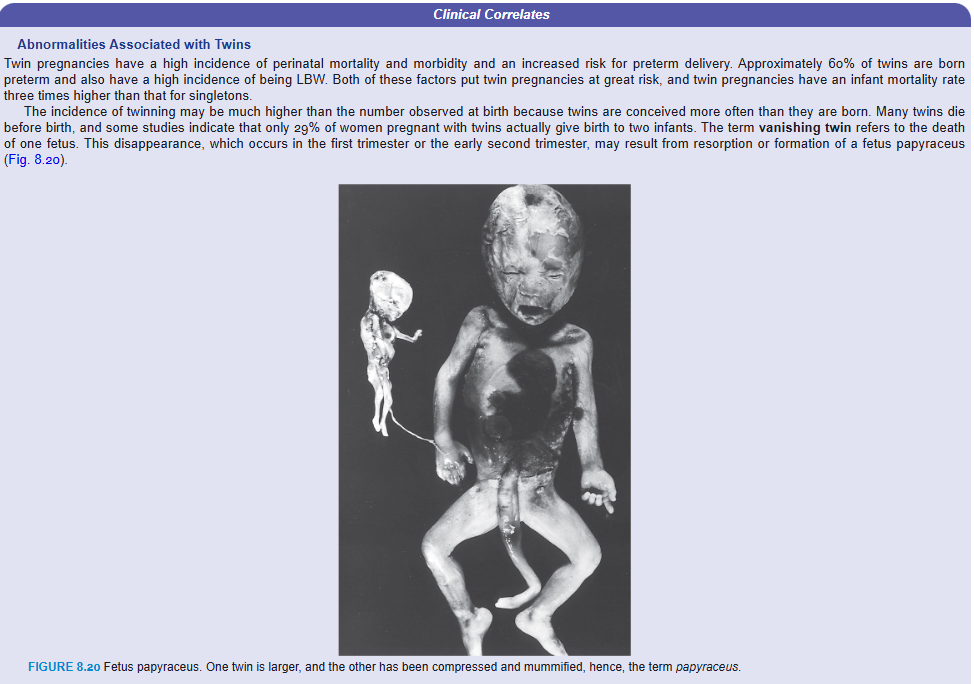
Twin pregnancies have a high incidence of perinatal mortality and morbidity and an increased risk for preterm delivery. Approximately 60% of twins are born preterm and also have a high incidence of being LBW. Both of these factors put twin pregnancies at great risk, and twin pregnancies have an infant mortality rate three times higher than that for singletons.
The incidence of twinning may be much higher than the number observed at birth because twins are conceived more often than they are born. Many twins die before birth, and some studies indicate that only 29% of women pregnant with twins actually give birth to two infants. The term vanishing twin refers to the death of one fetus. This disappearance, which occurs in the first trimester or the early second trimester, may result from resorption or formation of a fetus papyraceus (Fig. 8.20).
Another problem leading to increased mortality among twins is the twin–twin transfusion syndrome, which occurs in 15% of monochorionic monozygotic pregnancies. In this condition, placental vascular anastomoses, which occur in a balanced arrangement in most monochorionic placentas, are formed such that transfer of blood from one twin (donor/pump) to the other (recipient) occurs. As a result, the donor twin develops anemia, hypovolemia (decreased blood volume), and growth restriction, while the recipient twin develops polycythemia (excessive numbers of red blood cells) and hypervolemia that cause cardiac hypertrophy and congestive heart failure (Fig. 8.21). The outcome is poor, with the death of both twins occurring in 50% to 70% of cases.
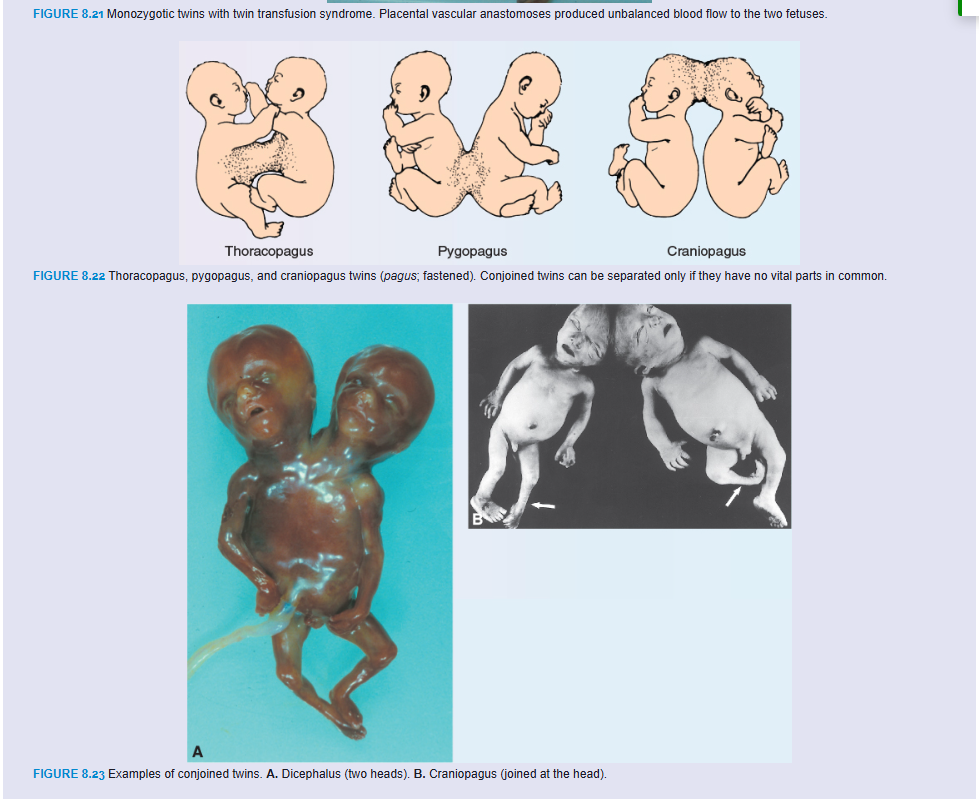
At later stages of development, partial splitting of the primitive node and streak may result in formation of conjoined twins. These twins are classified according to the nature and degree of their union (Figs. 8.22 and 8.23). Occasionally, monozygotic twins are connected only by a common skin bridge or by a common liver bridge. The type of twins formed depends on when and to what extent abnormalities of the node and streak occurred. Misexpression of genes, such as GOOSECOID, may also result in conjoined twins. Many conjoined twins have survived, including the most famous pair, Chang and Eng, who were joined at the abdomen and who traveled to England and the United States on exhibitions in the mid-1800s. Finally settling in North Carolina, they farmed and fathered 21 children with their two wives.
In brother–sister pairs of dizygotic twins, testosterone from the male twin can affect development of the female. Thus, females in such pairs tend to have square jaws, larger teeth, perform better on spatial ability tests and have better ball skills than most girls. They are 15% less likely to get married and they have fertility problems, producing 25% fewer children.
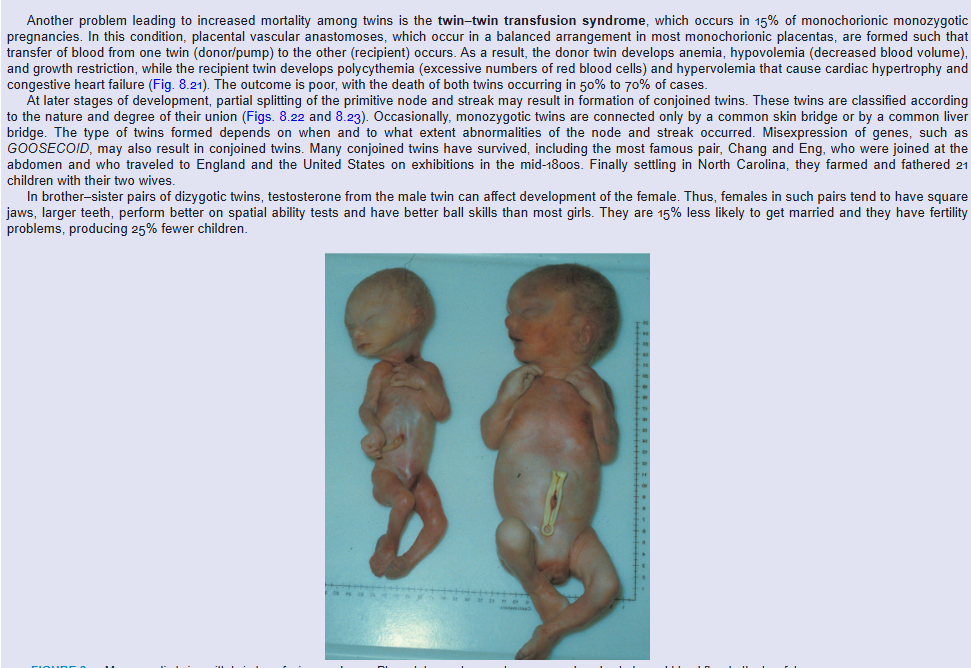
Another problem leading to increased mortality among twins is the twin–twin transfusion syndrome, which occurs in 15% of monochorionic monozygotic pregnancies. In this condition, placental vascular anastomoses, which occur in a balanced arrangement in most monochorionic placentas, are formed such that transfer of blood from one twin (donor/pump) to the other (recipient) occurs. As a result, the donor twin develops anemia, hypovolemia (decreased blood volume), and growth restriction, while the recipient twin develops polycythemia (excessive numbers of red blood cells) and hypervolemia that cause cardiac hypertrophy and congestive heart failure (Fig. 8.21). The outcome is poor, with the death of both twins occurring in 50% to 70% of cases.
At later stages of development, partial splitting of the primitive node and streak may result in formation of conjoined twins. These twins are classified according to the nature and degree of their union (Figs. 8.22 and 8.23). Occasionally, monozygotic twins are connected only by a common skin bridge or by a common liver bridge. The type of twins formed depends on when and to what extent abnormalities of the node and streak occurred. Misexpression of genes, such as GOOSECOID, may also result in conjoined twins. Many conjoined twins have survived, including the most famous pair, Chang and Eng, who were joined at the abdomen and who traveled to England and the United States on exhibitions in the mid-1800s. Finally settling in North Carolina, they farmed and fathered 21 children with their two wives.
In brother–sister pairs of dizygotic twins, testosterone from the male twin can affect development of the female. Thus, females in such pairs tend to have square jaws, larger teeth, perform better on spatial ability tests and have better ball skills than most girls. They are 15% less likely to get married and they have fertility problems, producing 25% fewer children.
Clinical Correlates
Preterm Birth
Factors initiating labor are not known and may involve “retreat from maintenance of pregnancy,” in which pregnancy-supporting factors (e.g., hormones) are withdrawn, or active induction caused by stimulatory factors targeting the uterus. Probably, components of both phenomena are involved. Unfortunately, a lack of knowledge about these factors has restricted progress in preventing preterm birth. Preterm birth (delivery before 37 completed weeks) of premature infants occurs in approximately 12% of births in the United States and is a leading cause of infant mortality and also contributes significantly to morbidity. It is caused by preterm PROM, premature onset of labor, or pregnancy complications requiring premature delivery. Risk factors include previous preterm birth; black race; multiple gestations; infections, such as periodontal disease and bacterial vaginosis; and low maternal body mass index.
SUMMARY
The fetal period extends from the beginning of the ninth week of gestation until birth and is characterized by rapid growth of the body and maturation of organ systems. Growth in length is particularly striking during the third, fourth, and fifth months (approximately 5 cm per month), whereas increase in weight is most striking during the last 2 months of gestation (approximately 700 g per month) (Table 8.1, p. 108). Most babies weigh between 2,700 and 4,000 g (6 to 9 lb) at birth. Those babies weighing <2,500 g (5 lb 8 oz) are considered low birth weight; those below 1,500 g (3 lb 5 oz) are considered very low birth weight. IUGR is a term applied to babies who do not achieve their genetically determined potential size and are pathologically small. This group is distinct from babies that are healthy but are below the 10th percentile in weight for their gestational age and are classified as SGA.
A striking change is the relative slowdown in the growth of the head. In the third month, it is about half the size of the CRL. By the fifth month, the size of the head is about one-third of the CHL, and at birth, it is one-quarter of the CHL (Fig. 8.2).
During the fifth month, the mother can clearly recognize fetal movements, and the fetus is covered with fine, small hair.
A fetus born during the sixth or the beginning of the seventh month has difficulty surviving, mainly because the respiratory and central nervous systems have not differentiated sufficiently.
In general, the length of pregnancy for a full-term fetus is considered to be 280 days, or 40 weeks after onset of the last menstruation, or, more accurately, 266 days or 38 weeks after fertilization.
The placenta consists of two components: (1) a fetal portion, derived from the chorion frondosum or villous chorion, and (2) a maternal portion, derived from the decidua basalis. The space between the chorionic and decidual plates is filled with intervillous lakes of maternal blood. Villous trees (fetal tissue) grow into the maternal blood lakes and are bathed in them. The fetal circulation is at all times separated from the maternal circulation by (1) a syncytial membrane (a chorion derivative) and (2) endothelial cells from fetal capillaries. Hence, the human placenta is of the hemochorial type.
Intervillous lakes of the fully grown placenta contain approximately 150 mL of maternal blood, which is renewed three or four times per minute. The villous area varies from 4 to 14 m2, facilitating exchange between mother and child.
Main functions of the placenta are (1) exchange of gases; (2) exchange of nutrients and electrolytes; (3) transmission of maternal antibodies, providing the fetus with passive immunity; (4) production of hormones, such as progesterone, estradiol, and estrogen (in addition, it produces hCG and somatomammotropin); and (5) detoxification of some drugs.
The amnion is a large sac containing amniotic fluid in which the fetus is suspended by its umbilical cord. The fluid (1) absorbs jolts, (2) allows for fetal movements, and (3) prevents adherence of the embryo to the amnion. The fetus swallows amniotic fluid, which is absorbed through its gut and cleared by the placenta. The fetus adds urine to the amniotic fluid, but this is mostly water. An excessive amount of amniotic fluid (hydramnios) is associated with anencephaly and esophageal atresia, whereas an insufficient amount (oligohydramnios) is related to renal agenesis.
The umbilical cord, surrounded by the amnion, contains (1) two umbilical arteries; (2) one umbilical vein; and (3) Wharton jelly, which serves as a protective cushion for the vessels.
Fetal membranes in twins vary according to their origin and time of formation. Two-thirds of twins are dizygotic, or fraternal; they have two amnions, two chorions, and two placentas, which sometimes are fused. Monozygotic twins usually have two amnions, one chorion, and one placenta. In cases of conjoined twins, in which the fetuses are not entirely split from each other, there is one amnion, one chorion, and one placenta.
Signals initiating parturition (birth) are not clear, but preparation for labor usually begins between 34 and 38 weeks. Labor itself consists of three stages: (1) effacement and dilatation of the cervix, (2) delivery of the fetus, and (3) delivery of the placenta and fetal membranes.
Problems to Solve
An ultrasound at 7 months’ gestation shows too much space (fluid accumulation) in the amniotic cavity. What is this condition called, and what are its causes?
Later in her pregnancy, a woman realizes that she was probably exposed to toluene in the workplace during the third week of gestation but tells a fellow worker that she is not concerned about her baby because the placenta protects her infant from toxic factors by acting as a barrier. Is she correct?