Chemistry - Separating Mixtures
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
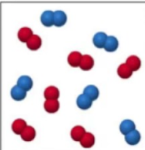
What is this?
It is a mixture

What is this?
It is a element
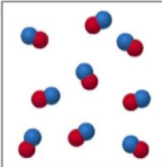
What is this?
It is a compound
What is an element?
A pure substance made of only one atom
What is a compound?
A substance formed from two or more different elements chemically joined together
What is a mixture?
A combination of two or more substances that are not chemically joined together
What is a pure substance?
Something made of just one element or compound
What is a physical change?
A type of change in which no new substance is formed (e.g. melting ice)
What is a chemical change?
A change that results in new substances being formed (e.g. rusting iron)
What does filtration separate?
Filtration separates solids from liquids (e.g. sand from water)
What does evaporation separate?
Evaporation separates dissolved solids from a liquid (e.g. salt from seawater)
What is simple distillation used for?
It is used for separating a liquid from a solution based on boiling points (e.g. water from seawater)
What does fractional distillation used for?
It is used for mixtures of liquids with different boiling points (e.g. crude oil)
What does a separating funnel separate?
It separates immiscible liquids (e.g. oil and water)
What does chromatography separate?
It separates components of a mixture based on different rates of movements (e.g. ink colours)
What is a saturated solution?
A solution that has so much solute in it that it can’t dissolve any more
What does miscible mean?
Two liquids that dissolve together
What does immiscible mean?
Two liquids that don’t mix (e.g. oil and water)