Topic 12: Acid-base Equilibria
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Brønsted–Lowry acid
A Brønsted–Lowry acid is a proton (H+ ion) donor.
Brønsted–Lowry base
A Brønsted–Lowry base is a proton (H+ ion) acceptor.
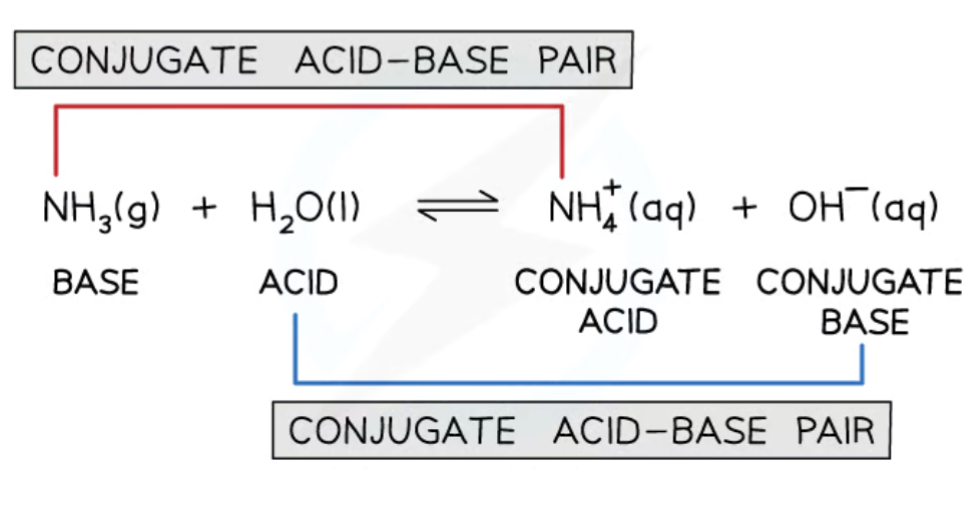
Acid-base reactions
In an acid-base reaction, protons are transferred from the acid to the base.
The acid loses a proton and forms a conjugate base, forming an acid-conjugate base pair that have one proton difference.
The base gains a proton and forms a conjugate acid, forming a conjugate acid-base pair that have one proton difference.
Difference between a strong acid and a weak acid
A strong acid completely dissociates into ions in aqueous solution. For example, HCl (aq) → H+ (aq) + Cl- (aq) shows the dissociation of a strong acid. It is said to be an irreversible reaction because the equilibrium position is so far to the right.
A weak acid only partially dissociates into ions in an aqueous solution. For example, CH3COOH (aq) ⇌ CH3COO- (aq) + H+ (aq) shows the dissociation of a weak acid. It is a reversible reaction, and the equilibrium position is on the left.
Difference between a strong base and a weak base
A strong base completely dissociates into ions in an aqueous solution. It is said to be an irreversible reaction because the equilibrium position is so far to the right.
A weak base only partially dissociates into ions in an aqueous solution. It is a reversible reaction, and the equilibrium position is on the left.
Amphoteric substance
An amphoteric substance can act as both an acid and a base.
pH definition
The pH of an aqueous solution is defined as pH = -log [H+] where [H+] is the hydrogen ion concentration, measured in mol dm-3.
Acid dissociation constant, Ka
Ka quantifies how much a weak acid dissociates into its ions at equilibrium.
For a weak acid, HX: HX (aq) ⇌ H+ (aq) + X- (aq)
Ka = [H+][X-] / [HX], where [HX] is the concentration of the acid and [H+] and [X-] are the concentrations of the ions at equilibrium.
The initial concentration of the acid can be used because often the equilibrium position is so far to the left that the initial concentration and the equilibrium concentration is almost the same.
The Ka value of a weak acid can be used to calculate its pH.
The higher the Ka, the stronger the acid.
pKa
pKa = -log Ka
The lower the pKa, the stronger the acid.
Ionic product of water, Kw
Water self-ionises: H2O (l) ⇌ H+ (aq) + OH- (aq)
Kw is the equilibrium constant for water’s self-ionisation.
Kw = [H+][OH-], where [H+] and [OH-] are the concentrations of the ions at equilibrium.
[H2O] is excluded from the calculation because the concentration of water is constant.
pKw
pKw = -log Kw
Finding the pH of a strong base
A base contains the H+ ions from the self-dissociation of water.
The addition of OH- ions to water in a basic solution shifts the equilibrium position of the self-dissociation of water to the left, but the value of Kw must stay the same.
To find the new concentration of H+ ions, rearrange [H+][OH-] = Kw to Kw / [OH-] = [H+], and input the concentration of [OH-] from the base.
End point of titration
When the indicator changes colour.
Equivalence point of titration
When all of the analyte has reacted with the titrant.
Equivalence point of titration curve
The midpoint of the vertical section of the curve
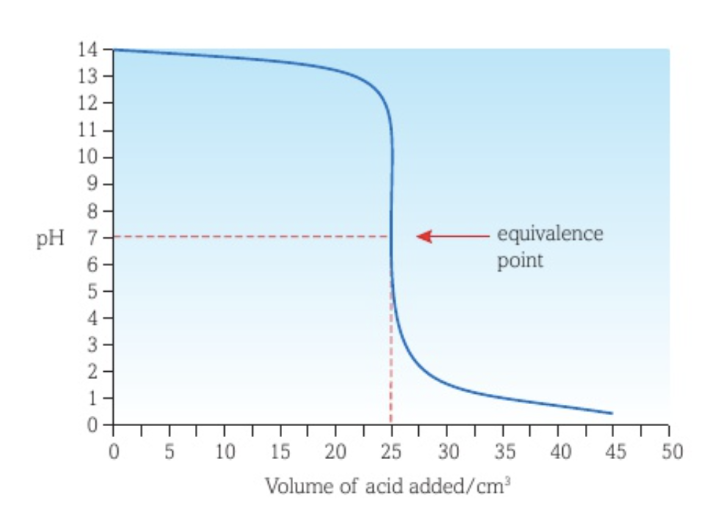
Titration curve between a strong acid and a strong base with the acid as titrant
There is a long vertical section of the curve.
The equivalence point is at a pH of 7.
The minimum pH reached is very low.
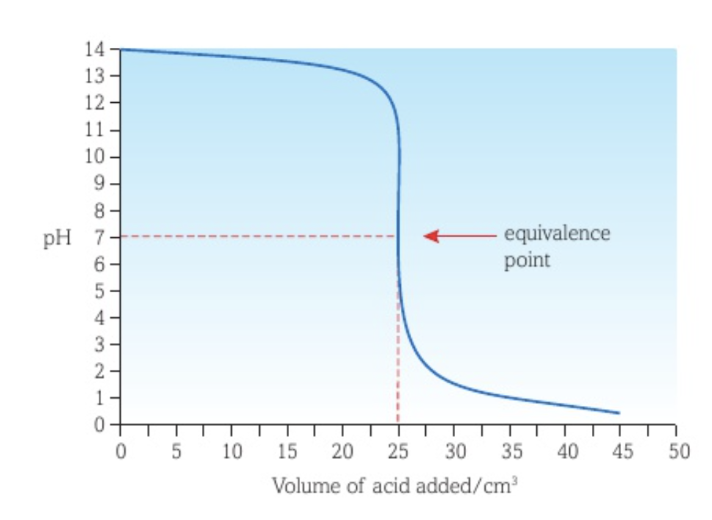
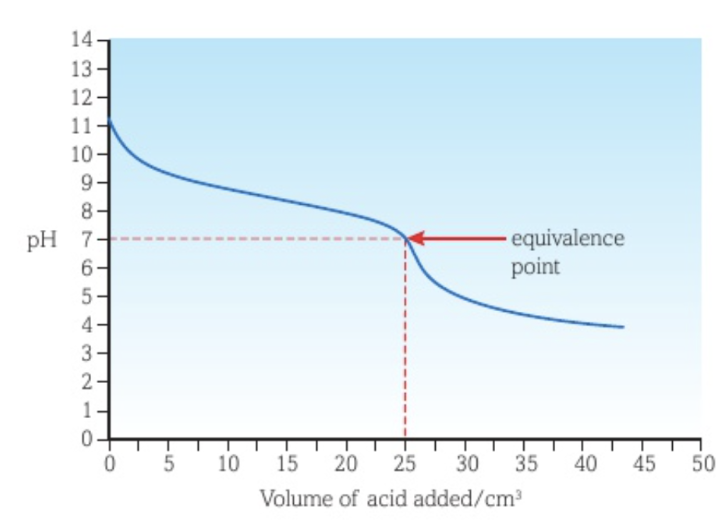
Titration curve between a weak acid and a strong base with the acid as titrant
The vertical section of the curve is shorter than the curve of a strong acid - strong base titration.
The equivalence point is at a pH higher than 7.
There is little pH change before the vertical section of the curve because the acid added is weak.
There is a buffer region after the vertical section of the curve.
The lowest pH reached is greater than that of the curve of a strong acid - strong base titration.
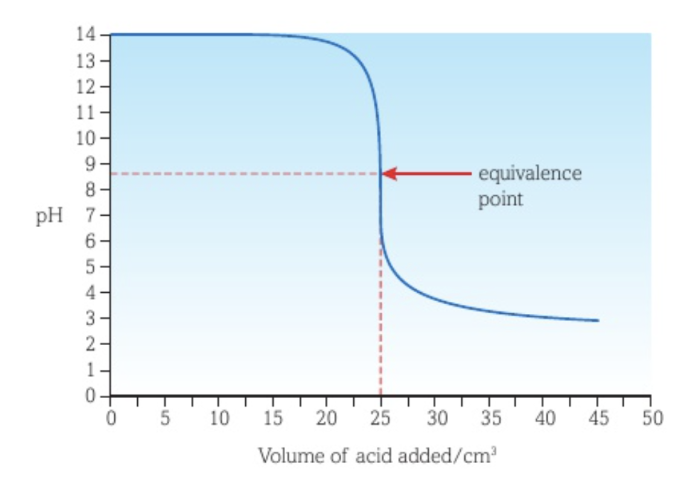
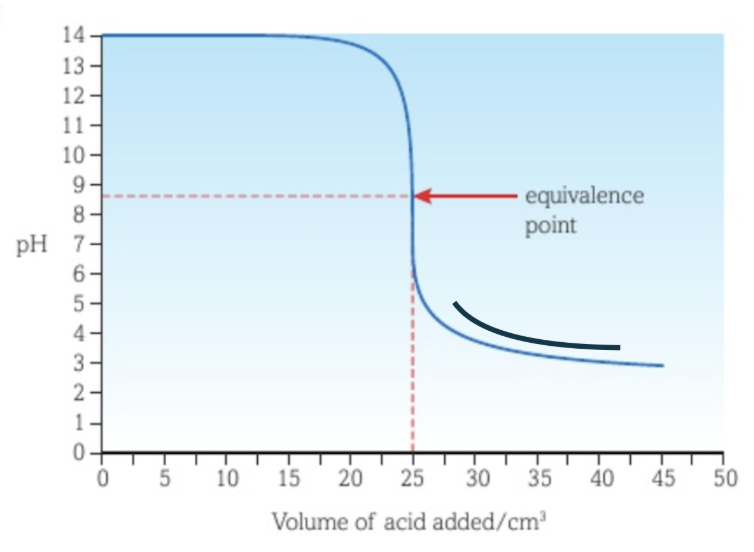
Titration curve between a strong acid and a weak base with the acid as titrant
The vertical section of the curve is shorter than the curve of a strong acid - strong base titration.
There is a buffer region before the vertical section of the curve.
The equivalence point is at a pH lower than 7.
The lowest pH reached is similar to that of the curve of a strong acid - strong base titration.
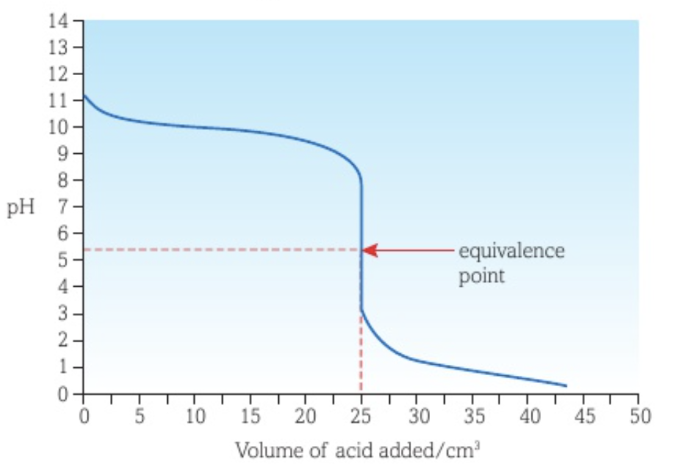
Titration curve between a weak acid and a weak base with acid as titrant
There is no vertical section of the curve, only a point of inflexion.
The lowest pH reached is greater than that of the curve of a strong acid - strong base.
The equivalence point is at a pH of 7.
KIn and pKIn
An indicator is a weak acid or base, so it dissociates into ions reversibly: HIn (aq) ⇌ H+ (aq) + In- (aq)
KIn is the equilibrium constant for an indicator.
KIn = [H+][In-] / [HIn]
When the indicator is in the middle of its colour range, [HIn] will be equal to [In-], so the equation simplifies to KIn = [H+]
Since pH = -log [H+] and pKIn = -log KIn, pKIn = pH. This means that the pH when an indicator is in the middle of its colour range is equal to the pKIn of the indicator.
How to select a suitable indicator
The pH range of the indicator’s change in colour should be in the pH range of the vertical part of the curve.
The pKIn value of the indicator should be as close as possible to the pH at the equivalence point of the titration.
Buffer solution
A buffer solution minimises the change in pH when a small amount of acid or base is added.
It is made from excess weak acid with its conjugate base or from excess weak base with its conjugate acid.
How a buffer solution of ethanoic acid sodium ethanoate works
Ethanoic acid partially dissociates into ions: CH3COOH (aq) ⇌ CH3COO- (aq) + H+ (aq)
Sodium ethanoate fully dissociates into ions: CH3COONa (aq) → CH3COO- (aq) + Na+ (aq)
The buffer consists of ethanoic acid as the weak acid and CH3COO- ions as its conjugate base.
Sodium ethanoate provides a large reservoir of ethanoate ions, as it fully dissociates, and ethanoic acid provides a large reservoir of itself because the equilibrium position is far to the left.
When H+ ions are added, most of them react with ethanoate ions to make ethanoic acid: CH3COO- (aq) + H+ (aq) ⇌ CH3COOH (aq). Not all of the H+ ions added can be removed because this reaction is reversible.
When OH- ions are added, they react with ethanoic acid: CH3COOH (aq) + OH- (aq) → CH3COO- (aq) + H2O (l).
When OH- ions are added they also react with H+ ions to make water, decreasing the concentration of H+ ions: H+ (aq) + OH- (aq) ⇌ H2O (l). This causes the equilibrium position to shift to the right to increase their concentration again.
Since there is a large reservoir of ethanoic acid and ethanoate ions, the small changes to their concentrations as a result of the addition of H+ or OH- ions does not change the ratio of their concentrations significantly, so the pH is controlled.
What is the pH of the buffer solution containing 0.35 mol dm-3 methanoic acid and 0.67 mol dm-3 sodium methanoate? The Ka of methanoic acid is 1.6 x 10-4 mol dm-3.
HCOOH (aq) ⇌ HCOO- (aq) + H+ (aq) and HCOONa (aq) → HCOO- (aq) + Na+ (aq)
Ka = [H+][HCOO-] / [HCOOH]
Rearrange to obtain ( Ka [HCOOH] ) / [HCOO-] = [H+]
Methanoic acid is assumed to only negligibly dissociate, so the concentration of it at equilibrium is said to be the same as the starting concentration.
[H+] = (1.6)(10-4)(0.35) / 0.67 = 8.4 x 10-5 mol dm-3
pH = -log (8.4 x 10-5) = 4.08
Buffer action in a weak acid - strong base titration
When the strong base and the weak acid are mixed, the acid reacts with the base to form its conjugate base, forming a buffer solution: XH (aq) ⇌ X- (aq) + H+ (aq) and XH (aq) + OH- (aq) → X- (aq) + H2O (l) where XH is the weak acid and X- is the conjugate base.
This makes the pH change more gradual, shown by a gradient of the titration curve that is smaller.
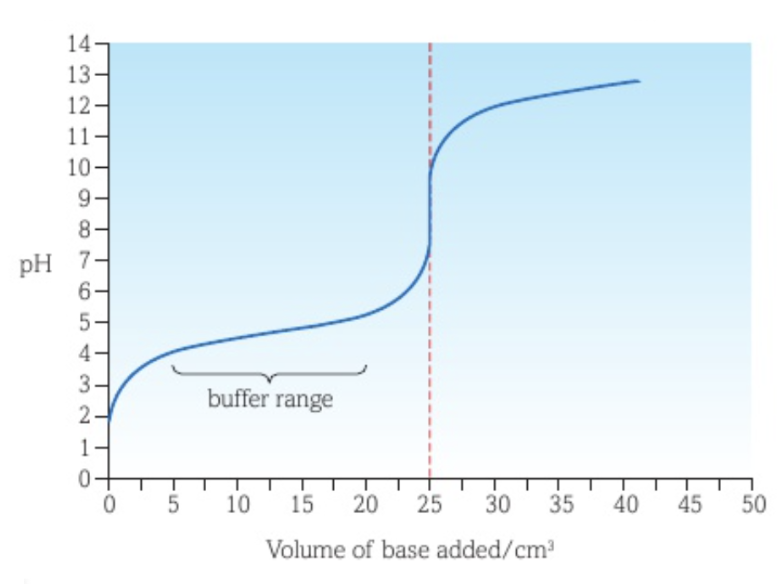
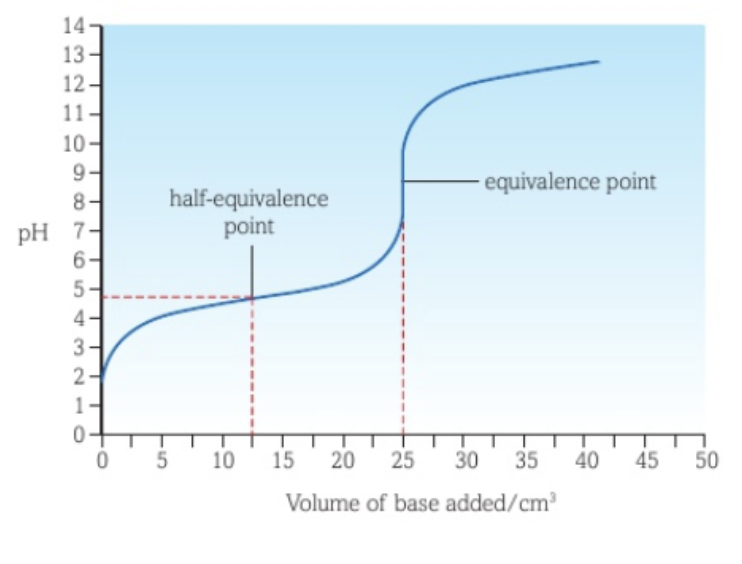
Half-equivalence point
The half-equivalence point is the point where half of the analyte has reacted.
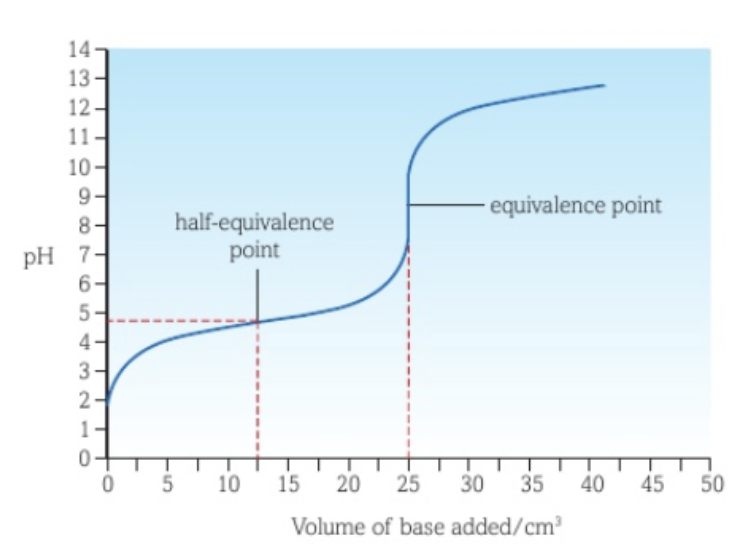
Determining Ka of a weak acid in a weak acid - strong base titration curve
When a strong base is added to a weak acid as titrant, the pH at the half-equivalence point is equal to the pKa value of the weak acid, as half of the weak acid has been converted to its conjugate base, making their concentrations the same.
Half of the weak acid has produced its salt, which then dissociates into the weak acid’s conjugate base.
This can be mathematically proven: XH (aq) ⇌ X- (aq) + H+ (aq) and YX (aq) → X- (aq) + Y+ (aq). Ka = [X-][H+] / [XH]. Since [X-] = [XH], this simplifies to Ka = [H+]. -log(Ka) = -log[H+], so pKa = pH.
The Ka of the acid can be found by using Ka = 10-pH
Diprotic acid
An acid that can donate two protons (H+)
Enthalpy change of neutralisation difference between strong and weak acids
The enthalpy change of neutralisation of a strong acid and a strong base will always have a similar value because they are almost completely dissociated into ions, so the same reaction of H+ (aq) + OH- (aq) → H2O (l) will occur each time.
Weak acids often have enthalpy changes of neutralisation that are much less than that of a strong acid with a strong base because some energy must be used to weaken the bond connecting the H+ ion to the acid and to therefore dissociate the weak acid molecules so they can react.
Buffer action in blood
Blood has a buffer system to maintain an almost constant pH of blood.
H2CO3 (aq) ⇌ HCO3- (aq) + H+ (aq)
When carbon dioxide dissolves in the blood, carbonic acid is produced.
The equilibrium position shifts to the right, producing more H+ ions.
The large reservoir of HCO3- ions react with any added H+ ions to help control the pH.
If the number of H+ ions decreases, the equilibrium position will shift to the right to increase the number of H+ ions back to a normal level.
NH3
A weak base
The reason the proton gets donated from the carboxylic group rather than the methyl group in ethanoic acid
For a proton to be donated, the bond holding it must be highly polarised.
The O-H bond is highly polarised because oxygen is much more electronegative than hydrogen, so it is easier for the H+ ion to be removed.
The C-H bond has low polarity because carbon and hydrogen have similar electronegativities, so the H+ ion is not easily removed.