UV spectroscopy
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
What is UV spectroscopy? Why is it used in pharmacy? Why is it beneficial?
-an analytical technique
-When light passes through a sample, certain wavelengths of the light are absorbed by the molecules present, and by measuring this absorption we can both identify compounds and determine their concentrations.
-it’s quick, precise, and non-destructive – the sample can be recovered afterwards for other tests.
What do wavelengths tell us?
-the energy required to promote electrons within molecules to higher energy states, particularly in certain types of chemical bonds. Different molecules absorb different wavelengths of light depending on their chemical structure.
What does the wavelengths of light that a molecule absorbs depend on?
-its chemical structure
Do all molecules absorb light in the UV-visible region?
no
In order for molecules to absorb light in the UV-visible region, what must they contain?
contain conjugated double bonds and/or aromatic rings.
200-400nm= UV light
400-700nm=visible light
The longer the conjugation of the molecule…
-the longer the UV wavelength absorption
Explain the laws of spectrophotometry
-When a beam of radiation passes through a sample, e.g. a solution, several things happen:
(a) some of the radiation is absorbed by the sample
(b) some of the radiation is reflected/scattered
(c) some radiation passes straight through.
-For analytical purposes we’re interested in measuring the amount of radiation absorbed by the sample and therefore need to eliminate/minimise the effects of reflection and scattering. This is done by taking 𝐼0 as equal to the intensity passing through the cell when filled with a blank solution (everything except the substance being measured), and 𝐼 as the intensity passing through the cell when filled with the sample solution.
How do we describe/quantify the amount of radiation which passes through the sample in spectrophotometry?
By calculating transmittance
I₀ represents the incident light intensity — the amount of light that initially hits the sample before passing through it.
I represents the transmitted light intensity — the amount of light that successfully passes through the sample.
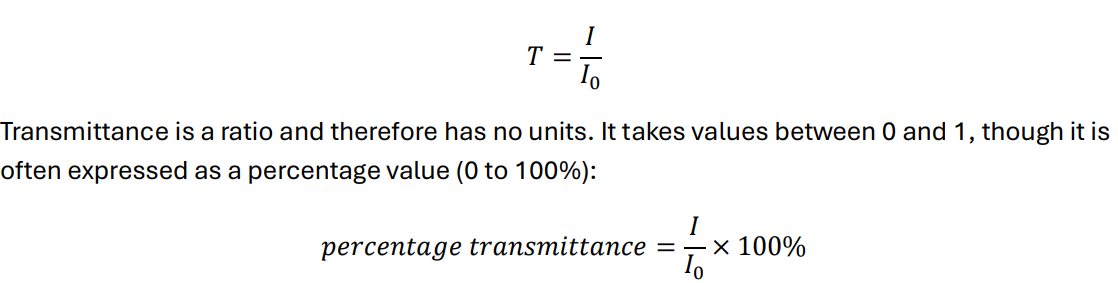
What does transmittance value depend on? (3) (i.e. T or I)
-the path length of the cell
-the concentration of the sample
-the nature of the substance
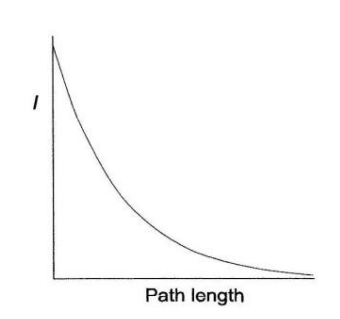
What is the relationship between T or I and the length of the cell? How does this look graphically?
- 𝐼 decreases exponentially with an increase in path length
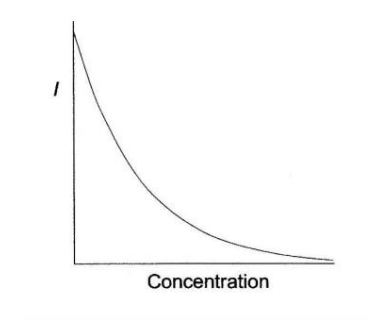
What is the relationship between transmittance and the concentration of the solution? How does this look graphically?
-transmittance decreases exponentially with an increase in concentration
-This aligns with the Beer-Lambert Law, which states that as the concentration of a solution increases, more light is absorbed, and less light is transmitted through the solution.
What is absorbance directly proportional to?
-concentration. Higher concentration=higher absorption (but too high concentration, then no)
Explain the Beer-Lambert Law
𝐴 = 𝑎𝑐l
𝑎 (or 3 backwards)= molar absorption coefficient. The units for the absorption coefficient will depend upon those used for concentration and path length. If 𝑙 is in cm and 𝑐 is in mol dm-3, then 𝑎= molar absorption coefficient and given the symbol 𝜖 (epsilon). It will have the units dm3 mol-1 cm-1 or (M-1 cm-1 ). The molar absorption coefficient, therefore, represents the absorption of a 1M solution in a cell of path length 1 cm.
A=absorbance
A 2.305 x 10-5 M solution of a substance had an absorbance of 0.497 in a 0.5 cm path length cell. Calculate the molar absorption coefficient for the substance.
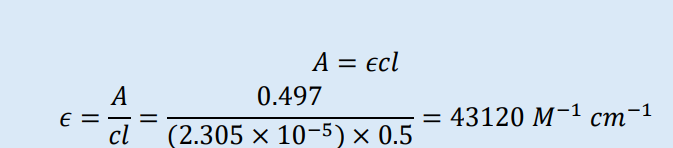
What is specific absorbance? How do you calculate it?
-If 𝑙 is in cm and 𝑐 is in % m/v (i.e. g/100 cm3 )
-symbol= 𝐴(1%, 1 cm).
-The specific absorbance represents the absorbance of a 1% m/v solution in a cell of path length 1 cm.
-Specific absorbance= A/c x length
A is normal abs.
A (1%, 1cm)= specific abs
The absorbance of a 0.00140% m/v solution of tolbutamide in methanol when measured in a 1 cm path length cell was found to be 0.715 at 228 nm. Calculate the specific absorbance.
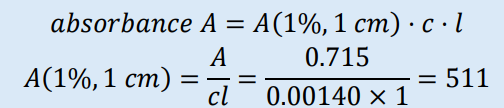
Can a single compound have multiple peaks on an absorption spectrum?
yes
What is the equation to find transmittance?
I ÷ I0
Why does transmittance have no units?
-because it’s a ratio
What kind of values does transmittance take?
-Values between 0 and 1
How is transmittance often expressed as and what is the equation for this?
-as a %
What is the dependence of absorption on conc. and path length known as?
-The beer-lambert law A= a x c x L
What does the molar absorption coefficient represent?
-the absorption of a 1M solution in a cell of path length 1cm
What is the molar absorption coefficient useful for?
-For comparing the light absorbing abilities of different substances on a molecule for molecule basis
What is the symbol for specific absorbance?
A(1%, 1cm)
What does specific absorbance represent?
-The absorbance of a 1%m/v solution in a cell of path length 1cm
How should the wavelength for an assay be chosen?
-So that only the substance of interest (analyte) absorbs, with no absorbance from any impurities
What absorbance should the wavelength for a sample correspond to and why?
-The wavelength should correspond to an absorbance maximum and not on the sloping portion of the curve otherwise a small error in setting the wavelength will have a large effect on the absorbance value
What are the two methods for converting absorbance from UV spectroscopy to concentration?
->calibration curve
-> the beer-lambert law -> needs given value for the absorption coefficient
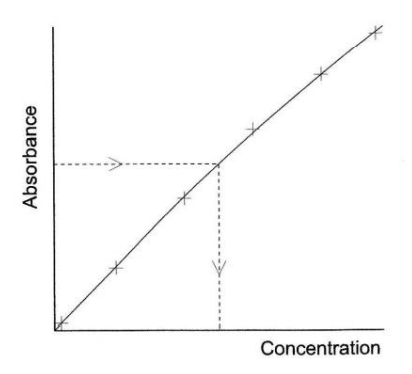
Walk through a calibration curve:
-> typically 6-8 standard solutions are prepared from a sample of the pure material that is to be determined and their absorbance measured (how much light is absorbed by the sample or doesn’t get transmitted) not absorbed=transmitted=goes through sample
-> The values are then used to construct a calibration curve
-> The sample absorbance is measured and its concentration read off the calibration curve
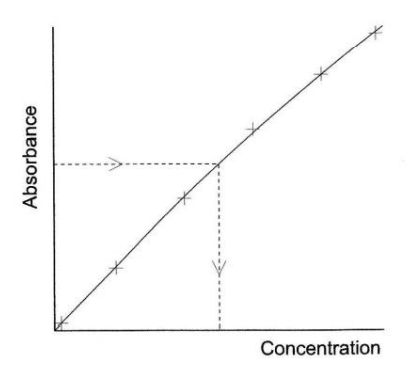
Why is the calibration curve method in UV analysis good?
-It can be used even if the Beer-Lambert law doesn’t hold (i.e. absorbance vs. concentration plot is curved)
Why is the calibration curve method in UV analysis bad?
-A pure sample of the analyte must be available -> Note that this is a very time consuming process
Walk through using the beer lambert law and absorbance coefficient in UV analysis
-The absorbance for the sample solution is measured and the concentration calculated using the Beer-Lambert law and a given for the specific absorbance of molar absorption coefficient
What is the equation for mass of drug per average tablet?
-Mass per tablet = mass determined x (Mass of tablet ÷ mass of sample)
How is it possible to determine each component from UV analysis even though in a mixture of substances substances it is quite probable that no region of the UV-visible spectrum can be found in which just one component absorbs?
-By making measurements at a number of wavelengths -> If we assume that the components of the mixture do not react with one another, then the absorbance A(l) at some wavelength will be given by the sum of the absorbances for all the individual components
What is the equation for absorbance from individual components of a mixture?
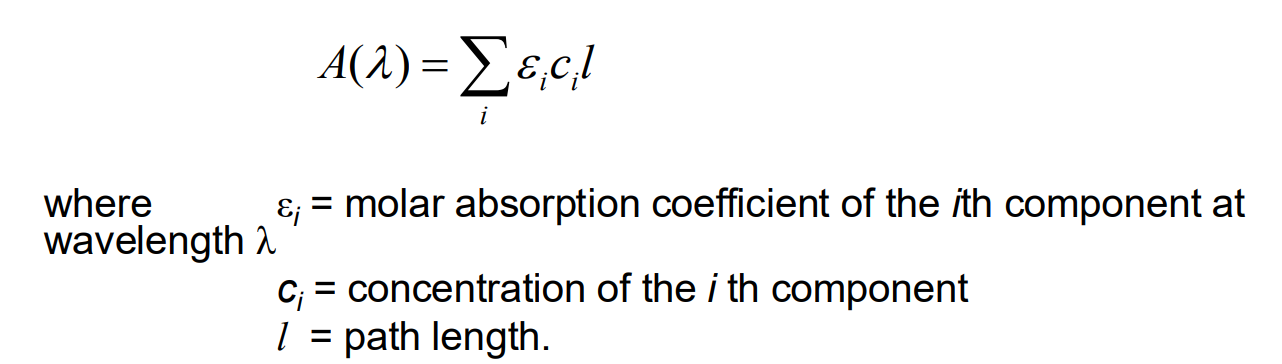
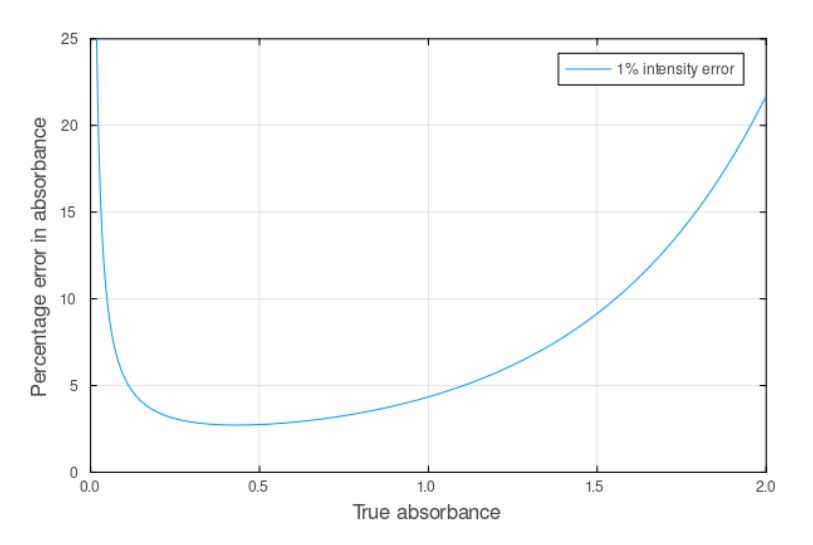
Explain the precision of absorbance measurements
error=curved
Optimal range: 0.2-0.8 AU. What you want to aim for. (highest precision measurements)
• Low A (<0.2): Poor precision as measuring tiny changes in strong signal (I ≈ I₀) poor precision of measuring strong signal
• High A (>0.8): Poor precision of measuring weak signal
Why might calibration curves show non-linear behaviour? (i.e. not a straight slope) (3)
-as you’re increasing the concentration, you’re changing the chemistry. E.g. more association between the molecules, which could lead to the way that they interact with light to change, which could cause a change in the absorption coefficient because you’re forming dimers or aggregates, change in configuration
-there was an interference with stray light. This could make it seem as if more light is going through the sample, but in reality the light isn’t passing through the sample; it’s light bouncing back. This is why the inside of a spectrophotometer is black
-another possibility is non-monochromatic radiation
What is optical density?
-all light losses, including scattering
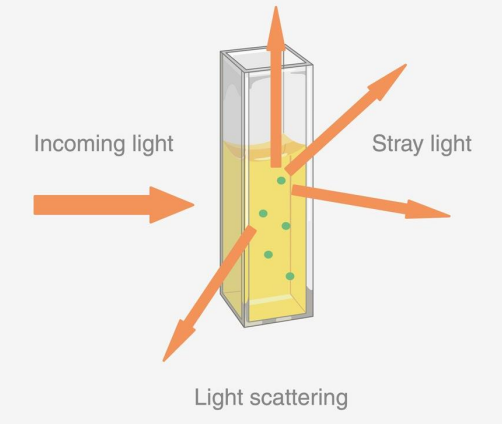
What is scattering?
-light deflected in all directions
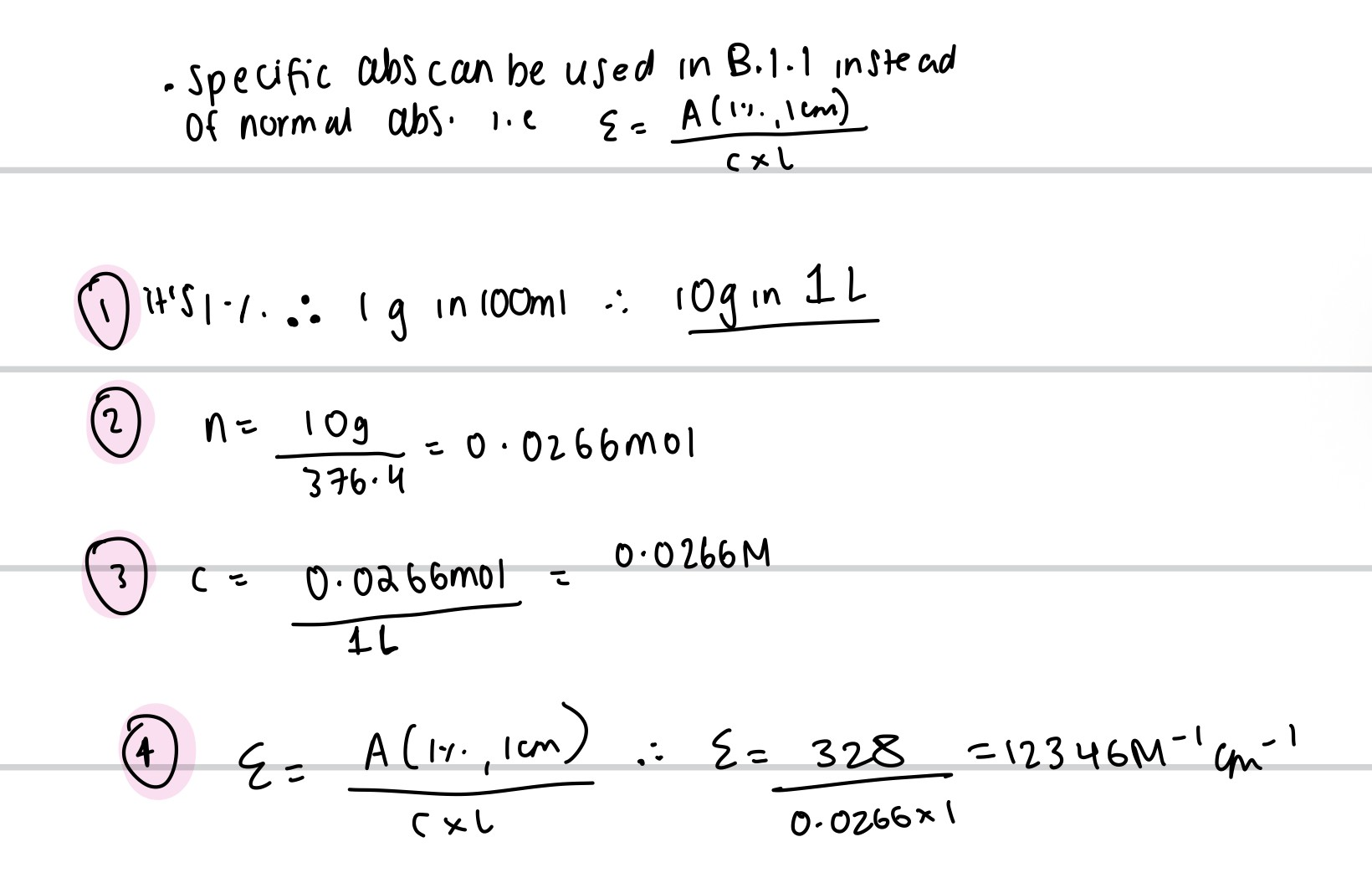
The BP gives the specific absorbance A(1%,1cm) for riboflavin (Mr = 376.4) as 328 at 444 nm. Calculate the value of the molar absorption coefficient for riboflavin at 444 nm.
(1%,1cm) means absorbance for a 1% solution with path length 1cm
-remember, molar absorption coefficient means 1M solution, 1 cm path length. so, we use 10g/L instead of 1g/100ml

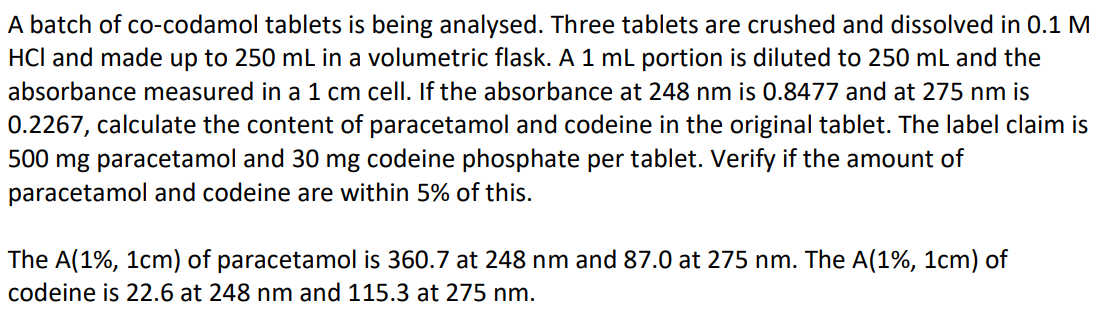
First, identify what we know:
• 3 tablets in 250 mL (Solution A)
• 1 mL of Solution A diluted to 250 mL (Solution B)
• Absorbances of Solution B: A₂₄₈ = 0.8477, A₂₇₅ = 0.2267
• Path length = 1 cm •
Specific absorbances (A(1%, 1cm)):
• Paracetamol: 360.7 (248 nm), 87.0 (275 nm) • Codeine: 22.6 (248 nm), 115.3 (275 nm)
Then, check image

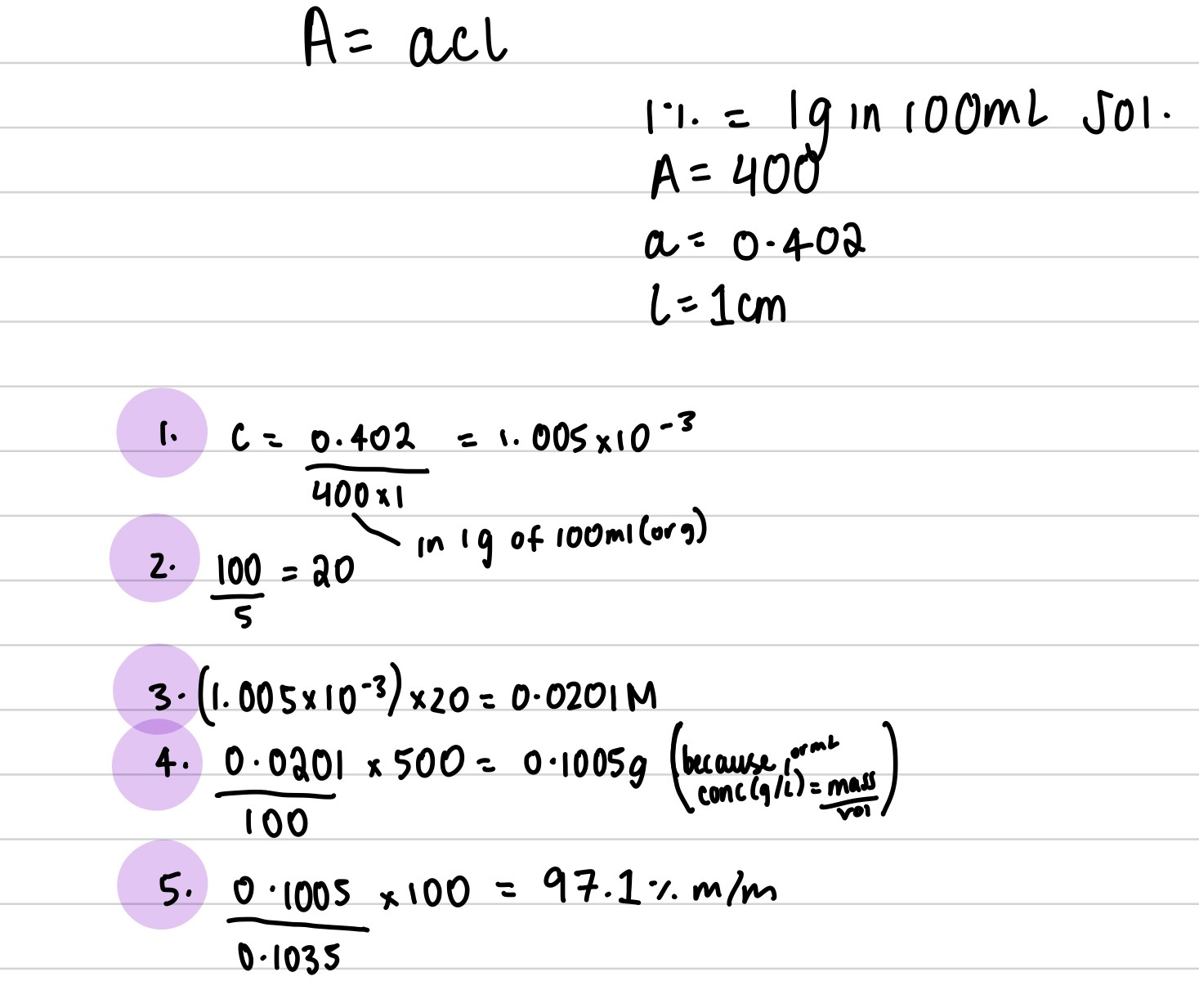
In a spectrophotometric assay for the local anaesthetic cyclomethycaine sulphate, 0.1035 g of sample was dissolved in 0.01 M hydrochloric acid to give 500.0 cm3 of solution. 5.0 cm3 of this solution was then diluted to 100.0 cm3 with 0.01 M hydrochloric acid and the absorbance at 261 nm measured. The absorbance was found to be 0.402 in a 1 cm path length cell. Given A(1%, 1 cm) = 400, calculate the percentage purity of the sample.
0.1097 g of a pure sample of chloramphenicol was dissolved in water to give 500.0 cm3 of solution. 10.0 cm3 of this solution was diluted to 100.0 cm3 with water and the absorbance in a 1 cm path length cell measured. The absorbance was 0.652 at 278 nm. Calculate the specific absorbance for chloramphenicol at 278 nm.
A(1%, 1 cm) = 297.2

The absorbance of a 0.00100% m/v solution of doxycycline hydrochloride in methanol when measured in a 2 cm path length cell was found to be 0.581 at 349 nm. Calculate the specific absorbance and molar absorption coefficient for doxycycline hydrochloride. Mr(doxycycline hydrochloride) = 512.9
A(1%, 1 cm) = 290.5 (all you do is A[1%,1cm]=A/cl no conversion at all
ε = 14900 dm3 mol-1 cm-1
![<p>A(1%, 1 cm) = 290.5 (all you do is A[1%,1cm]=A/cl no conversion at all</p><p>ε = 14900 dm3 mol-1 cm-1 </p>](https://knowt-user-attachments.s3.amazonaws.com/aa36001d-f9c9-4c62-b8e8-d29fe9413108.png)
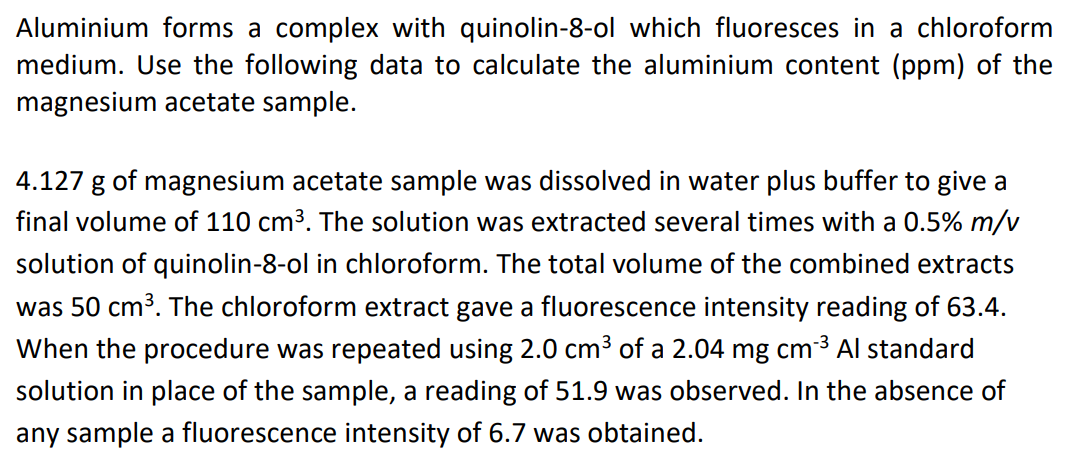
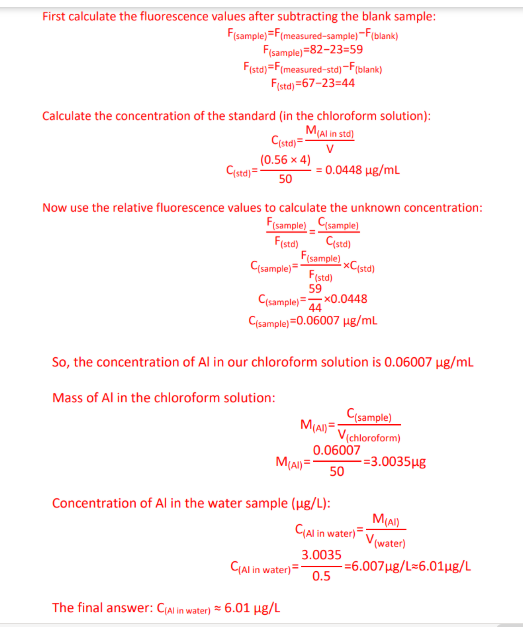
Aluminium content = 1.24 ppm