GCSE chemistry Bonding, structure, and properties of matter
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
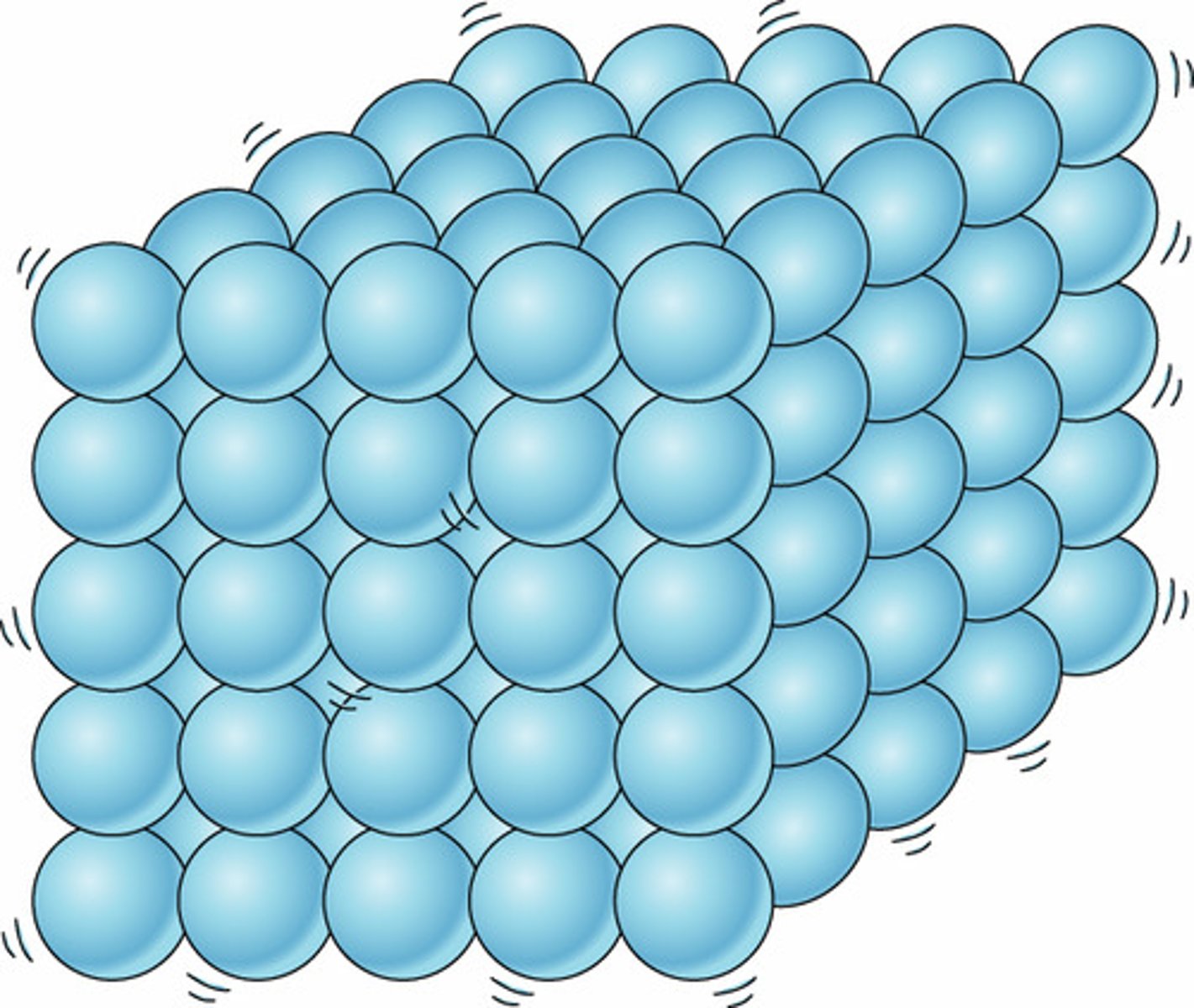
particle arrangement in solids
solids are really hard to compress because the particles in a solid are packed together in a regular pattern with almost no spaces in between the particles with strong forces of attraction
solids have a fixed place and cannot flow from place to place because they keep a definite shape and volume
particles vibrate about their positions - hotter = more they vibrate (causing solids to expand slightly when heated)
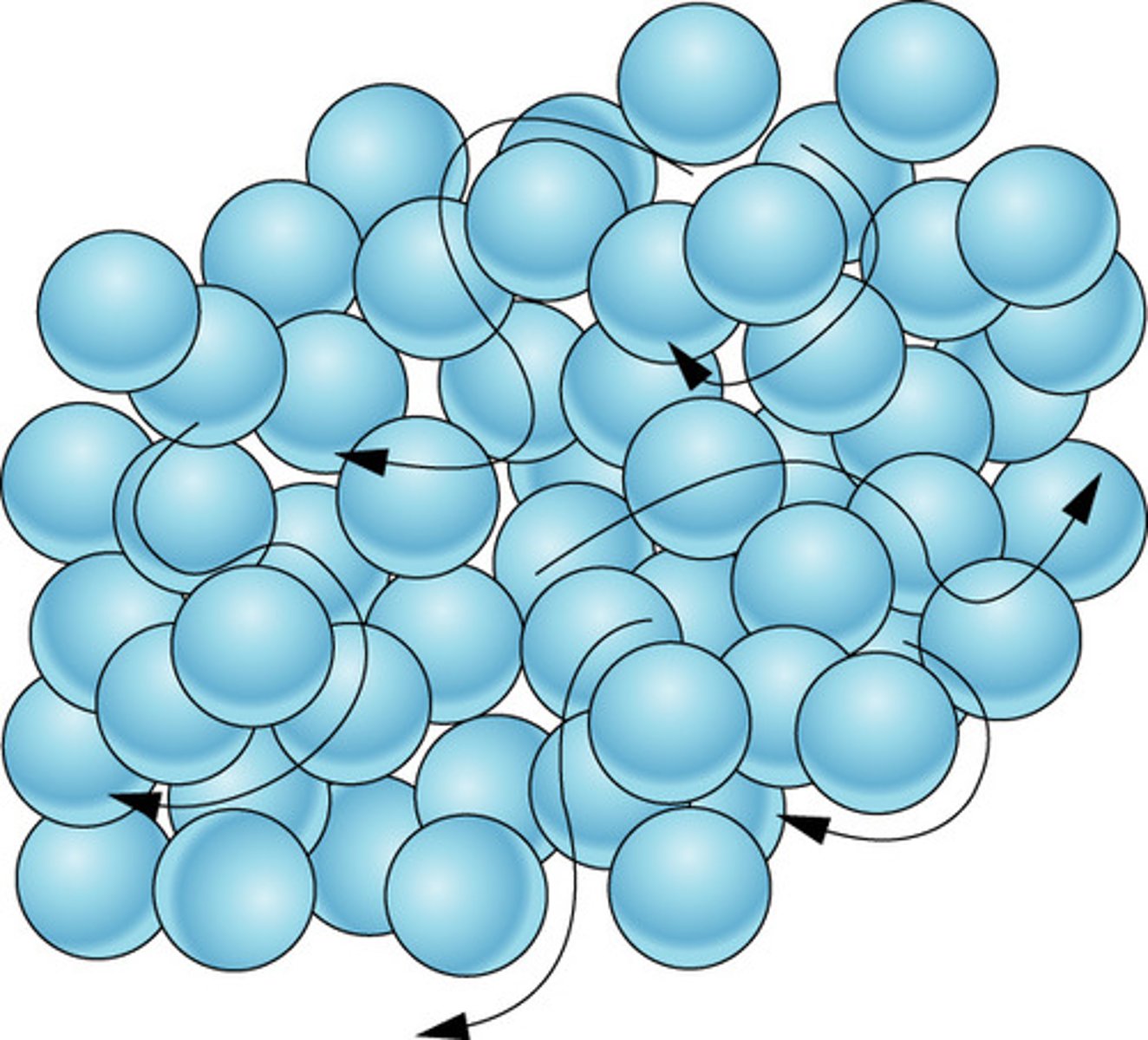
particle arrangement in liquids
hard to compress particles close with not that many spaces in between them .Weaker force of attraction between the particles are randomly arranged and free to move past each other but still have a strong force of attraction
liquids have a definite volume but will not keep a definite shape and will flow to fill the bottom of a container the particles are constantly moving with random motion.
The whole of the liquid gets the faster they move. This causes liquids to expand slightly when heated.
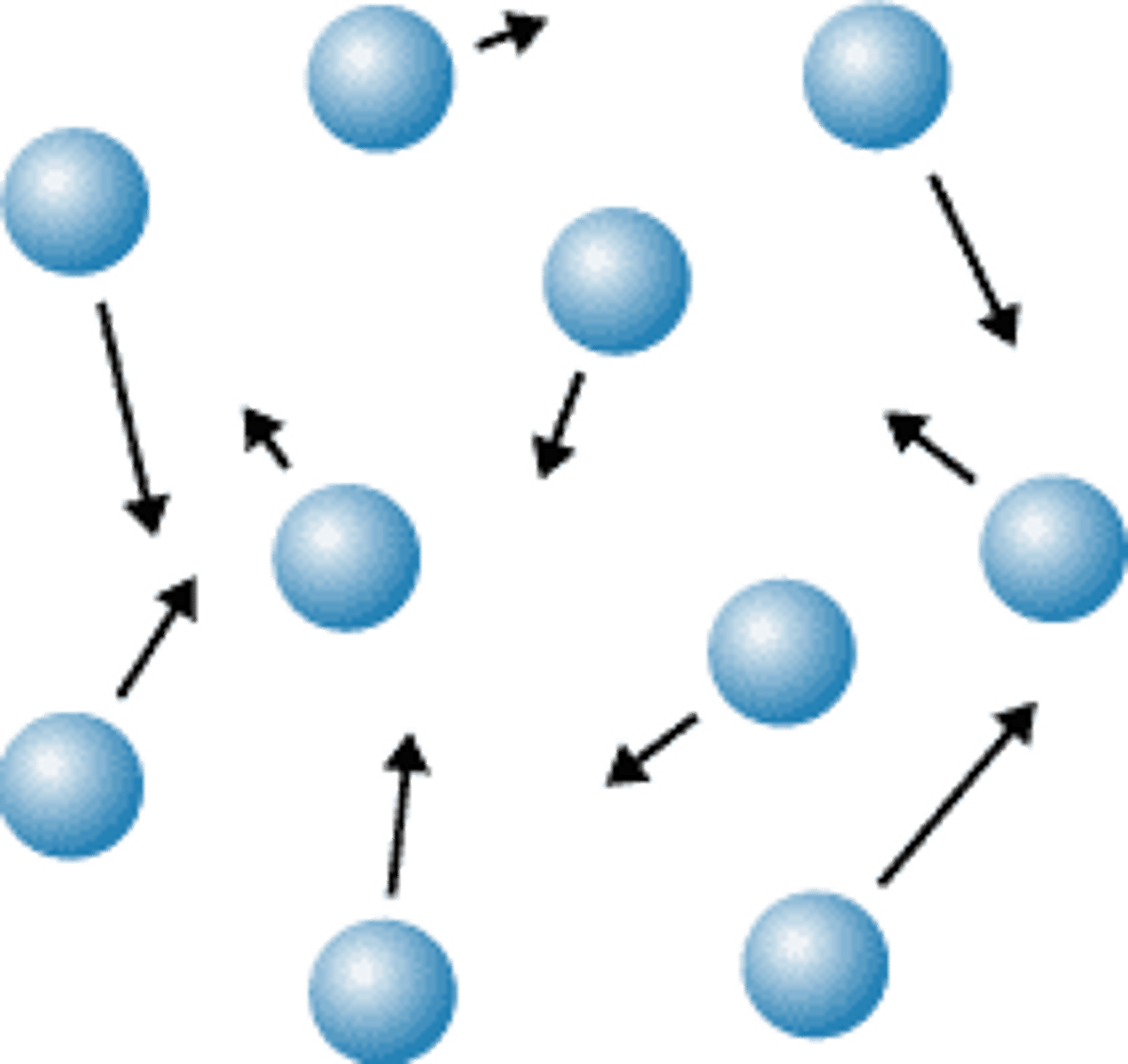
particle arrangement in gases
hard to compress because there is lots of empty space between them and they are very far apart
Gases don't keep a definite shape or volume and always for the space of any container because they are constantly moving
the particles move constantly with random motion the hotter the gas gets the faster they move
gases are expand when heated or the pressure increases.
states of matter
The amount of energy needed to change state from solid to liquid and from liquid to gas depends on the strength of the forces between the particles of the substance.
The nature of the particles involved depends on the type of bonding and the structure of the
substance.
The stronger the forces between the particles the higher the melting point and boiling point of the substance.
ionic bonding facts
elements react in order to achieve a full outer energy level
by doing this, they achieve the stable electronic structure of a group 0 noble gas (full outer shell)
happens between metals and non metals, metals form + ions (ca+ions) and non from anions - ions
ex tip - (you wont get any marks for e.g. chlorine ion, only chloride ion)
Ex q : describe what is happening in this reaction [ionic bonding] (3 marks)
x electron(s)
passes from atom (a) to atom (b)
a becomes ion b becomes ion
both atoms have a full outer energy level
ionic compounds facts
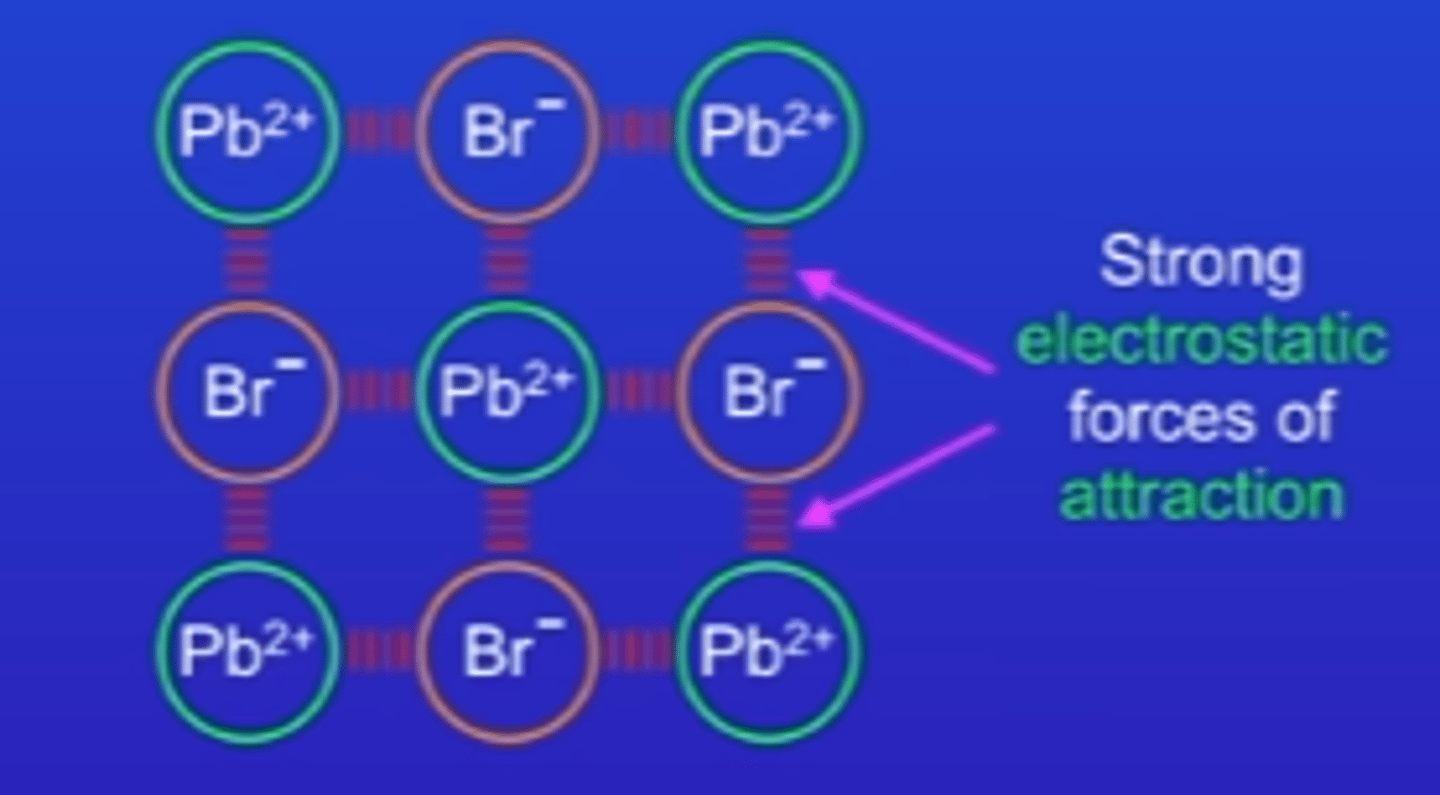
many ionic compounds form crystals - which are giant structures (giant ionic lattice)
every positive ion is surrounded by negative ions and every negative ion is surrounded by positive ions
giant ionic lattices are three dimensional structures
very strong forces of attraction between + - ions (electrostatic forces of attraction) which are also called ionic bonds {ionic bonds act in all directions}
ionic compound properties
ionic compounds have very high melting and boiling points , because the strong electrostatic forces of attraction require a great deal of energy to the thermal store to break - ions vibrate
they cannot conduct electricity when they are solids, because the ions are locked in place, they cannot move, but only vibrate in place
but they can conduct electricity when they are melted or dissolved in water , because the ions can now move and carry the electrical charge
when ions are conducting electricity, it is the ions and not the electrons
covalent bonding facts
1) When non-metal atoms bond together, they share pairs of electrons to make covalent bonds.
2) The positively charged nuclei of the bonded atoms are attracted to the shared pair of electrons by electrostatic forces, making covalent bonds very strong.
3) Atoms only share electrons in their outer shells (highest energy levels).
4) Each single covalent bond provides one extra shared electron for each atom.
covalent bonding more facts
5) Each atom involved generally makes enough covalent bonds to fill up its outer shell.
Having a full outer shell gives them the electronic structure of a noble gas, which is very stable.
6) Covalent bonding happens in compounds of non-metals (e.g. H,°) and in non-metal elements (e.g. Cl2).
in more than one covalent molecule, they are held by intermolecular forces and not electrostatic forces
properties of small covalent molecules (held together by intermolecular forces)
low melting and boiling points so they are gases or liquids at room temperature, there are strong covalent bonds between atoms , but weak intermolecular forces between molecules - they do not require that much energy to overcome
When boiled, it's only the intermolecular forces that are broken, and not the actual covalent bonds between the atoms
as the size of the covalent molecule increases, the (number) intermolecular forces increase - therefore creating an ascending boiling point e.g. alkanes
These substances do not conduct electricity because the molecules do not have an overall electric charge.
giant covalent structures (melting point)
Substances that consist of giant covalent structures are solids with very high melting points.
All of the atoms in these structures are
linked to other atoms by strong covalent bonds.
These bonds must be overcome to melt or boil these substances. - there are also millions (lots) of bonds to overcome
Diamond and graphite (forms of carbon) and silicon dioxide (silica) are examples of giant covalent structures.
diamonds and sillica (sillicon dioxide)
diamonds are made of carbon atoms and have 4 valence electrons (sillica is made of sillicon and oxygen covalently bonded)
each carbon atom forms covalent bonds to 4 other carbon atoms
contain a very large number of carbon atoms (millions for small amount) which makes diamonds a very hard substance
if we want to melt , all covalent bonds must be overcame and that takes a lot of energy - very high melting and boiling point
diamond cannot conduct electricity, because all of the outer electrons are in covalent bonds so there are no delocalised electrons to carry electrical charge
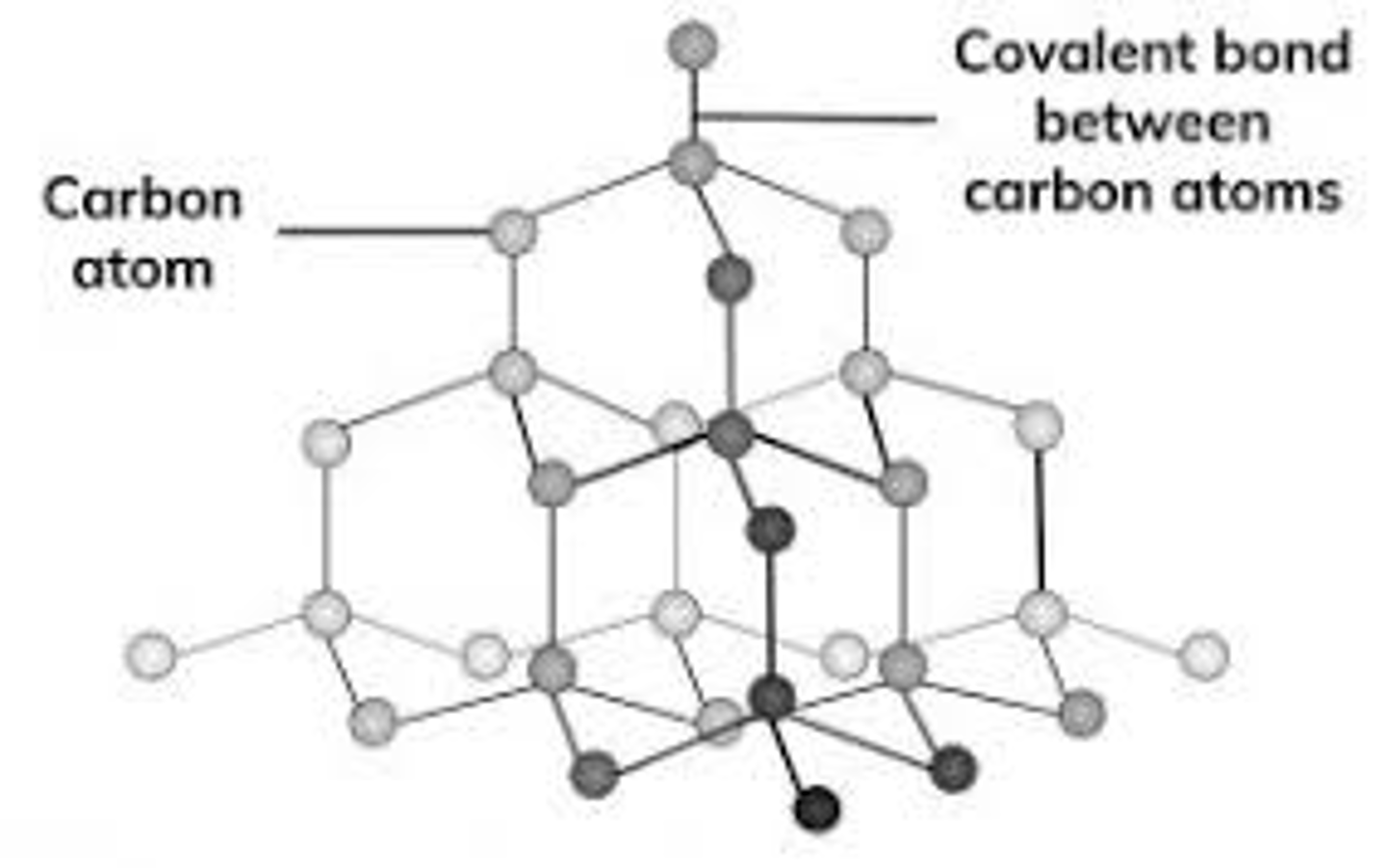
graphite bonding and structure
soft and slippery, very high melting and boiling point, good conductor of electricity and heat
each carbon atom forms covalent bonds to 3 other carbon atoms - which form hexagonal rings (C6)
The hexagon rings of carbon atoms are arranged into And there are no covalent bonds between the layers This means that the layers can slide over each other Which makes graphite soft and slippery
graphite properties in relation to structure
Often used as a lubricant machine machines to reduce friction between moving parts
Contains a large number of strong covalent bonds so boiling point is self-explanatory
good conductor of heat and electricity, because they have electrons that are not in covalent bonds that are released from carbon atoms (delocalised electrons) because they can move, they can conduct thermal energy and electricity (metals also have this property)
structure of graphene
single layer of graphite so only one atom thick and 2d
good conductor of electricity because of delocalised electrons,
useful in electrons
extremely strong and has a high melting and boiling point - large no of strong covalent bonds which will make graphene useful for producing new materials
structure of fullerene
carbon atoms but are hollow and pent, hex, hep rings
first fullerene to be discovered is buckminsterfullerene c60 in a hollow sphere from hex and pent carbon rings
can be used to deliver drugs e.g. pharmaceuticals into the body, can be used as lubricants in machines where they reduce friction in between moving parts, can be used as catalysts in chemical reaction
carbon nanotubes from fullerenes structure and uses
carbon nanotubes are fullerenes shaped into long cylinder's with a relatively small diameter (very high length to diameter) ratio [with rings from hex carbon rings]
high tensile strength, so great deal of force can be applied to a carbon nanotube until it breaks
delocalised electrons for electricity conductivity and heat
can be used to reinforce materials e.g. high end tennis rackets
still being investigated
polymer facts
polymers are very large molecules we make a polymer by thousands of small identical molecules and these starting molecules are monomers - often alkenes
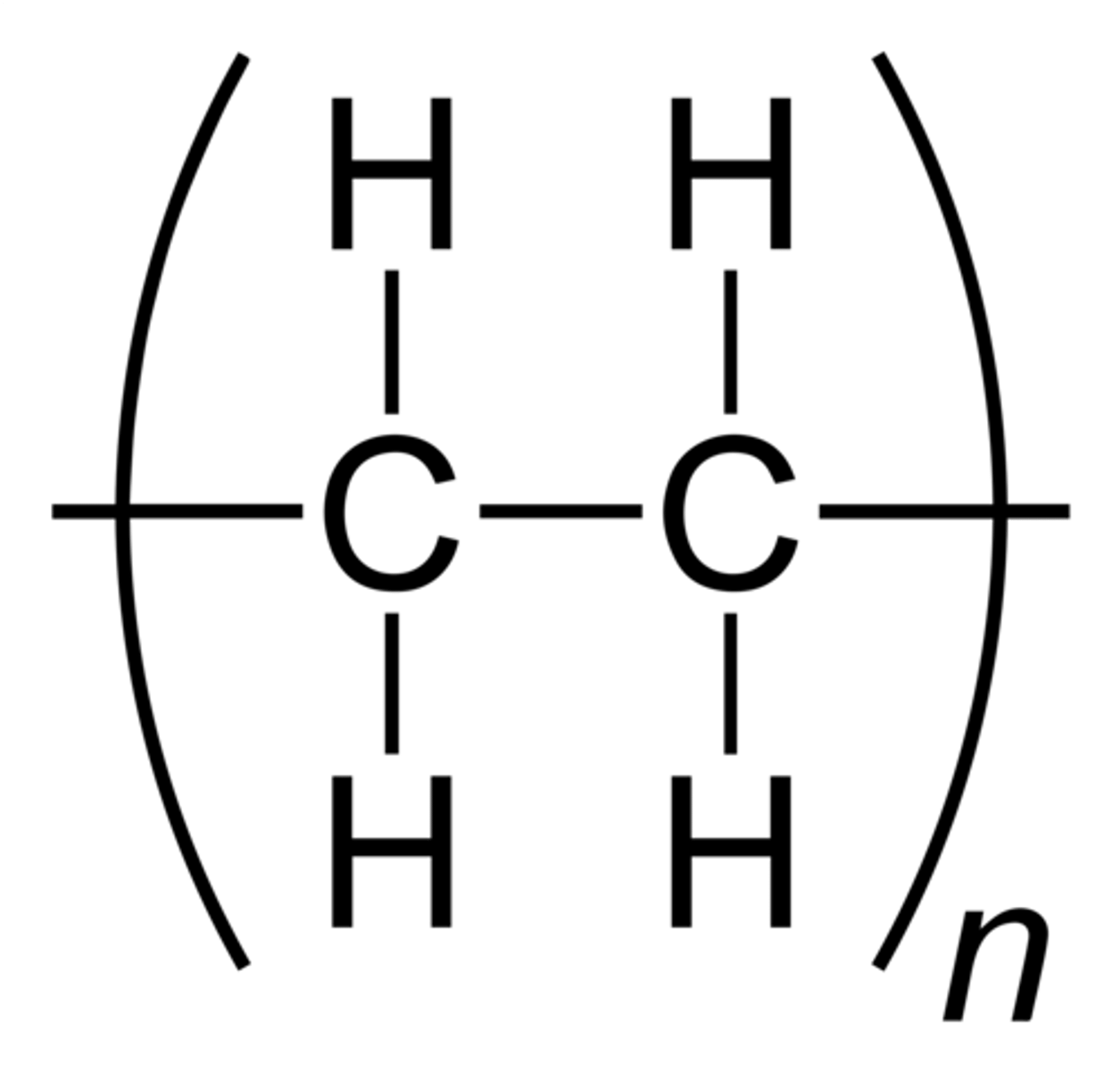
example is ethene, so thousands of monomer ethene molecules are joined to form poly(ethene)
in the ethene monomer, the c atoms are in a double covalent bond but in polyethene, the c atom covalent bonds are single
polymers are really strong molecules - dont need to know what happens at the end of a polymer molecule to the sticking out parts
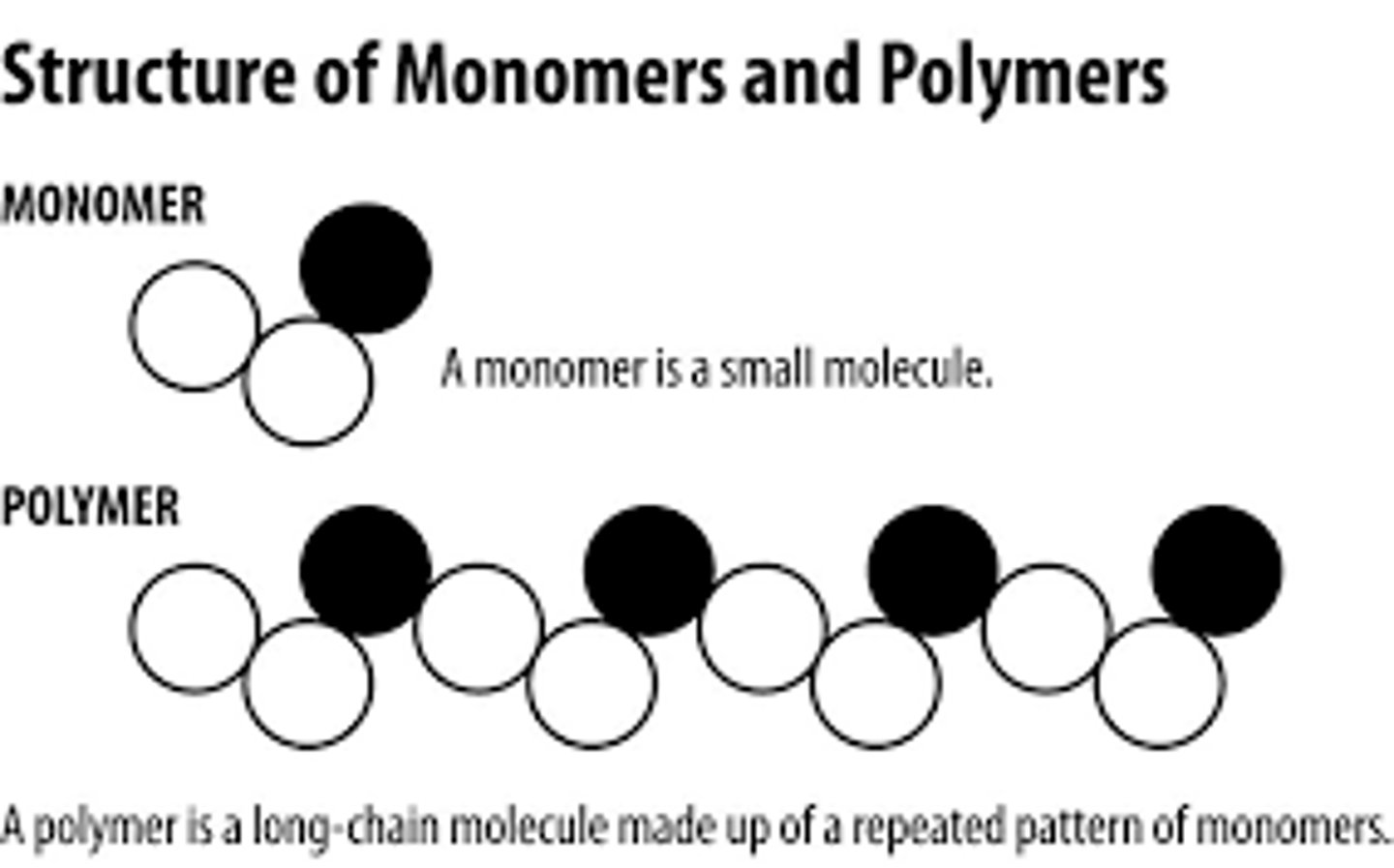
repeating unit facts (polymers)
because polymers can be very long and large, scientists take the monomer diagram, make the c bond single
covalents bonds on either side have to extend out of the brackets, this tells us that the polymer molecule extends out in both directions
lower case n represents a large number
why polymers are solids at room temp
intermolecular forces of attraction between polymer molecules are relatively strong - require a lot of energy to overcome - so polymers have a high melting point - so most polymers are solids in room temperature
metallic bonding facts
metals consist of a giant structure of atoms arranged in regular layers, in a metal the electrons in the outer energy level of each atom are delocalised.
we can call a substance having a sea of delocalised electrons, because each metal atom has lost its outer electron, we now refer to them as + metal ions
between the sea of delocalised negative electrons and the + metal ions, there is a strong electrostatic attraction (attraction between a + and - object) - this attraction is a metallic bond and it is strong
delocalised electrons
in metals the outer electrons are not attached to any individual atom, they are free to move through the whole structure
why metals have very high boiling points - conduction of electricity and thermal energy
in order to melt a metal we have to break the strong metallic bonds, and that requires a great deal of energy
metals are also very good conductors of heat and electricity because the delocalised electrons can move, because electrons are charged, the moving electrons can carry an electric current and thermal energy
why metals can be bent or shaped - and problem with this
alloys
layers of atoms can slide over each other , but this presents a problem, because some pure metals are not hard enough to be useful - e.g. copper, gold, iron, and al so we need to make these metals harder so we need to make an alloy
an alloy is a mixture of metals. In alloys, the different sizes of atoms distorts the layers which makes it more difficult for the layer to slide over each other therefore harder
Nanoscience refers to structures that are 1-100 nm in size, of the order of a few hundred atoms. Nanoparticles, are smaller than fine particles
Coarse particles (PM10) have
diameters between 1 x 10-5 m and 2.5 x 10-6 m.
(contain many thousands of atom)
(PM2.5), which have diameters between 100 and 2500 nm
(1 x 10-7 m and 2.5 x 10-6 m). (contain several thousand atoms)
Coarse particles
are often referred to as dust
Surface area to volume ratio of nano particles
As the side of cube decreases by a factor of 10 the surface area to volume ratio increases by a factor of 10. This gives nanoparticles a huge SA:V - this means that we need a much smaller quantity of nanoparticles compared to materials with normal particle size e.g. a catalyst
uses and disadvantages of nanoparticles
this makes nano particles extremely useful in items like medicines, suncreams, cosmetics, deodorants, electronics, and catalysts
however there are some risks, (especially in cosmetics, suncreams, and deodorants) because it is possible that nanoparticles can be absorbed into the body
no one knows that potential long term effects of this, so it is important that nanoparticles are studied and used carefully
Dot and cross diagrams EVAL + 2D Stick Diagram EVAL
3D stick diagram EVAL
BC one dot is used to represent e- of one atom and crosses for the other, it is very clear where the electrons are coming from, but dot and cross diagrams dont tell us about the shape of the molecule
because the covalent bond is shown as a stick ,cannot tell which electron in the covalent bond came from which atom, also give no idea of outer shell electrons, dont give us accurate info on the shape of the molecule
shows the shape of the molecule (3D)
Ball and stick diagram for ionic lattices EVAL
Space filling diagram EVAL ionic lattice
allows us to clearly see the ions in 3 dimensions, however the ions are shown as widely spaced, when in reality the ions are packed together
space filling diagrams give us a better idea of how closely packed the ions are but it can be difficult to see the 3 dimensional packing
one problem with both is that they only show a tiny part of a giant crystal lattice, in reality a crystal lattice is a giant structure
so these diagrams give the impression that the structures are much smaller than they acc are
Make sure that you understand convention of drawing ionic, covalent diagrams, and stuff that you need to add for marks