vaccine and immunisation
1/34
Earn XP
Description and Tags
week 11 immunology
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
vaccination controversy
vaccination ethics
fears of iatrogenic diseases
preferences for natural lifestyles
religious opposition to vaccines
increased sense of responsibility for small risks of vaccines vs. responsibility for risks of exposing children to infectious diseases
active and passive immunisation
active immunisation | passive immunisation |
long term | short term |
induced by using inactivated or attenuated live organisms or their products | administration of human iGs or immune cells |
vaccines induce humoral/ cell mediated immunity | immediate protection |
passive immunisation
human normal immunoglobulins (HNIG): derived from pooled plasma of donors and containing AB to infectious agents
specific immunoglobulins for tetanus, hep B, rabies
obtained from pooled blood donors recently immunised with relevant vaccine
mammalian humoral response to infection
viral infection induces at least 3 classes of ABs: IgG, IgM, IgA
requirements for an effective vaccine

active immunisation strategies
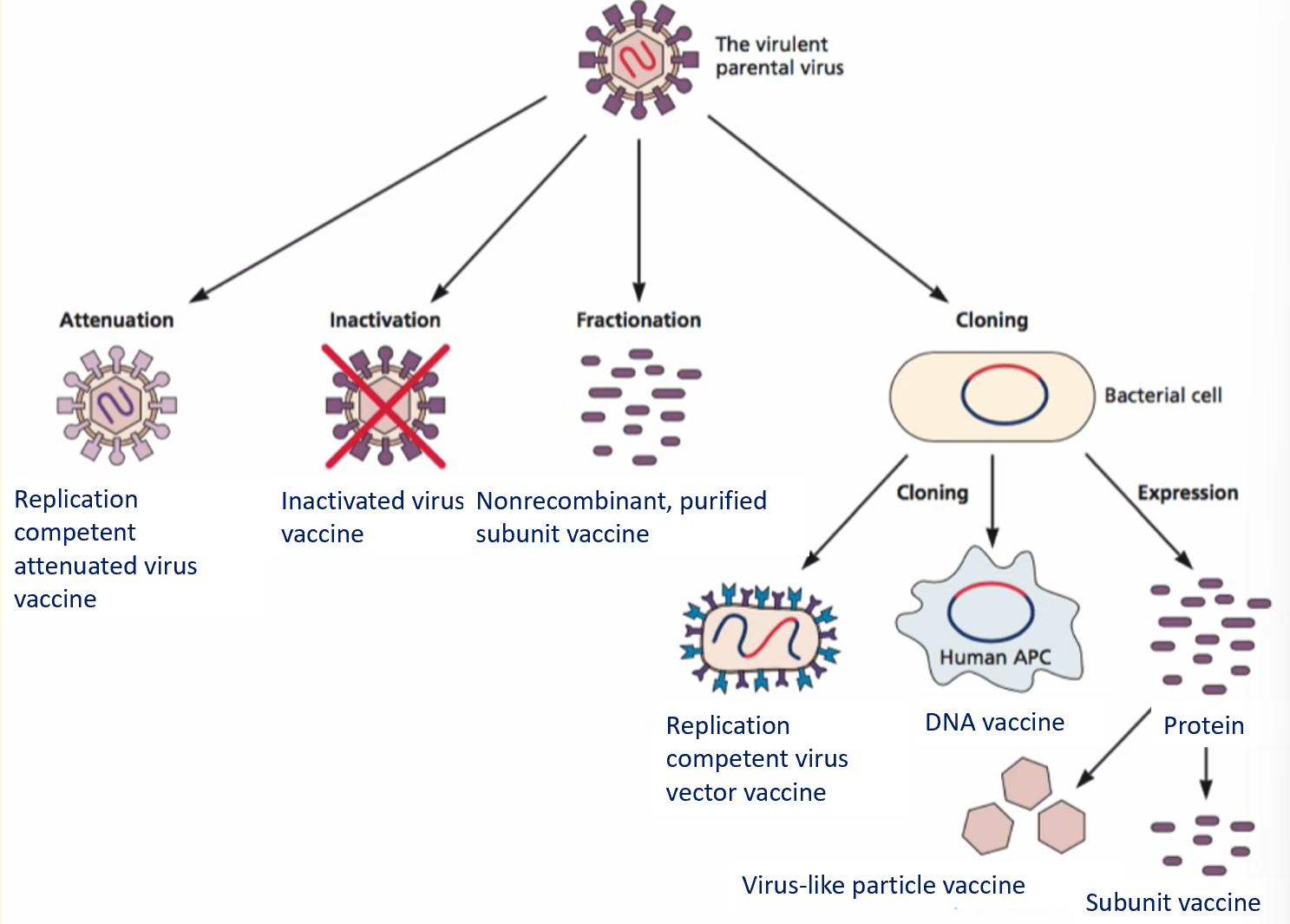
inactivated vaccines
chemical procedure
ineffectivity is eliminated, antigenicity not compromised
relatively stablem carrying little or no risk of vaccine-associated virus infection
denaturation of virus proteins may lead to loss of antigenicity
possible contamination with viral components
polio virus
enterovirus of picornaviridae
3 antigenic types
minor illness: aseptic meningitis
major illness: flaccid weakness of various muscle groups
IPV: inactivated polio vaccine
enhanced potency inactivated polio vaccine contains polio viruses of all 3 types
influenza virus
acute viral infection of respiratory tract
symptoms: fever, chills, headache, myalgia
secondary bacterial infections (chronic respiratory and cardiac diseases can aggravate illness)
formalin-inactivated or detergent or chemically disrupted
prepared each year using virus strains considered most likely to be circulating in winter
purified viruses grow in embryonated hen’s eggs and chemically inactivated
live attenuated vaccine given by aerosol may soon be licensed
attenuated vaccines
viral replication occurs, stimulates immune response
infection induces mild or inapparent disease
viruses traditionallt attenuated by sleeting for growth in non human cells
sabin oral poliovirus vaccine
virs must first be isolated by growing it in cultured human cells
adaptation to growth in cultured human cells mat cause some attenuation
virus adapted to grow in cells of a different species until it grows poorly in human cells (adaptation result of mutation)
life attentuated viral vaccines pose particular risk to immunodeficient recipients
risk of reversion
safer live-attenuated viral vaccines using recombinant DNA technology
if a gene that is required for virulence but not growth or immunogenicity can be identified, this gene can be mutated or deleted from genome using recombinant DNA
creates an avirulent (non pathogenic) virus that can be used as vaccine
usually large mutations in virulence gene, making it difficult for virus to revert to wild type
subunit vaccines
consist of only some components of virus, sufficient to induce a protective immune response but not enough to allow danger of infection
antigen usually a capsid or membrane protein
subunit vaccines categories
several categories of subunit vaccines:
synthetic vaccines
short, chemically synthesied peptides
recombinant vaccines
produced by genetic engineering
virus vectors
recombinant virus genomes genetically manipulated to express protective antigens from pathogenic viruses
hep B virus vaccination
cancer vaccine
HbsAg protein produced in yeast
currently prepared from yeast cells using recombinant DNA technology
action of HBV vaccine
injected HBV vaccine containing HbsAg proteins are engulfed and processed by APCs
APCs process antigen and attach the same to the surface of the APCs
APCs present antigen to the Th cells, leading clonal expansion, of T cells as well as production oof memory T cells
antigen recognised directly from B cells → weak immune response
Ag binding to Fab region on weak B cell receptor, secondary signalling from cytokines released by Th cells
B cells begin somatic hypermutation at the Fab region, further increases corresponding fit between the Fab and the antigen
B cells mature to plasma cells to produce neutralising ABs
also undergo clonal expansion and memory cell formation for future defence
recomb
recombinant zoster vaccine
herpes virus
causes chickenpox in childhood
later reactivates causing shingles
recombinant gE produced in mammalian
adjuvanted with AS01
injected
adjuvants
enhance immunogenicity of vaccines but few are approved for use in humans
adjuvants are tested in combination with specific vaccines and are approved for use in that configuration
adjuvants are diverse
particulates
liposomes
microparticles
virus-like particles
bacterially derived adjuvants
natural/synthetic surfactants
oil/water emulsions
adjuvants enhance immunogenicity of vaccines
vaccines without adjuvants inhance modest production of Th polarising cytolines, ABs and activated T cells
vaccines with adjuvants promote maturation of more APCs increase interaction between APCs and T cells
promotes production of greater numbers and more types of Th polarising cytokines, multifunctional T cells and ABs
core of mechanism of action of adjuvants
classified as immunostimulants and delivery systems
immunostimulants such as PAMPs, DAMPs, chemically synthesised small molecules agonists provide danger signals to activate PRRs on APCs
enhance antigen presentation on MHC molecules
activation of PRRs lead to upregulation of cytokines/co-stimulatory molecules
enhance co-stimulatory signalling
delivery systems such as LNPs, PGLA and self loaded protein nanoparticles act by facilitating presentation of antigens on MHC molecules
molecular targets of adjuvants: toll-like receptors
TLR1/TLR6: heterodimerise with TLR2 and signal through myeloid differentiation primary response 88
activated NF-κB and MAP kinases, leading to secretion of pro-inflammatory and anti-inflammatory cytokines
TLR4/TLR5: function as homodimers and signal to MyD88 pathway
TLR7/TLR9: also use MyD88 pathway, rapidly activate IRF7 to induce type I interferons
TLR3: uses TIR domain-containing adapter inducing IFNβ (TRIF) signalling to induce type I interferons through IRF3
molecular targets of adjuvants: cytosokic pattern recognition receptors
nucleotide binding oligomerisation domain (NOD like receptors) are cytosoic sensors of bacterial PAMPs but also recognise multiple cellular products including ATP, uric acid and K+
activate NF-κB pathway and induce cytokines driving Th 2 differentiation
retinoic acid inducible gene I and melanoma differentiation associated protein 5 are intracellular viral sensors that drive type I interferon response through IRF3 and IRF7
cGAS stimulator of interferon genes STING pathway recognised double stranded DNA to induce NF- κB pathway
molecular targets of adjuvants: C type lectin receptors
CLR are cell surface molecules expressed on multiple myeloid cell subsets
dectins 1 and 2/MINCLE recruit SYK1 and activate NF-κB through the CARD9-BCL-10-MALT1 complex
dectin 1 has been shown to induce NFAT and AP1 pathways in macrophages and dendritic cells and in vitro experimental models
dectins are also specialised in inducing antifrugal immunity
dendritic cell specific ICAM3 DC SIGN activates NF-κB via p65 acetylation
resulting gene expression is poorly understoof but IL-10 expression ahs been shown to be induced
DEC205 and DNGR1 are known to induce cross presentation but signalling pathways are unknown
SARS-CoV-2
causes COVID-19
divided into 3 phases
asymptomatic state (1-2 days of infection)
upper airway and conducting airway response (next few days)
hypoxia, ground glass infiltrates and ARDS progression
SARS-CoV-2 virion structure
lipid membrane includes 3 transmembrane proteins
spike protein is trimer of identical subunits (2 small proteins, transmembrane protein and envelope protein)
platforms of COVID 19 vaccines
schemaric diagram of SARS CoV 2 structure including ssRNA genome and 4 structural proteins: spike protein (S), envelope protein (E), membrane protein (M) and nucleocapsid protein (N)
diverse vaccine platforms including:
inactivated vaccine
live attenuated vaccine
viral vector vaccine DNA vaccine
RNA vaccine
recombinant subunit vaccine
virus-like particles vaccine
COVID-19 inactivated whole virus vaccines
inactivated whole virus vaccines are made using old technology, which delivers an inactivated version of SARS-CoV-2 virus if it later infects body
virus-like particle
COVID-19 adenoviral vaccines
adenoviral vector vaccines are made using common cold virus to deliver genetic instructions for human cells to make SARS-CoV-2 spike protein, priming immune system to attack SARS-CoV 2 virus if it later infects host
COVID-19 DNA vaccines
DNA vaccine consists of plasmid produced in bacteria that encodes protein of interest
antigen is transcribed and translated in transfected human cells
vaccine antigen is presented to APCs, via MHC pathways which will present to activate naive T cells
CD8+ T cell immunity is predominantly activated by endogenously expressed antigens presented on MHC class I molecules
active CD8+ T cells stimulated release of cytokines that inhibit viral replication and increase expression of MHC I molecules
macrophages are also activated to support cell mediated immune responses
CD4+ Th cell activation via MHC class II from APC
if vaccine proteins are secreted, these are recognised by BCRs in naive B cells, which also use ,MHC-II to get activated
activated B cells produce Abs to protect against disease
COVID-19 mRNA vaccine
mRNA vaccines are made using nanoparticle to deliver genetic instructions for human cells to make SARS CoV 2 spike protein, priming immune system to attack virus if it later infects host
autoimmune haemolytic anaemia (AIHA) of COVID-19 mRNA vaccination
AIHA may be considered as a very rare complication of COVID 19 vaccination that occurs following vaccination
direct casual relationship between COVID-19 and AIHA is still ambiguous
AIHA is caused by IgG and IgM autoABs that react with RBC related surface antigens
warm ABs (mainly IgG type)
detectable by direct antiglobulin test positivity for IgG, Abs can activate complement
cold autoABs (usually IgM class)
binds at cold temperatures and rapidly activate complement, causing IV RBC lysis
COVID-19 protein-based vaccine
made using older technology, which deliver CoV spike protein directly into body, with adjuvant, priming immune system to attack virus later
vaccine challenges
difficult to develop single vaccine to target all strains and mutations
immune escape
vaccination hesitancy
issues of distribution, affordability, accessibility and acceptability
dendritic cell based vaccines
CD14+ monocytes are isolated from leukapheresis products, differentiated into immature MoDCs with IL-4 and GM-CSF
adult dermal fibroblasts are directly reprogrammed to conventional type I DCs by lentiviral transduction of TFs without need of leukapheresis
resulting DCs are then loaded with tumour associated antigens
mature, TAA loaded DCs are reinfused to pt