Chapter 1 - Biochemistry in Space and Time
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Biochemistry
The study of the chemistry of life processes
A possible timeline for Biochemical Evolution
Key metabolic processes are common to many organisms
Different organisms have macromolecules of similar structure and common biochemical processes.
Suggests all organisms evolved from a common ancestor
Eukaryotes
Multicellular organisms
ex: animals, plants, human beings, and many microscopic unicellular organisms such as yeast
Prokaryotes
Unicellular organisms
lack a nucleus
All organisms can be placed in one of three domains
Eukarya, Bacteria, or Archaea
based on biochemical characteristics
DNA
is constructed from 4 building blocks
Is a linear polymer composed of monomers consisting of a sugar (deoxyribose), a phosphate, and one of the 4 nitrogenous bases (bases)
Has polarity which is important for many processes
Covalent Structure of DNA
the backbone of DNA contains linked sugars and phosphates
Variable bases extend from the backbone
The Double Helix
Base-paired DNA forms a double helix structure
Bases form specific base pairs held together by hydrogen bonds
The two strands are antiparallel
Watson-Crick Base Pairs
A-T pairs form two hydrogen bonds
G-C pairs form three hydrogen bonds
DNA Structure
Explains heredity and the storage of information
each strand serves as a template for a new partner
Due to specific base pairing
Allows for the generation of two identical daughter double helices from one parent strand
Two complementary DNA strands
Spontaneously assemble to form a double helix
The biochemical timescale
The timescale for biological interactions and processes
on the order of picoseconds to microseconds
Covalent bonds
formed by electron sharing between two adjacent atoms the strongest bonds
The strongest bonds
typical C-C covalent bonds have a distance of 1.54 Angstrom and a bond energy of 355 kJ mol-1 (85 kcal mol-1)
Angstrom is 0.1 nm, or 10-10 m.
Resonance
Some molecules, such as adenine, exhibit multiple covalent structures called resonance structures
Ionic Interactions
Noncovalent interactions that occur between fully charged atoms or molecules
Coulomb energy
The energy (E) of electrostatic attraction between opposite charges or repulsion between like charges is given by ___
E = kq1q2/Dr
Where:
k is a proportionality constant
q1 and q2 are the charges on the two atoms
D is the dielectric constant of the solvent
k is a proportionality constant r is the distance between atoms
Electrostatic Interactions in Water have
a bond distance of ~ 3 Angstrom
a bond energy, given by Coulomb energy, of 5.86 kJ mol-1 (1.4 kcal mol-1)
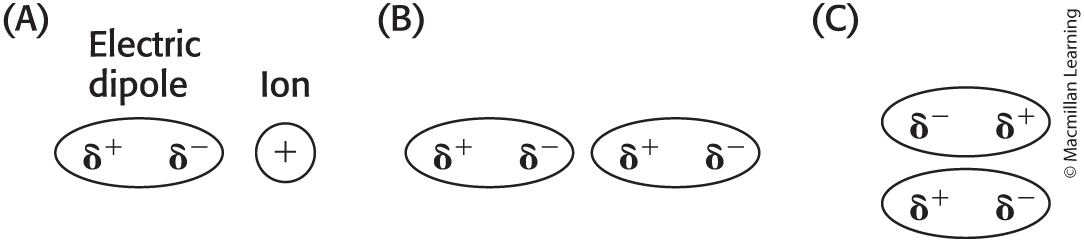
Electric Dipoles
molecules with no overall charge can have regions where electron distribution is uneven
Leads to ____
Dipoles can interact with ions or with other dipoles
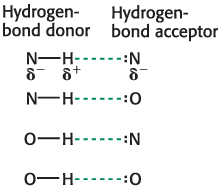
Hydrogen Bonds
occur between an electronegative atom and a hydrogen covalently bonded to another electronegative atom.
vary in bond distance from 1.5 Å to 2.6 Å.
have bond energies from 4 to 20 kJ mol−1 (1–5 kcal mol−1).
Hydrogen-bond donor
the group that includes both the atom to which the hydrogen atom is covalently bonded and the hydrogen atom itself
hydrogen-bond acceptor
The lone pair of electrons that is on the atom less tightly linked to the hydrogen atom
Van der Waals Interactions
occur when two atoms are sufficiently close.
occur when transient asymmetry in electron distribution in one atom induces complementary asymmetry in a neighboring atom.
involve neighboring atoms attracting each other.
are weak.
have bond energies from 2 to 4 kJ mol−1 ( 0.5–1.0 kcal mol−1).
Van der Waals Contact Distance
Attraction increases as two atoms come closer to each other, until they are separated by the van der Waals distance.
At distances shorter than the van der Waals contact distance, strong repulsive forces become dominant.
Properties of Water
water is a polar molecule with a partial positive and partial negative end
Water is highly cohesive
A large number of hydrogen bonds are formed in liquid water, and the maximum number of hydrogen bonds are formed in crystalline ice
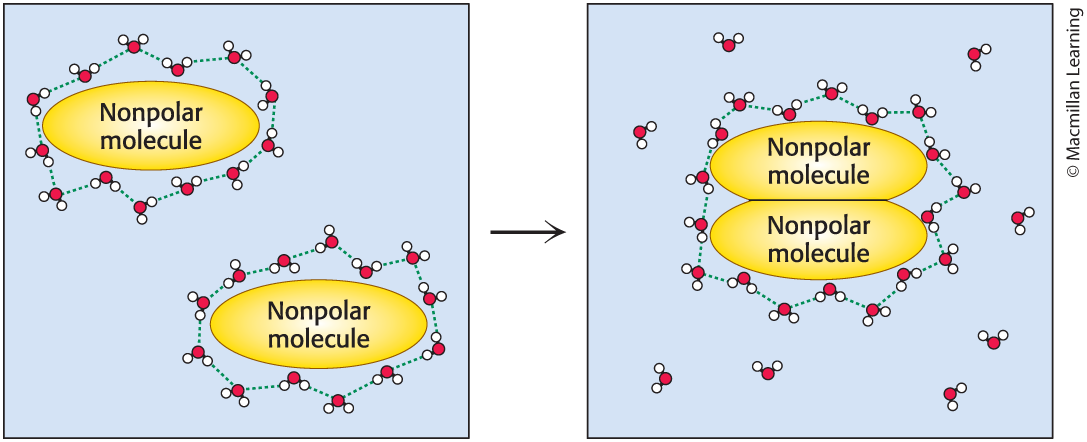
The Hydrophobic Effect
Nonpolar molecules in water can be driven together by the ____
–powered by the increase in entropy of water
–associated interactions are called hydrophobic interactions
The Double Helix is an Expression of the Rules of Chemistry
When a double helix forms, charge repulsion occurs between the negatively charged phosphates of the backbone.
These repulsive forces are reduced by the high dielectric constant of water and interaction of positively charged ions with the phosphate groups.
Hydrogen Bonds Between Complementary Bases
Explain the Specificity of Sequence Pairing
each individual nitrogenous base hydrogen bonds equally well with water as with its complementary base
Hydrogen bonding explains the specificity of sequence pairing
DNA Base Pairs Are the Optimal van der Waals Distance Apart
In the interior of the helix, bases are stacked and interact through van der Waals interactions.
The hydrophobic effect also contributes to the favorability of base stacking.
Surface complementarity occurs when hydrogen-bond donors align with hydrogen-bond acceptors and nonpolar surfaces
First Law of Thermodynamics
the total energy of a system and its surroundings is constant
Second Law of Thermodynamics
the total entropy of a system plus that of its surroundings always increases
states that, for a process to take place, the entropy of the universe must increase, which is possible only if
ΔSsystem > ΔHsystem /T
or TΔSsystem > ΔHsystem
•In other words, entropy will increase if and only if
ΔG = ΔHsystem − TΔSsystem < 0
•Biochemical reactions will occur only if the ΔG is negative, which is only when the entropy of the universe increases.
Entropy
can decrease locally in the system if there is a corresponding increase in entropy in the surroundings.
Gibbs Free Energy
−TΔStotal is the free energy or Gibbs free energy.
The change in Gibbs free energy is used to describe the energetics of biochemical reactions
ΔG = ΔHsystem − TΔSsystem
The Formation of the Double Helix
Heat is Released
The spontaneous formation of a double helix reduces the entropy of the DNA molecules.
appears to violate the Second Law of Thermodynamics
Heat released by helix formation increases the entropy of the surroundings.
Acid-Base Reactions
Are Central in Many Biochemical Processes
involve the addition or removal of a hydrogen, H+, ion.
pH is a measure of the H+ concentration and is defined by
pH = −log [H+]
H+ and OH− ions are formed upon the dissociation of H2O
H2O ↔ H+ + OH−
The Equilibrium Constant of Water
The equilibrium constant (K) for the dissociation of water is defined as
K = [H+][OH−]/[H2O]
KW, the ion constant of water, is defined as
KW = K[H2O]
This can be simplified to
KW = [H+][OH−]
Proton or Hydroxide Ion Concentration Can Be Calculated if the Other Is Known
KW has a known value
KW = [H+][OH−] = 10−14
From this, we can calculate
[H+] = 10−14/[OH−] and [OH−] = 10−14/[H+]
At exactly pH 7.0, [H+] = [OH−] = 10−7 M.
Acid-Base Reactions Can Disrupt the Double Helix
As base is added to a solution of double helical DNA, the helix is disrupted or denatured.
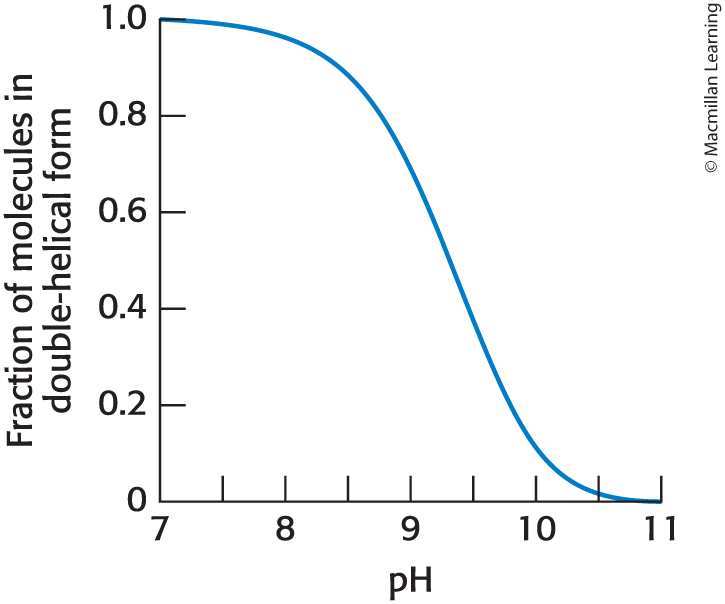
High pH Causes Loss of Hydrogen-Bond Donors in DNA
The chemical basis of the denaturation is the disruption of base-pairing.
example: the loss of a proton by the base guanine prevents base-pairing with cytosine.
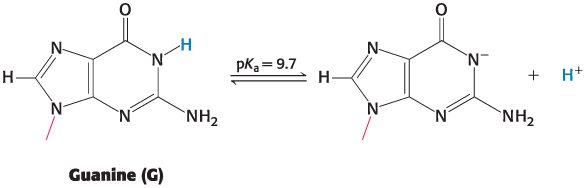
The pKa Value Describes the Susceptibility of Proton Removal
Proton dissociation for a substance HA has an equilibrium constant defined by the expression
Ka = [H+][A−]/[HA]
The pKa value indicates the susceptibility of proton to removal by reaction with a base:
pKa = −log(Ka)
When pH is equal to the pKa,
−log[H+] = −log([H+][A−]/[HA])
and
[H+] = [H+][A−]/[HA]
When the pH is at the pKa, the Group is 50% Likely to be Deprotonated
Dividing [H+] = [H+][A−]/[HA] by [H+] reveals that
1 = [A−]/[HA] or [A−] = [HA]
When the pH is equal to the pKa, the concentration of the protonated form of HA is equal to the deprotonated form A−.
The N-1 Proton of Guanine
The N-1 proton of guanine has a pKa of 9.7.
When pH is near to or exceeds 9.7:
the proton is increasingly likely to be lost
Base-pairing is disrupted
The helix becomes denatured
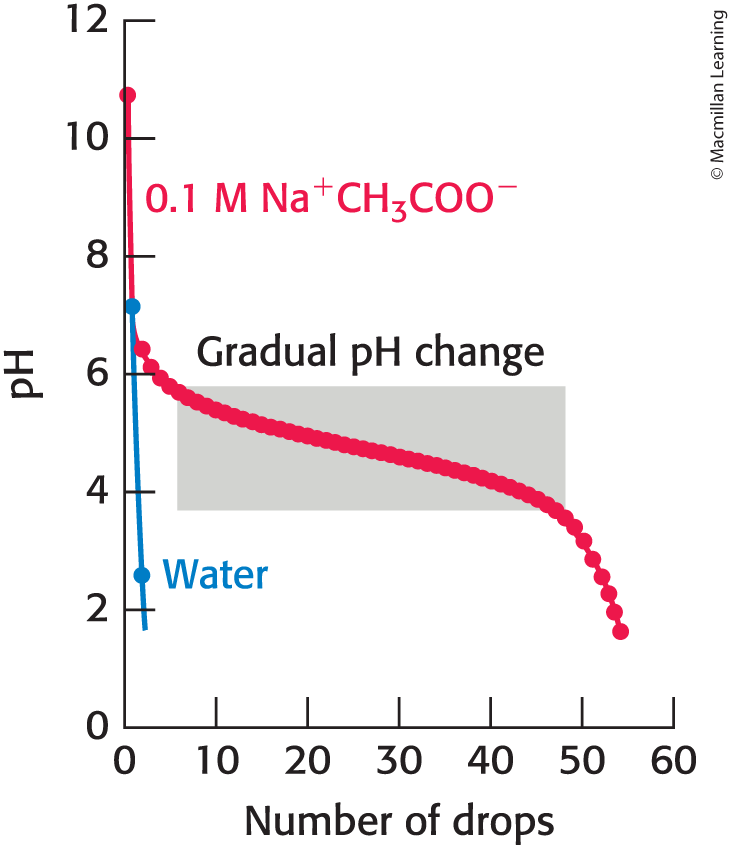
Buffers Regulate pH in Organisms and in the Lab
Buffers resist changes in the pH of a solution.
Buffers are most effective at a pH near its pKa.
When a Buffer is Present
pH Change is Gradual
Titration
gradually adding known amounts of reagent to a solution with which the reagent reacts while monitoring the results
The ionization reaction of a weak acid is given by ___ & the equilibrium constant for this reaction is ___
Taking the logarithms of both sides yields ___
HA ↔ H+ + A−
Ka = [H+][A−]/[HA]
log(Ka) = log([H+]) + log([A−]/[HA])
The Henderson-Hasselbach Equation
Recalling the definitions of pKa and pH and rearranging yields the Henderson–Hasselbalch equation
pH = pKa + log ([A−]/[HA])
Weak acids are most effective as buffers at pH near the pKa value of its acid component.
A Buffer Functions Best
Close to the pKa Value of Its Acid Component
Phosphoric Acid
Is an Important Buffer in Biological Systems
Physiological pH is typically near 7.4.
Given the pKa values shown, inorganic phosphate exists as a nearly equal mixture of H2PO4− and HPO42− in physiological systems
Genomic Sequences
Encode Proteins
The most fundamental role of DNA is to encode the sequences of proteins.
Proteins are built from 20 building blocks, called amino acids, rather than 4, as in DNA.
Proteins spontaneously fold into elaborate three-dimensional structures, determined almost exclusively by their amino acid sequences.