Partial Pressure
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
Partial Pressure: the pressure exerted by each gas is proportional to its …..
mole fraction
Mole fraction

A 5.00L tank at 19.1 C is filled w 6.60g of dinitrogen difluoride gas (MM=66.01g/mol) and 13.9 g of sulfure hexafluoride gas (MM=146.06g/mol). You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank.
turn grams of N2F2 and SF6 into mols so we can do mol fraction
Do mole fraction add together total mols just calculated
Calculate total pressure w PV=nRT——P=nRT/V
Now that we have total pressure we can use partial pressure equation to solve partial pressure for each (Xa)(Ptotal)
add both partials for total
Partial Pressure
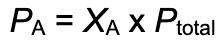
A mixture of N2 total pressure of 0.95 atom. What is the partial pressure (in atm ) of N2, if there are 0.56 mol N2 and 0.32 mol O2 in the mixture
Add mols for total mols so you can do mol fraction Mol A/total mol
calculate partial pressure PN2=(XN2)(Ptotal)- (mole fraction)(total pressure)