chem ch 1
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
chemistry
study of matter and the changes it undergoes
matter
anything that occupies space and has mass
substance
form of matter that has a definite composition and distinct properties
scientific method
systematic approach to research
hypothesis
tentative explanation for a set of observations
law
concise statement of a relationship between phenomena that is always the same under the same conditions
theory
unifying principle that explains a body of facts and/or those laws that are based on them
dalton’s atomic theory
All atoms of a given element are identical, having the same size, mass and chemical properties
element
Consists of only one kind of atom, cannot be broken down by physical of chemical methods
118
number of elements that have been identified
82
number of naturally occurring elements on earth
36
number of elements created by scientists
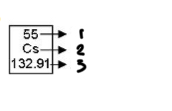
atomic num
1
chemical symbol
2
atomic mass
3
compound
substance composed of atoms of two or more elements chemically united in fixed proportions
chemical means
Compounds can only be separated into their pure components (elements) by ——— means
mixture
combination of two or more substances in which the substances retain their distinct identities
homogenous mixture
composition of the mixture is the same throughout
heterogenous mixture
composition is not uniform throughout
physical
——— means can be used to separate a mixture into its pure components
gas, liquid
solutes in gaseous solutions
gas, liquid, solid
solutes in liquid solutions
liquid, solid
solutes in solid solutions
physical
A ——— change does not alter the composition or identity of a substance
A ——— change alters the composition or identity of the substance(s) involved
extensive
An ——— property of a material depends upon how much matter is being considered
intensive
An ——— property of a material
does not depend upon how much
matter is being considered
mass
measure of the quantity of matter
weight
force that gravity exerts on an object
accuracy
how close a measurement is to the true value
precision
how close a set of measurements are to each other