Chem Paper 3 flashcards
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms



Explain why the product (more stable carbocation) shown in your answer to Question 2.2 is the major product. [2 marks]



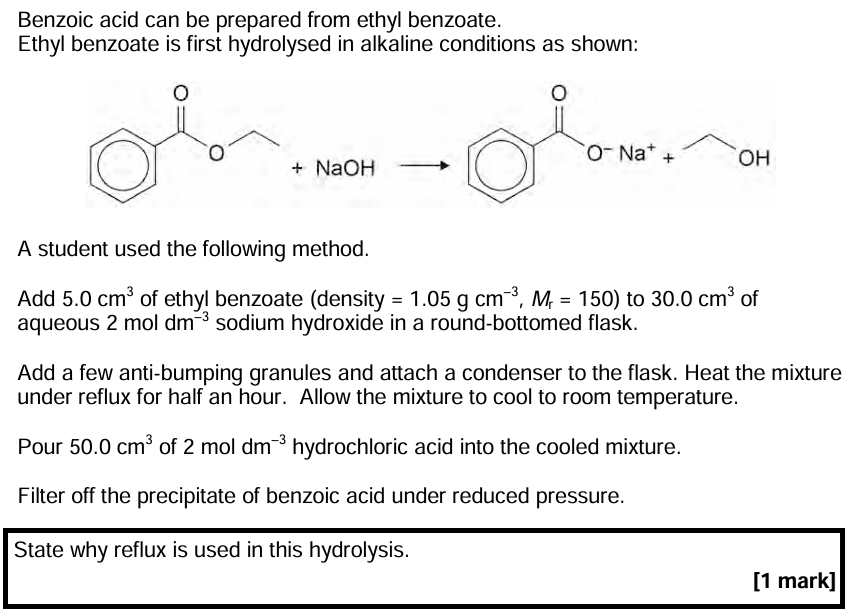
Allows reactant vapours (of volatile organic compounds) to be returned to the reaction mixture / does not allow any reactant vapour to escape
Suggest why sodium benzoate is soluble in cold water but benzoic acid is insoluble in cold water. [2 marks]
Sodium benzoate soluble because it is ionic
Benzoic acid insoluble because: despite the polarity of the COOH group / ability of COOH to form H-bonds, the benzene ring is non-polar.
After the solid benzoic acid has been filtered off, it can be purified.
Describe the method that the student should use to purify the benzoic acid. [6 marks]
Dissolve crude product in hot solvent/water
of minimum volume
Filter (hot to remove insoluble impurities)
Cool to recrystallise
Filter under reduced pressure / with Buchner/Hirsch apparatus
wash (with cold solvent) and dry
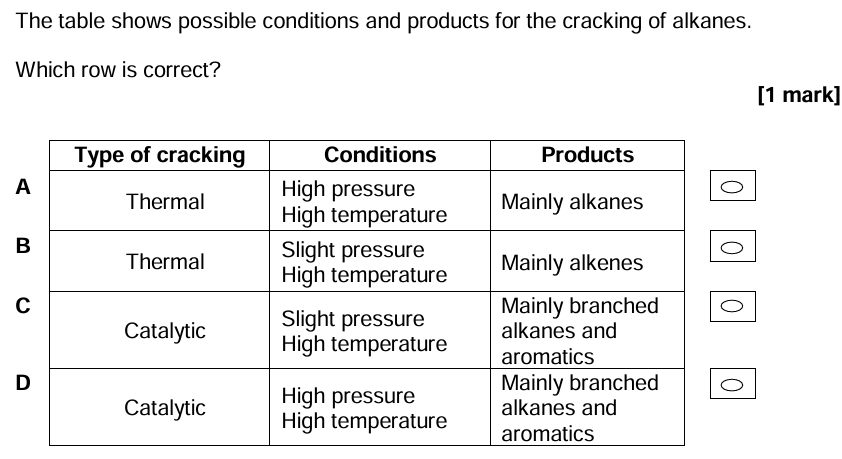
C
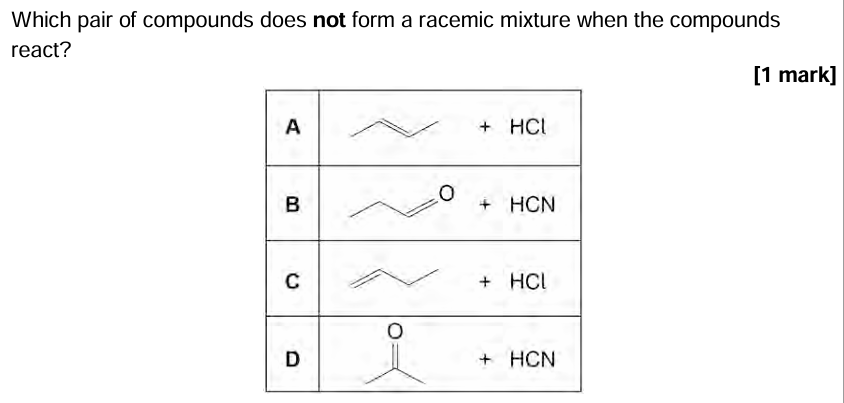
D
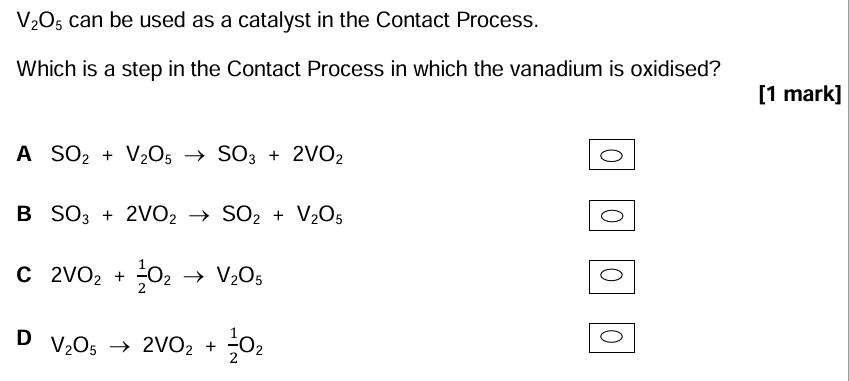
C

D