Bond Enthalpy
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
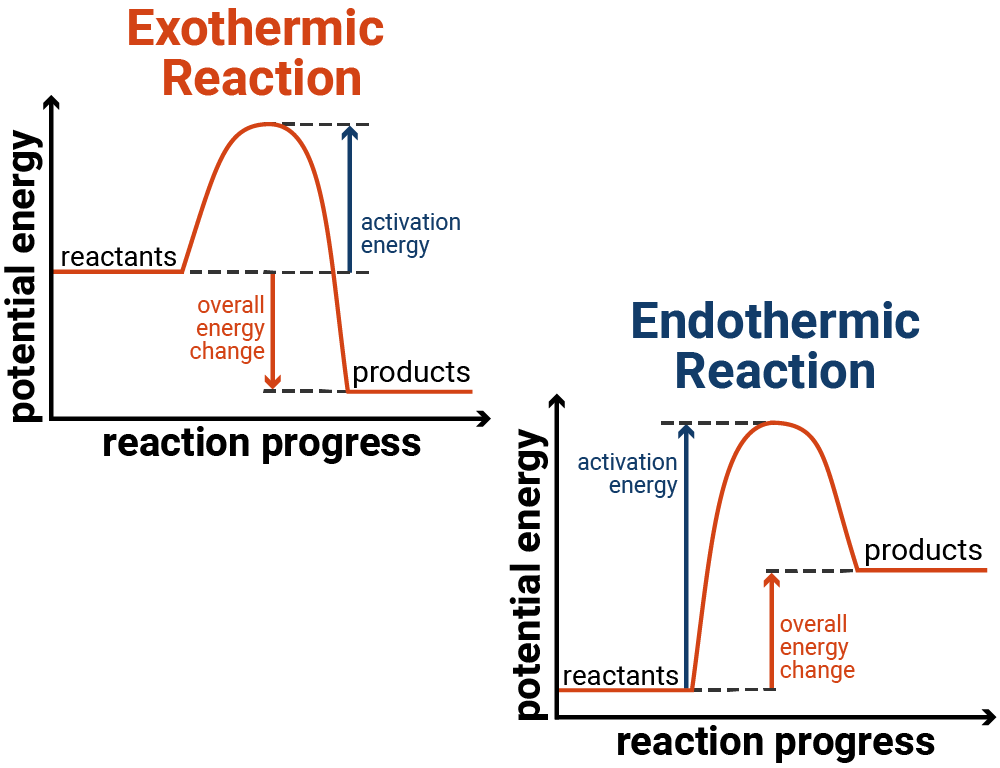
What is the definition of exothermic reactions ?
Reactions when the products have less energy than the reactant energy is given out to the surrounds ( temp goes up )
What is the definition of endothermic reaction ?
Reaction when the products have more energy than the reactant energy is taken in from the surroundings ( temp goes down )
Draw enthalpy level diagram of a exothermic and endothermic reaction
Draw the enthalpy level diagram of combustion of sulfur to form sulfur dioxide ?
Average bond enthalpy
Is the average enthalpy change that takes place when breaking (by homolytic fission) 1 mol of a given type of bond in the molecules of gaseous species
What does bond enthalpies do? (Energy)
The energy that takes to break or make a particular bond we can work out the overall enthalpy / energy change for the reaction
What is the equation for energy change
Sum of bond broken - sum of bond made
Is bond broken endo or exothermic reaction
Endothermic reaction
Is bond making endo Or exothermic reaction
Exothermic reaction
What does homolytic reaction means
The electrons are shared out equally making free radical
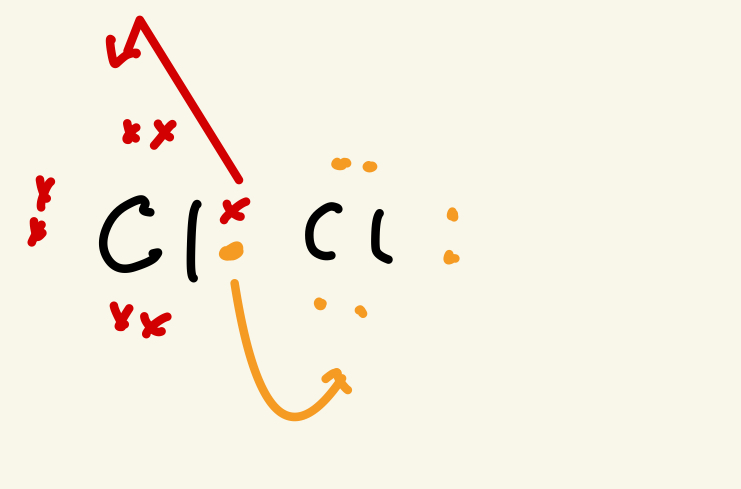
What does heterolytic fission mean
Both electrons se shared or transfer to one elements making ion
Why does using the energy change method to calculate the 🔺 H valves may not always be accurate
Will be less accurate than using formation or combustion data because the bond energies are slightly different depending on the compound the bond is found in