Lecture 1: Vaccines
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
40 Terms
define vaccines
biological products that induce protective immunity without causing disease → one of the most effective public health tools
what effects have vaccines had on disease eradication and reduction?
vaccination programs → reductions in incidence, basic reproductive number (R0, determines how likely illness is to spread or die out in a population), outbreak frequency
global eradicationL smallpox (1980, first coordinated global vaxx program), wold poliovirus types 2&3, type 1 still in Pakistan and Afghanistan
what are the consequences of vaccines not being 100% effective and define what factors determine the severity
breakthrough infections can occur in individuals despite prior immunity
factors influencing severity of breakthrough infections:
host factors : eg. elderly / immunocompromised
vax type (durability of protection) and schedule
hybrid immunity → natural infection + vax = lower severity
waning immunity
viral variants
exposure/viral load
BUT… overall, vaccination lowers severity of breakthrough infections
how do vaccines lead to herd immunity?
vaccines cannot protect every individual in a population directly. if enough individuals are immune (eg. ~90-95% immunity for highly transmissible pathogens), transmission can be interrupted → provides indirect protection for those that cannot get vaccinated
briefly describe the history of vaccination from ancient practices, 18th century, 19th century, 20th century, and 21st century
ancient practices: variolation in China, India, Ottoman Empire: deliberate exposure to dried small pox scabs. risky, but established principle that prior exposure to a pathogen confers protection
18th century: Edward Jenner used cowpox to protect against smallpox → concept of vaccination established → closely related but not identical virus still protected
19th century: Louis Pasteur developed vaccine for fowl cholera in chickens and rabies. Germ theory of disease (microbes vs miasma) strengthened vaccine science
20th century: YFV, influenza, polio, hepB, MMR vaccines developed
mass immunization programs dramatically reduce infectious diseases
21st century: advances technologies: mRNA, viral vectors, recombinant vaccines
why is the innate immune system important in vaccination?
early innate responses crucial for adaptive immune development
PRRs and cytosolic sensors (eg. TLRs, cGAS-STING, RIG-I) on APCs are activated by PAMPs from viruses and adjuvants designed to mimic PAMPs or DAMPs
APCs
what adaptive cells/processes are essential to vaccine-induced protection?
T cell-dependent B cell activation: initiated by antigen recognition and CD4+ T cell-derived signals
Germinal Centers: drive affinity maturation, isotype switching
Short-Lived Plasma Cells: rapidly produce vaccine-specific Abs
Long-Lived Plasma Cells: home to bone marrow, sustain Ab production
Memory B Cells: mediate long-term immune memory
Neutralizing Abs block viral entry; Fc-mediated functions enhance protection
CD8+ Effector T Cells: eliminate infected cells and limit disease severity
CD8+ Memory T Cells: expand rapidly upon re-exposure to eliminate infected cells
describe the differing pathways for adaptive immunity for vaccines presented on MHC I vs MHC II
MHC class I: activates CD8+ T cell, leading to CD8+ effector and memory T cells
MHC class II: activate CD4+ T cell which provides helper signals to B cells that encounter their soluble antigen → B cells proliferate and Ab response matures → memory B cell proliferation or plasma cell differentiation and antibody production
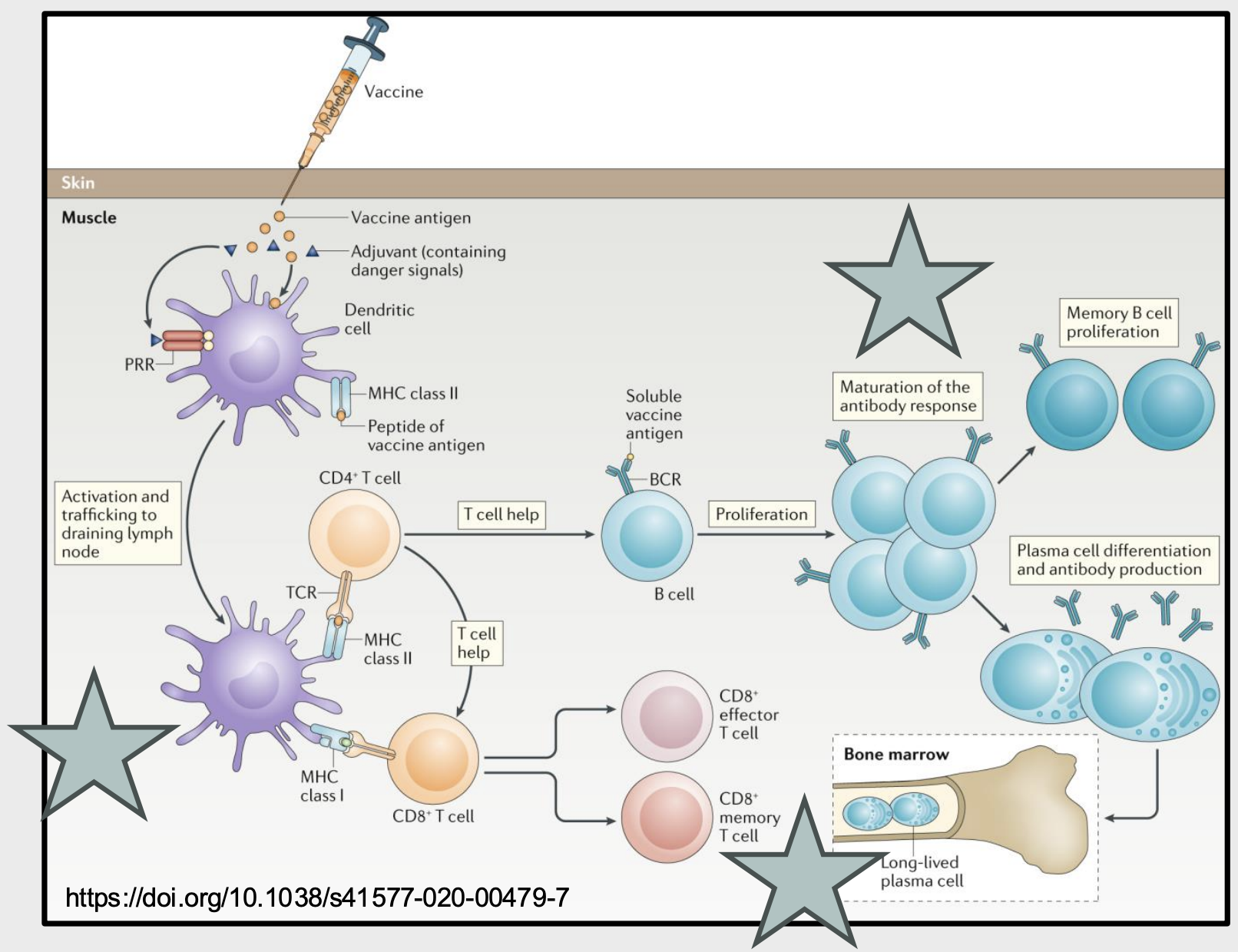
desribe the evidence of Abs as correlates of protection
neutralizing Abs block viral entry and strongly correlate with protection against infection
Ab deficiencies increase susceptibility to specific viral infections (eg. VZV), identifying key protective mechanisms
Passive Ab transfer provides immediate protection against some infections → eg. maternal Abs across placenta, purifies neutralizing Abs from immune donors
vaccine protection relies on what?
immune memory
the ability of immune memory to defend against a future pathogen encounter depends on what?
incubation time of the infection
quality of the memory response
level of Abs induced by memory B cells
describe how incubation period impacts the ability of immune memory to defend against a future pathogen encounter
following the primary exposure to the pathogen, the level of protective Abs decline below the protective threshold. When the pathogen has a long incubation period (eg. hep B), upon secondary infection this allows enough time for antibody levels to get above the protective threshold. with a short incubation period (h. influenzae B) , there is insufficient time to raise protective antibody levels
BUT in some cases Ab levels after primary vaccination remain above the protective threshold and can provide lifelong immunity

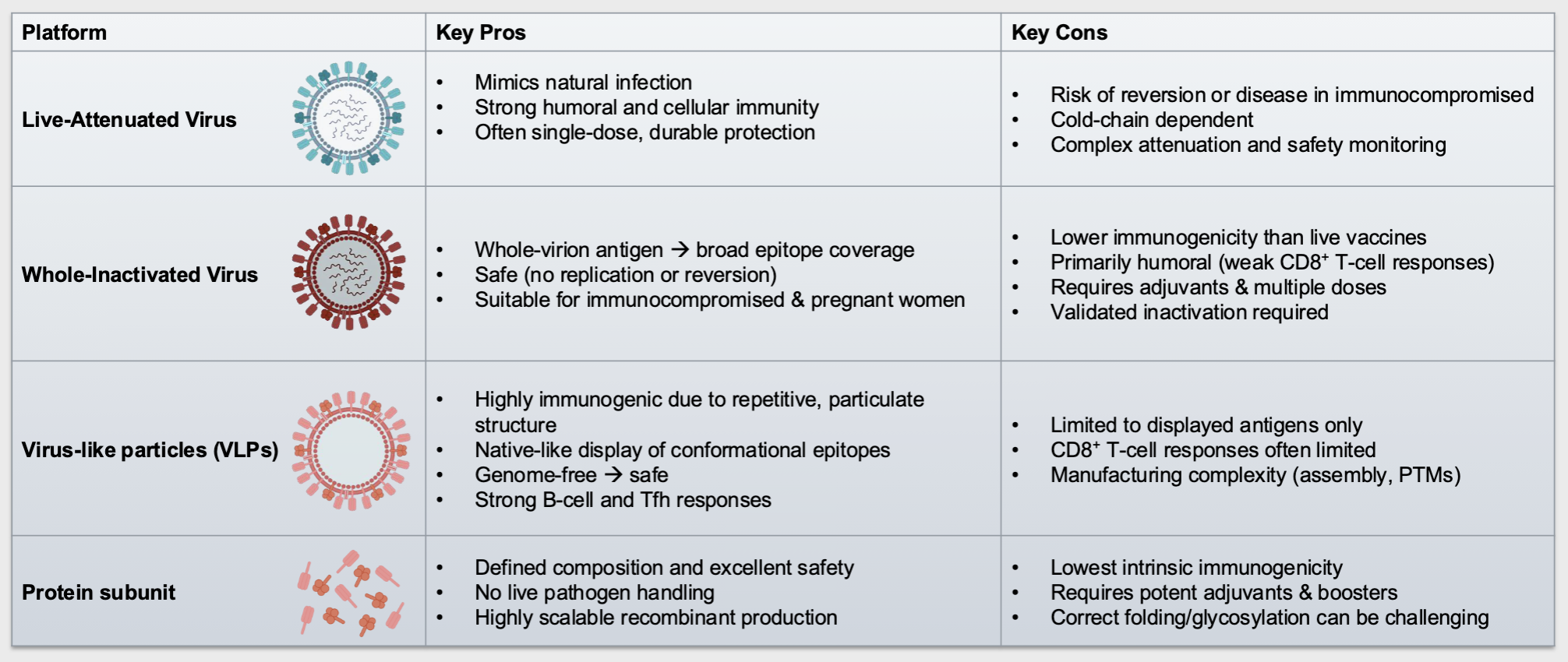
describe and provide examples of the classical vaccine platforms
Live-attenuated virus: whole, weakened viruses that can replicate but do not cause serious disease → eg. MMR vax
whole-inactivated virus: whole “killed” viruses that cannot replicate → eg. polio inactivated vax (IPV)
Protein Subunit: specific viral proteins or protein fragments → eg. hep B vax
virus-like particles (VLPs): viral proteins that mimic the virus but lack viral genetic material and cannot replicate → eg. HPV vax
describe live attenuated virus vaccines and how they can be produced
whole, weakened viruses that can replicate minimally within host cells, mimicking natural infection
often induce durable and broad immune responses
produced by:
serial passage in non-human hosts or cells (eg. chicken embryo fibroblasts) → virus adapts to new host, loses virulence in humans
Cold adaptation → virus passaged at low temperatures (25-36C), loses ability to replicate efficiently at high temperatures
genetic engineering: targeted mutations or deletions in virulence genes
describe an example of a live-attenuated vaccine
Measles virus (MeV) adaptation to CD46 in cell culture:
has several mutations that impact viral replication cycle
after passaging, virus lost ability to bind to CD150/SLAM and PVRL4/nectin so can no longer replicate in immune cells → inhibited at level of cell attachment
CD46/MCP found on all nucleated cells, CD150/SLAM found on immune cells (DCs, Mo), PCLR4/nectin found on epithelial cells and adenocarcinomas
describe the second example of a live-attenuated vax
cold-adapted influenza A virus → attenuated by affecting viral DNA/RNA synthesis
impacts viral RNA-dep RNA pol (RdRp) → trimeric, composed of PB1, PA, PB2
when passaged at lower temperatures, accumulated a few mutations in RdRp so cannot replicate at physiological temp → when given via nasal (has lower temp), can replicate but cannot replicate in the lungs
possibility of reversion
describe whole-inactivated vaccines and how they can be inactivated
whole viruses that cannot replicate within host cells → verification of inactivation is critical
often need adjuvants, higher Ag doses, and booster doses to help compensate for weaker immunogenicity compared to live vax
inactivated by chemical inactivation (more common) or physical inactivation
describe the methods for physical inactivation
formalin (formaldehyde): crosslinks proteins And nucleic acids (eg inactivated polio vax)
beta-propriolactone (BPL): alkylates nucleic acids (destroys genome), preserves protein structure better than formalin, used for flu, rabies, some COVID vax
describe the methods for physical inactivation
heat: Denatures viral proteins (e.g., capsid proteins and envelope glycoproteins → loss of receptor binding or fusion capability), BUT can strongly alter antigenic structures
Ultraviolet (UV) Inactivation : Direct photochemical damage to viral genomes, forms pyrimidine dimers in DNA or RNA, induces strand breaks, oxidative lesions, and cross-linking
can do more damage to viral structure
describe an example showing the importance of verifying complete inactivation of whole-inactivated virus vaccines
The Cutter Incident (1955, USA):
• Two production pools of IPV made by Cutter Laboratories (accounting for 120,000
doses) were given to healthy children
• Inadequate virus purification during production led to the presence of cell debris in
the vaccine pools
• Cell debris blocked proper exposure of virions to formalin, preventing complete
inactivation
Outcomes:
• 40,000 cases of abortive poliomyelitis, 51 cases of permanent paralysis, 5 deaths
• Immediate tightening of federal regulations for vaccine production
how can complete inactivation be measured?
extended cell culture amplification by taking inactivated virus and “infecting” highly permissive cell lines → monitor for evidence of replication: CPE observation, genome amplification (PCR), antigen detection (immunoassay)
describe protein subunit vaccines
• Contain designated viral proteins rather than whole viruses → minimized risk since no infectious virus present
• Often require adjuvants - immune system may generate weak responses to single antigen proteins
• Often expressed in yeast, mammalian, or insect cell systems
describe an example of a protein subunit vaccine
Hepatitis B virus (HBV) vaccine:
key proteinL HBV Surface Antigen HBsAg → S protein
HBsAg → viral envelope protein composed of 3 related forms: Small (S), S protein only, Middle (M), S protein +PreS2 domain, and Large (L), S protein + PreS2+PreS1 domains
why is only the S protein used in recombinant HBV vaccines?
major neutralizing epitopes: stimulates strong B and T cell responses
highly conserved: broad protection against HBV strains
established vaccine efficacy: induces protective anti-HB S antibodies after 3 doses
describe how the hepatitis protein subunit vaccine is produced using yeast
genetic material extracted from hepatitis virus
individual genes analyzed and identified
gene that directs production of surface protein is located
gene is removed from viral DNA and inserted into ‘plasmid’ → S protein moved to plasmid
plasmids inserted into yeast cells
yeast grown by fermentation
cells reproduce and generate more surface protein
result is large quantity of pure surface protein particles that provoke an immune response
surface proteins are combined with preserving agent (&adjuvant) and other ingredients to make vaccine
what are additional design and production considerations when making a protein subunit vaccine?
• Choice of antigen: must include protective epitope(s) that induce neutralizing Abs or effective T cell help
• Expression system: yeast (e.g., HBsAg), mammalian, or insect cells → affects glycosylation and folding; proper protein folding and post-translational modifications critical for immunogenicity
• Purification: removal of host contaminants while preserving native conformation
• Stability: formulation must maintain protein structure during storage and transport
• Dosing: multiple dosing and adjuvants often required for durable immunity
• Scalability: production must be compatible with large-scale manufacturing
describe virus-like particle (VLP) vaccines
• Viral structural proteins that self-assemble into particles that mimic that size, symmetry, and structure of native virions
• Genome-free → non-infectious and replication-incompetent
• Preserve conformational epitopes required for neutralizing antibodies
what effect do VLP vaccines have on B cells?
repetitive particle geometry of VLP-based vaccines optimally activated B cells:
• Strong B-cell activation requires at least 12–16 identical epitopes spaced ~5–10 nm apart (“immunons”) → VLPs naturally meet these criteria
• Enables efficient B cell receptor (BCR) cross-linking and recognition by IgM antibodies
• Promotes complement activation, plasma cell differentiation, and germinal center formation
• Induce durable antibody responses → especially valuable when target antigens are weakly immunogenic
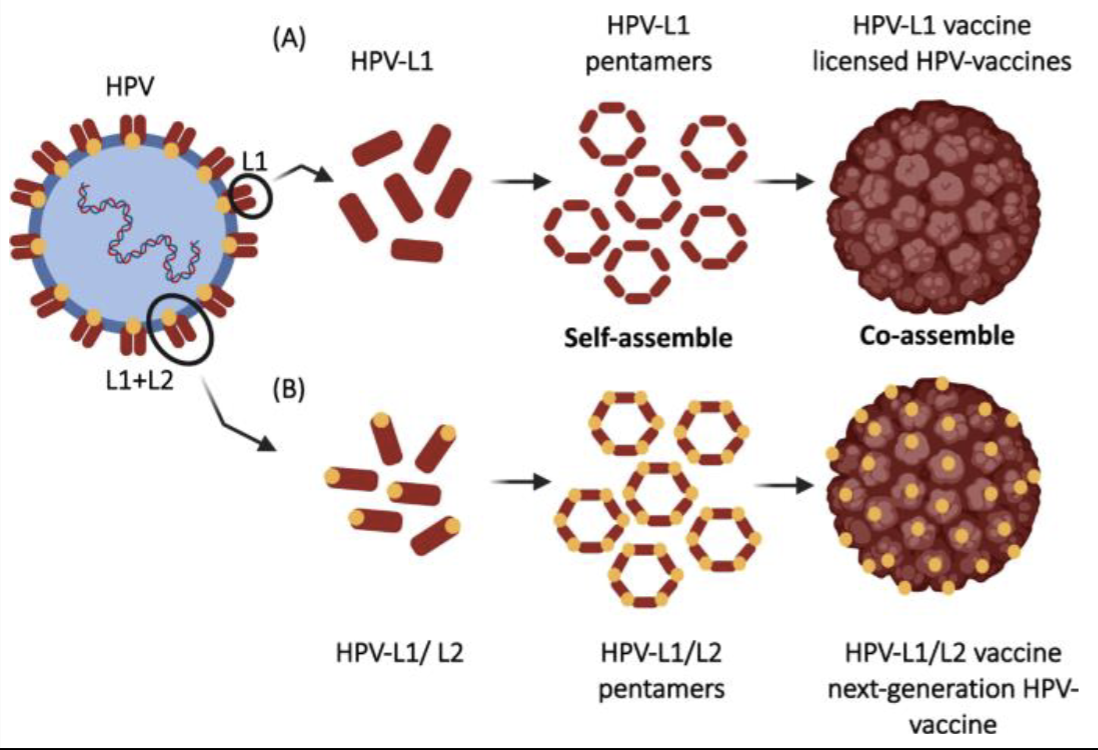
describe an example of a VLP vax
Human Papillomavirus (HPV) vaccine:
• Licensed HPV vaccine antigen = L1 major capsid protein (immunodominant, highly immunogenic)
• Recombinant L1 self-assembles into icosahedral VLPs
• Displays native conformational neutralizing epitopes
• L2 minor capsid protein (highly conserved, less immunogenic) → considered a target for the development of a next-generation Pan-HPV vaccine
describe the key pros and cons of the live-attenuated, whole-inactivated, VLP, and protein subunit vaccines
describe some modern vaccine platforms
mRNA and DNA vaccines: Use the host’s own protein synthesis machinery to produce immunogenic antigens → need to be delivered into cells for antigen expression
recombinant viral vector vaccines:
describe the core design elements of mRNA vaccines
produced by in vitro transcription (IVT) from DNA template
Conventional (non-replicating) mRNA:
• Synthetic mRNA is translated directly in the cytoplasm by host ribosomes
• Antigen output is dose-dependent
• Mimics the structure of endogenous mRNA with 5 sections: 5’ Cap, 5’ UTR, ORF, 3’ UTR, Poly-A Tail
Self-Amplifying mRNA (is replicating):
• Encodes antigen + viral replicase (RdRp); undergoes intracellular
RNA amplification
• Higher antigen expression at lower doses
what are the core design elements of DNA vaccines?
• Plasmid backbone: circular DNA molecule carrying the antigen gene; includes bacterial origin of replication and antibiotic resistance marker for propagation in bacteria
• Antigen coding sequence: gene encoding the target protein
• Eukaryotic expression elements: promoter, transcriptional terminator, and poly(A) signal to drive transgene expression in host cells
• Regulatory elements: sequences enhancing mRNA stability, nuclear localization, and efficient translation
• Codon optimization: adjusts gene sequence for efficient protein production in mammalian cells
what is a challenge with mRNA vaccines and how has this been overcome?
• mRNA is large (104-106 Da) & negatively charged → cannot pass through the anionic lipid bilayer of cell membranes
Techniques to deliver mRNA intracellularly:
In vitro: electroporation, gene guns, ex vivo transfection
In vivo: mRNA delivery vehicles (e.g., lipid nanoparticles)
lipid nanoparticles (LNPs)
• Encapsulate mRNA in their core → used in mRNA vaccine production
Consist of 4 Components:
• Ionizable lipids → help mRNA escape LNP
• Cholesterol or its variants → fusion to cell membrane
• Helper lipids → stabilize particle
• PEG-DMG → help extend circulation of LNP
what challenge do DNA vaccines have and how have we combatted this?
DNA must cross plasma membrane and enter nucleus → nuclear localization signals often intrinsic to DNA viruses but can be engineered into plasmids
variety of ways to transport DNA vax across plasma membrane → viral capsid, empty bacterial capsule, nanoparticle, liposome, gold beads
what are some current clinical applications of mRNA and DNA vaccines?
infectious disease vaccines → encode 1-2 Ags, often cell attachment proteins
eg. SARS-CoV2, flu, RSV, Zika prM
therapeutic cancer vaccines
genetic adjuvants
describe an example of an mRNA vaccine
mRNA-1345: examples of structure-based vaxx design
• LNP-based mRNA vaccine candidate developed by Moderna for respiratory syncytial virus (RSV) infection
• Encodes for the RSV prefusion F (pre-F) glycoprotein → responsible for entry of the virus and cell-to-cell spread
• Recently authorized for adults > 60 years of age
why choose the pre-F glycoprotein as the antigen for an mRNA vaccine?
metastable pre-F form exposes key neutralizing epitopes → epitopes lost in pre-F form
strong correlation with protection
high immunogenicity with lower Ag dose
proven success across RSV platforms