Chem Exam 2
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
Core electrons
remain close to the nucleus and usually don't participate
Valence electrons
The outermost electrons that interact with other atoms and where bonding occurs
Atoms
consists of protons neutrons and electrons
ex: Mg
Molecules
two or more atoms chemically bond together
ex: MgCl2
Ions
an atom or molecule that has gained or lost electrons, giving it a positive or negative electrical charge
ex: Mg^2+
Pure substance
-Made of only 1 type of particle (element or compound)
-fixed composition
Mixtures
-combine pure substances physically and can be separated
-can be homogeneous (same throughout) or heterogeneous (different parts visible)
Physical change
Alters a materials form/appearance without changing its chemical composition
Chemical change
creates a new substance with different molecular composition
Ionic bonds
usually form between metals and nonmetals. Metals lose electrons and become positively charged, while nonmetals gain electrons and become negatively charged. These opposite charges hold the atoms together
Covalent bonds
Form when 2 nonmetal atoms share electrons. This sharing allows both atoms to complete their outer shells, making the molecule stable. Covalent bonds are represented as a line, each line showing 2 shared electrons. There is no transfer, just sharing for stability
Conservation of mass
The law states matter cannot be created nor destroyed in a chemical reaction. The total mass before and after a reaction is the same
The mass of reactants= mass of product
NH+4
Ammonium
CO3 ^2-
Carbonate
OH-
Hydroxide
NO3 ^1-
Nitrate
SO4 ^2-
Sulfate
PO4 ^3-
Phosphate
covalent, ionic, or both?: KI
ionic
covalent, ionic, or both?: H2O2
covalent
covalent, ionic, or both?: Li2CO3
both
ionic or covalent?: NCI3
covalent
ionic or covalent?: MgCl2
ionic
ionic or covalent?: CCI4
covalent
ionic or covalent?: NF3
covalent
What is the symbol for BaO?
Ba^2+O^2-
What is the symbol for Na2O?
(Na^1+)2 O^2-
Does NaCN have covalent bonds?
yes
Does P4O10 have covalent bonds?
yes
Does Rb2S have covalent bonds?
no
draw the structure for: HBr
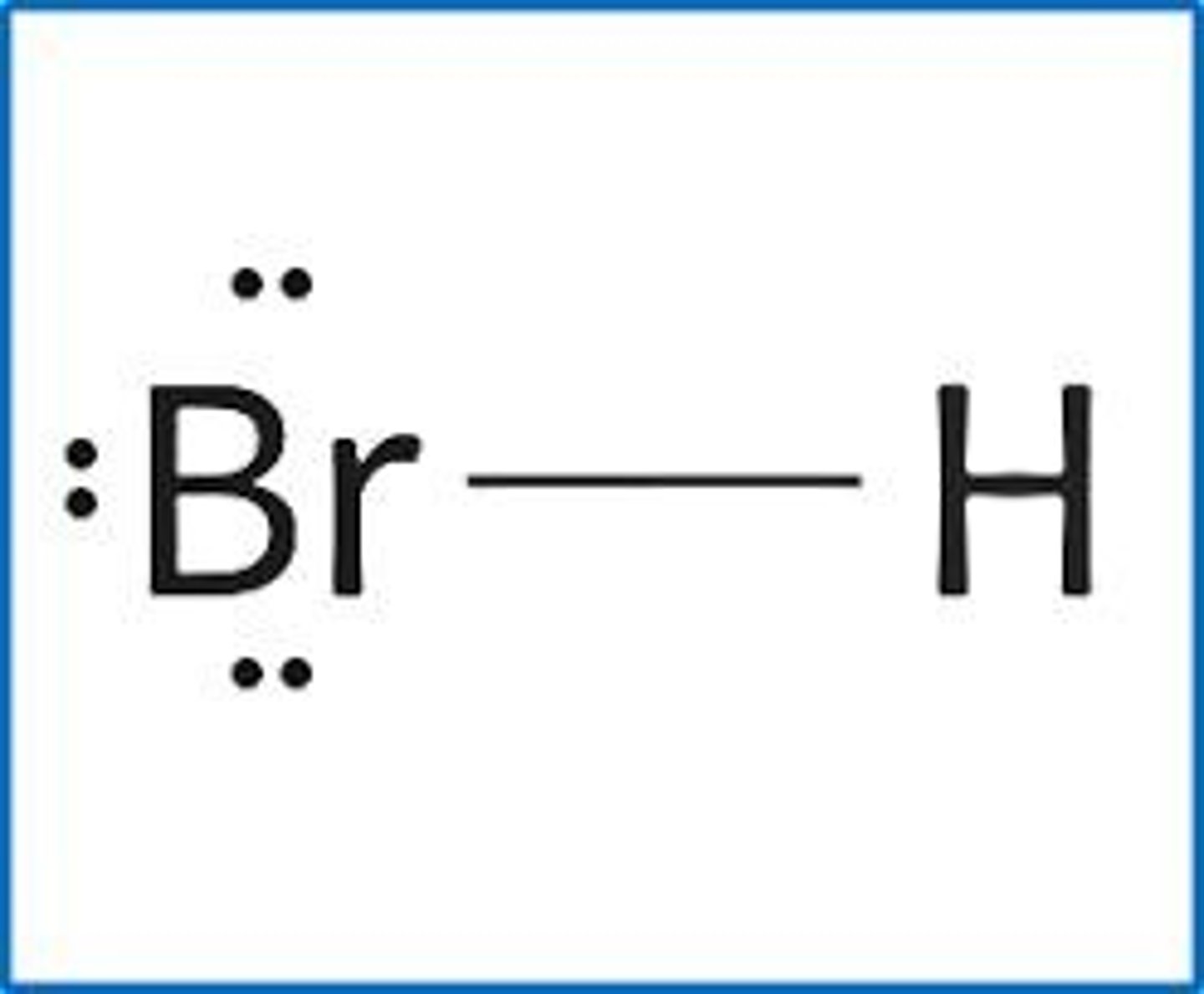
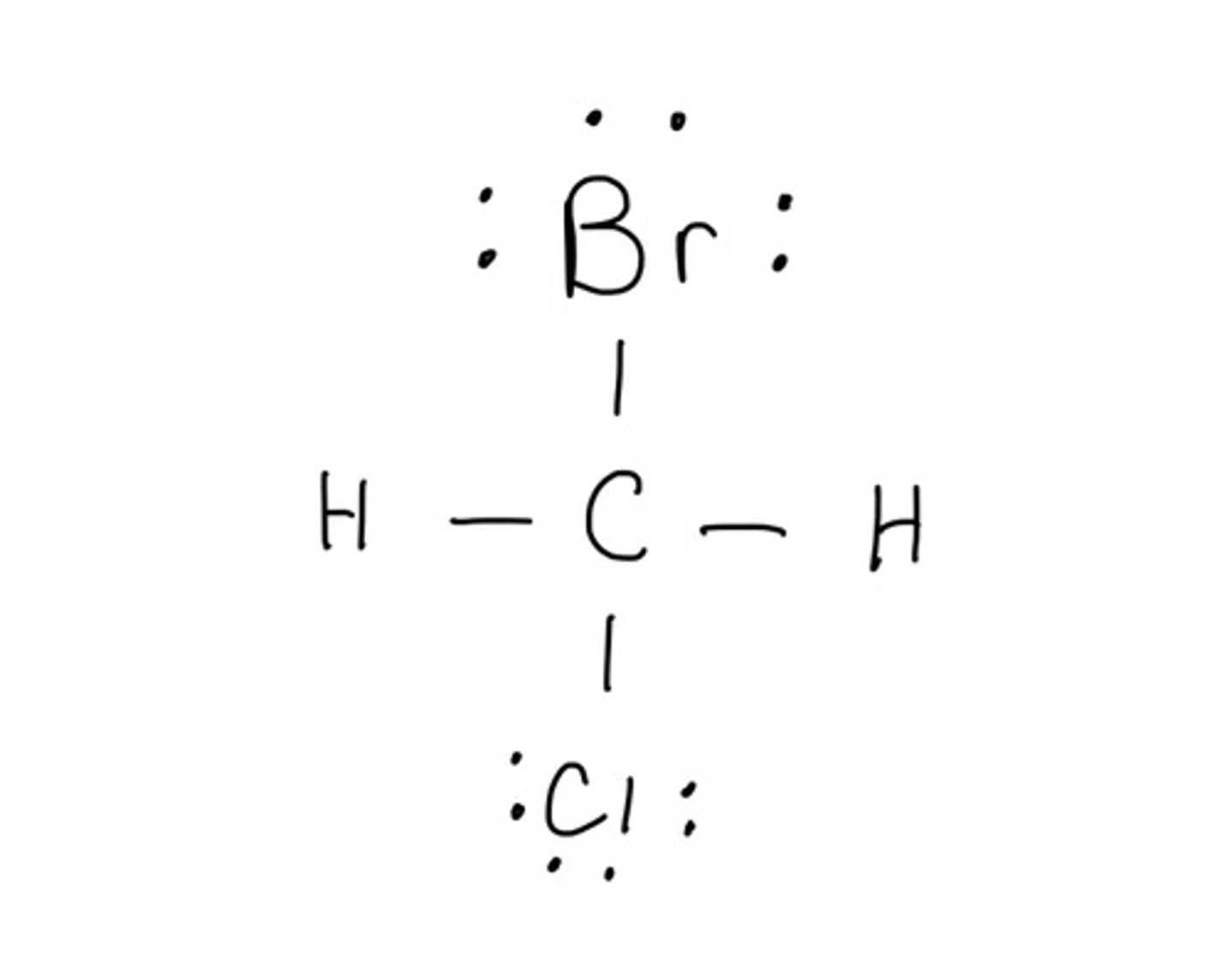
Draw the structure for: CICH2Br
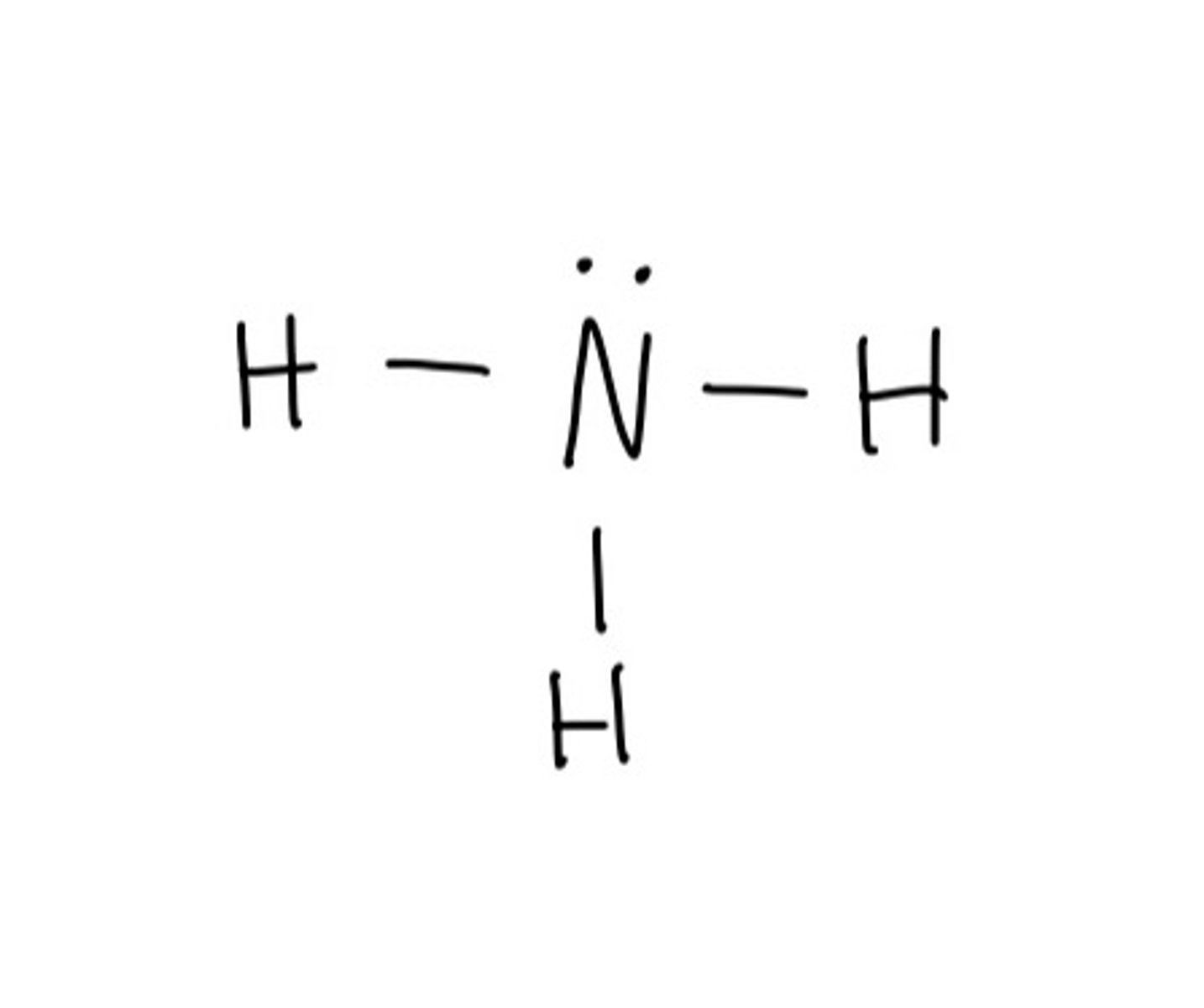
Draw lewis structure for: NH3
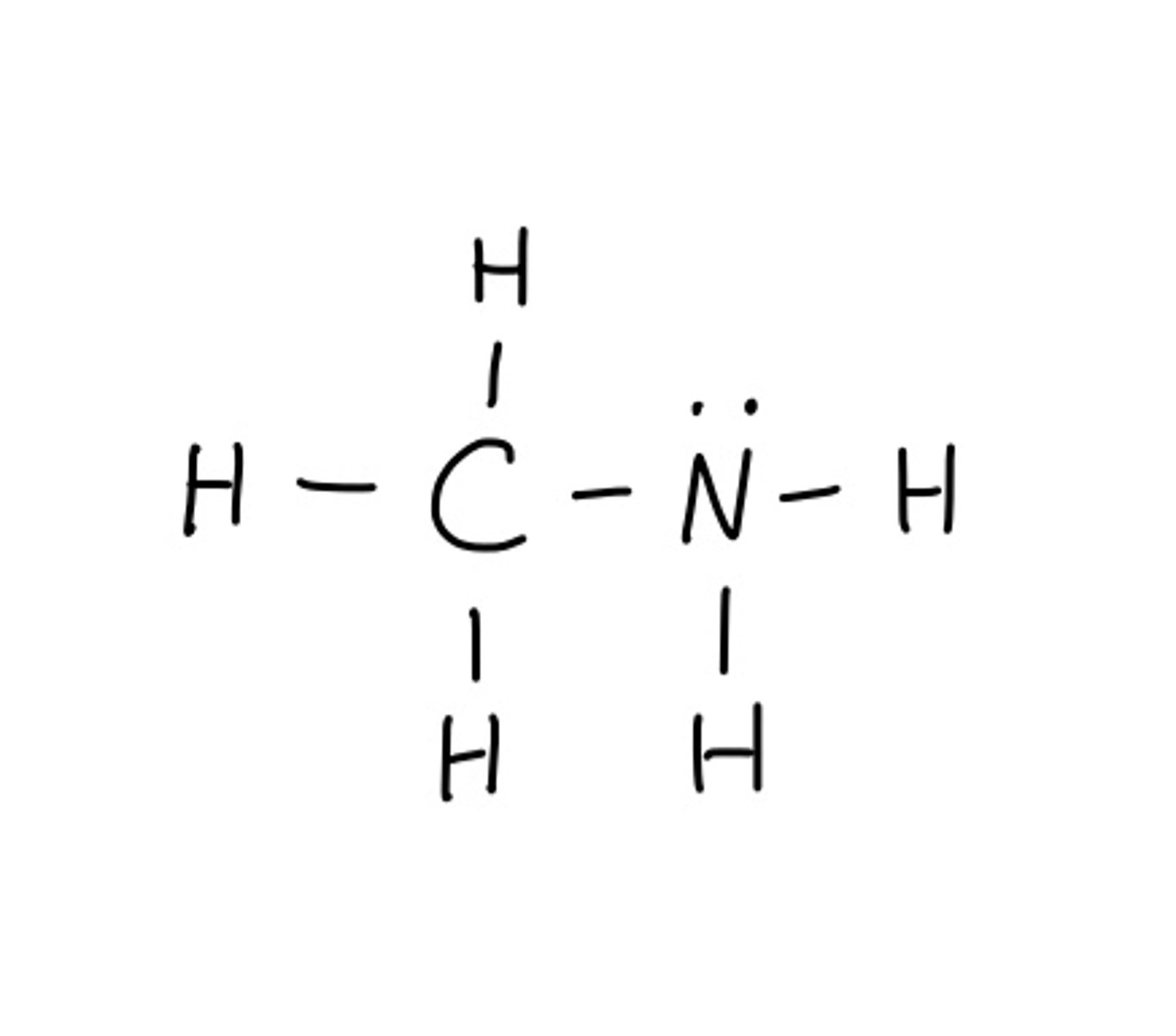
Draw lewis structure for: CH3NH2
Draw lewis structure in order written: CH3OCH3
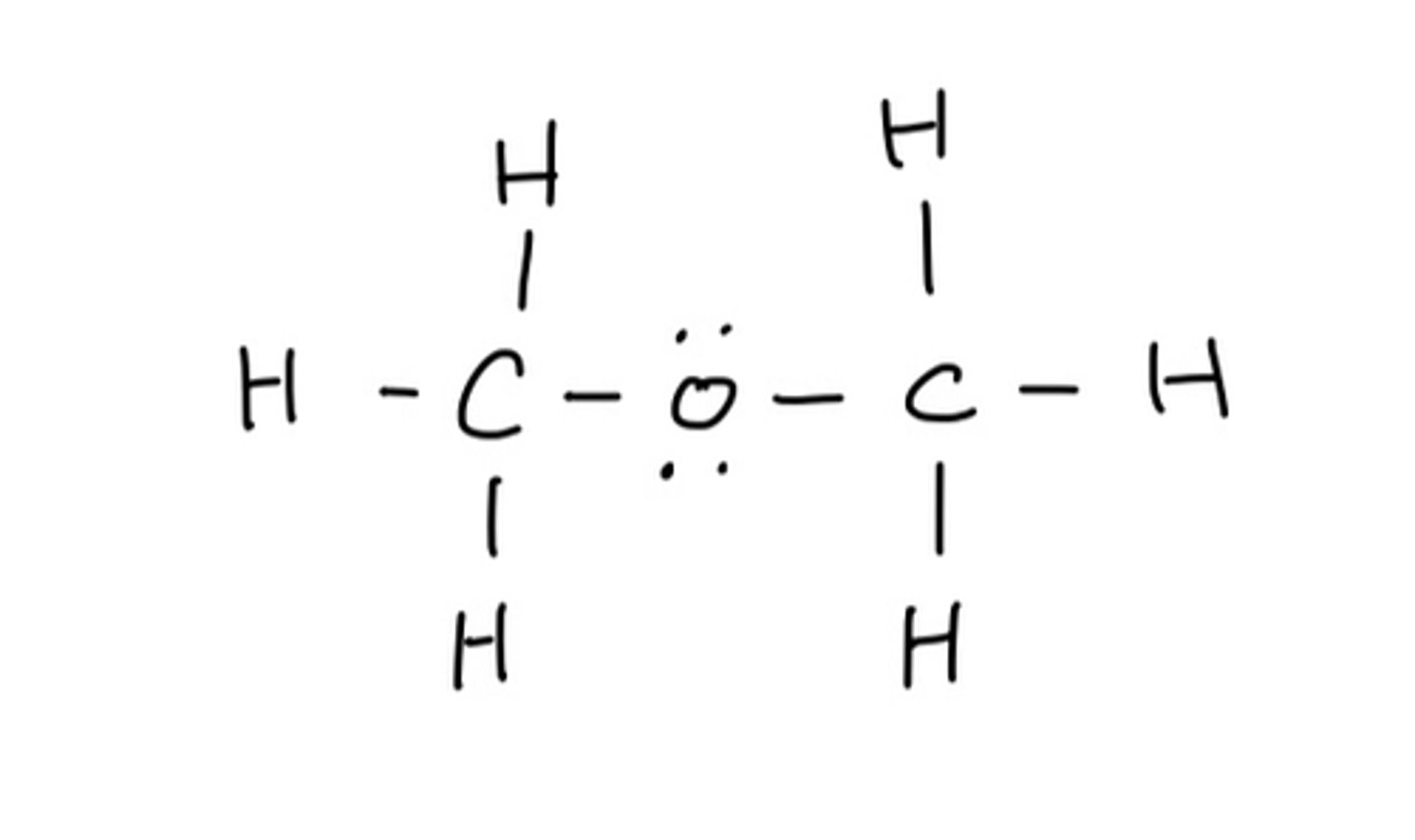
Draw lewis structure with 3 bonds between C and C: CHCH
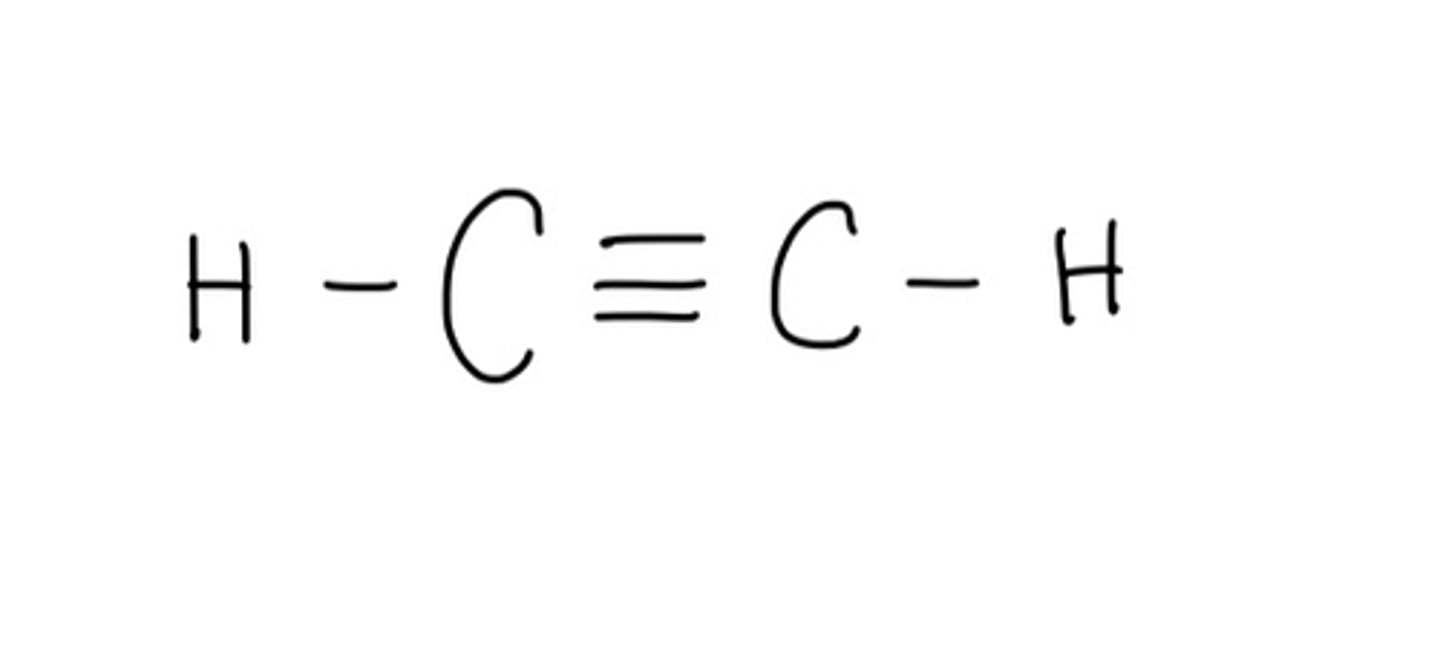
What is the chemical formula for: Zinc(III)phosphate
Zn3(PO4)2
What is the chemical formula for: Ammonium nitrate
NH4NO3
What is the chemical formula for: Sodium hydrogen sulfate
NaHSO4
what is the name for: V2O5
Vanadium (V) oxide
what is the name for: NH4NO3
ammonium nitrate
what is the name for: Hg(OH)2
Mercury (II) hydroxide
what is the name for: KNO3
potassium nitrate
VSEPR
Valence shell electron pair repulsion
Make as far as possible
Molecular geometry for: BeCl2
linear
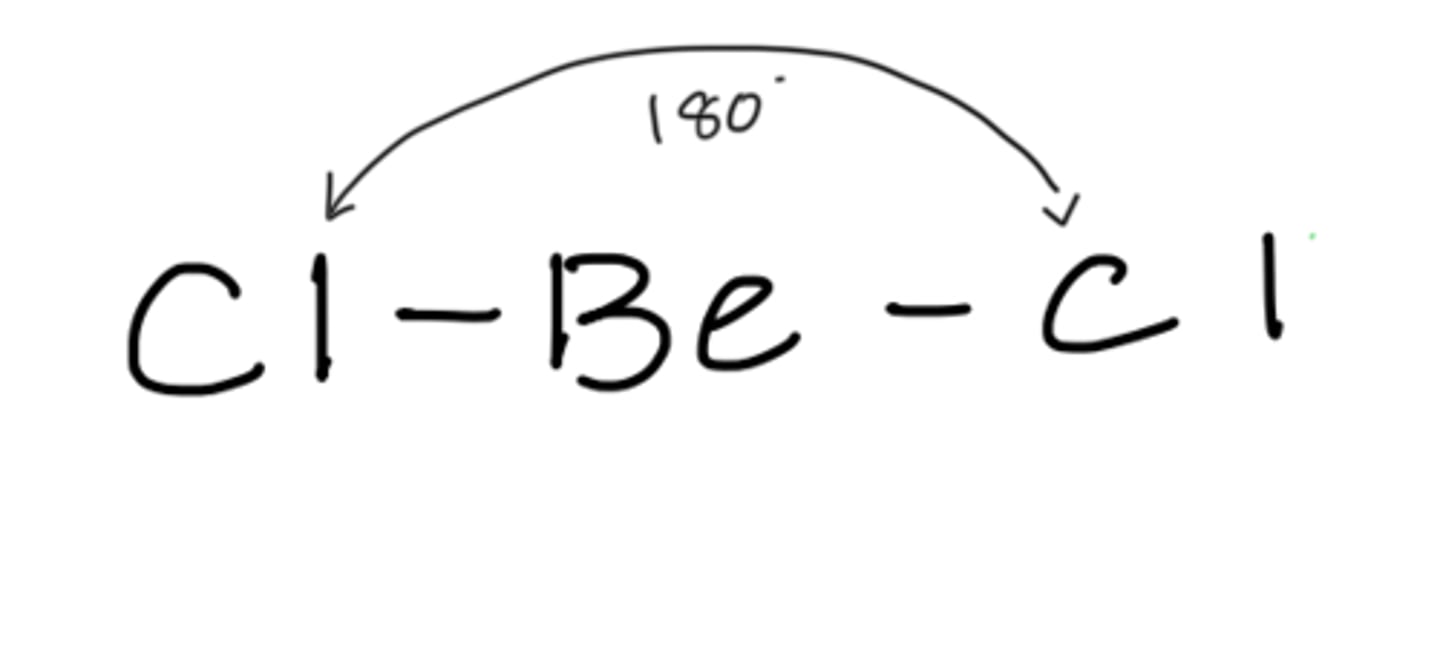
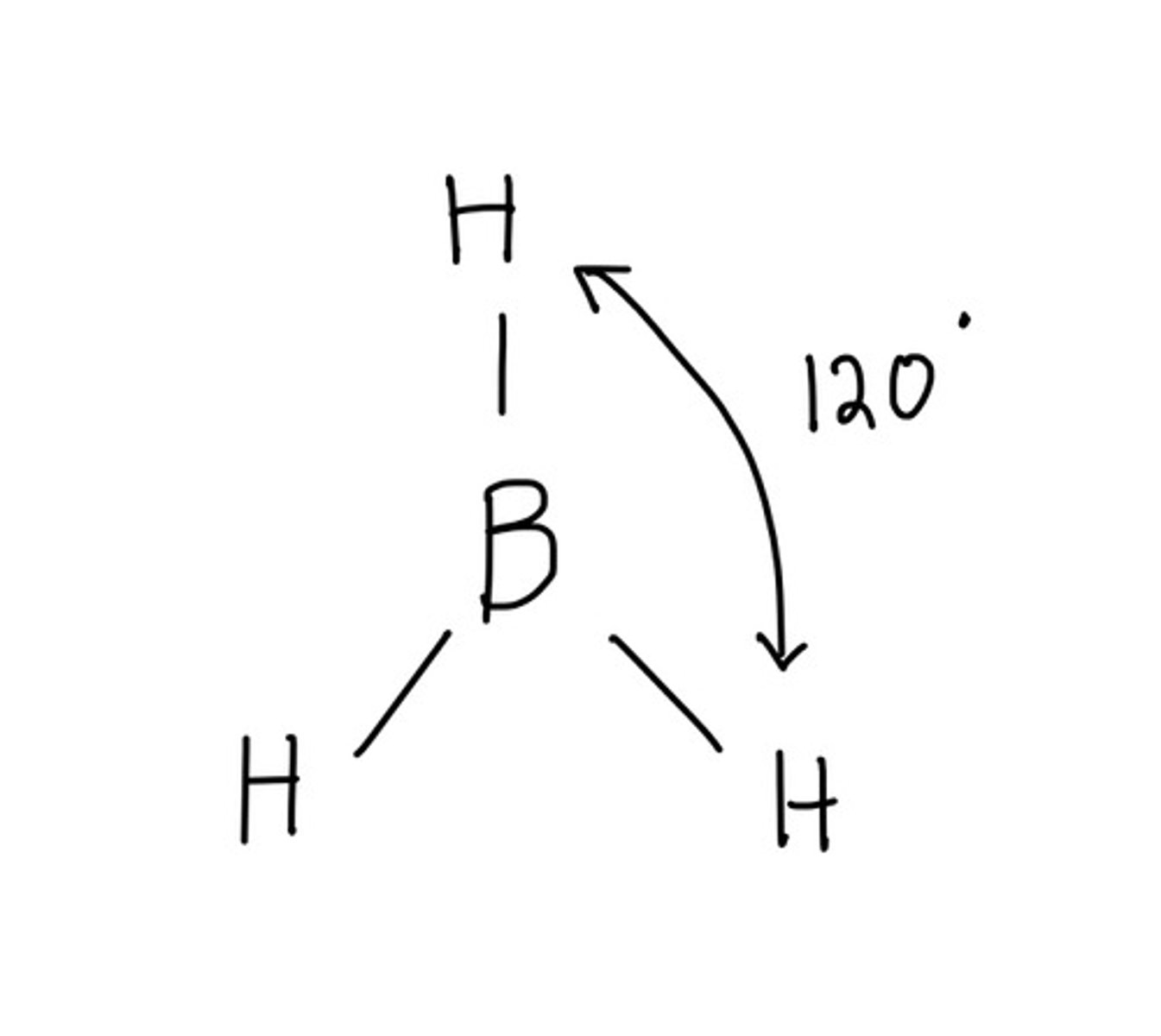
Molecular geometry for: BH3
Trigonal planar
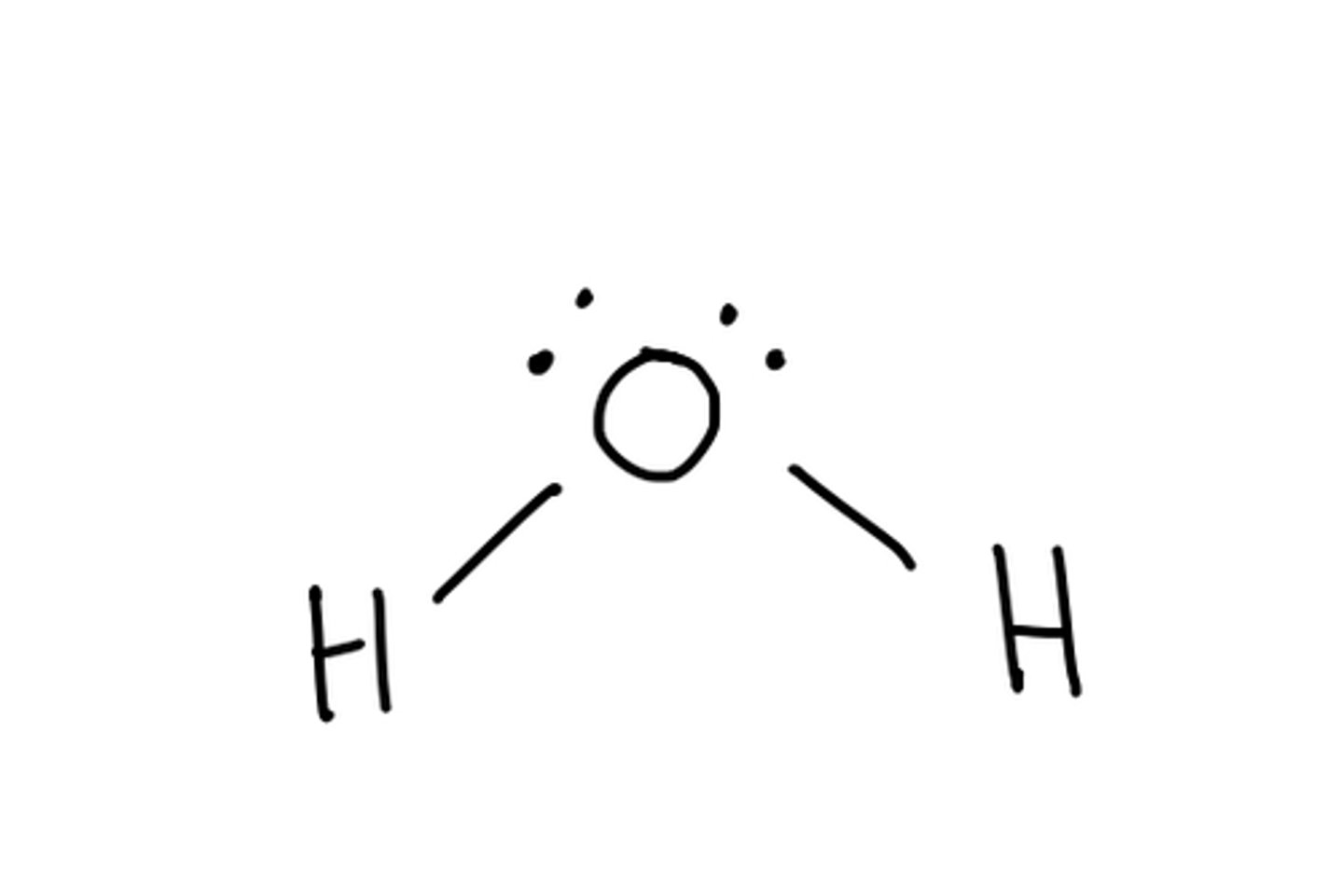
Molecular geometry for: H2O
Bent
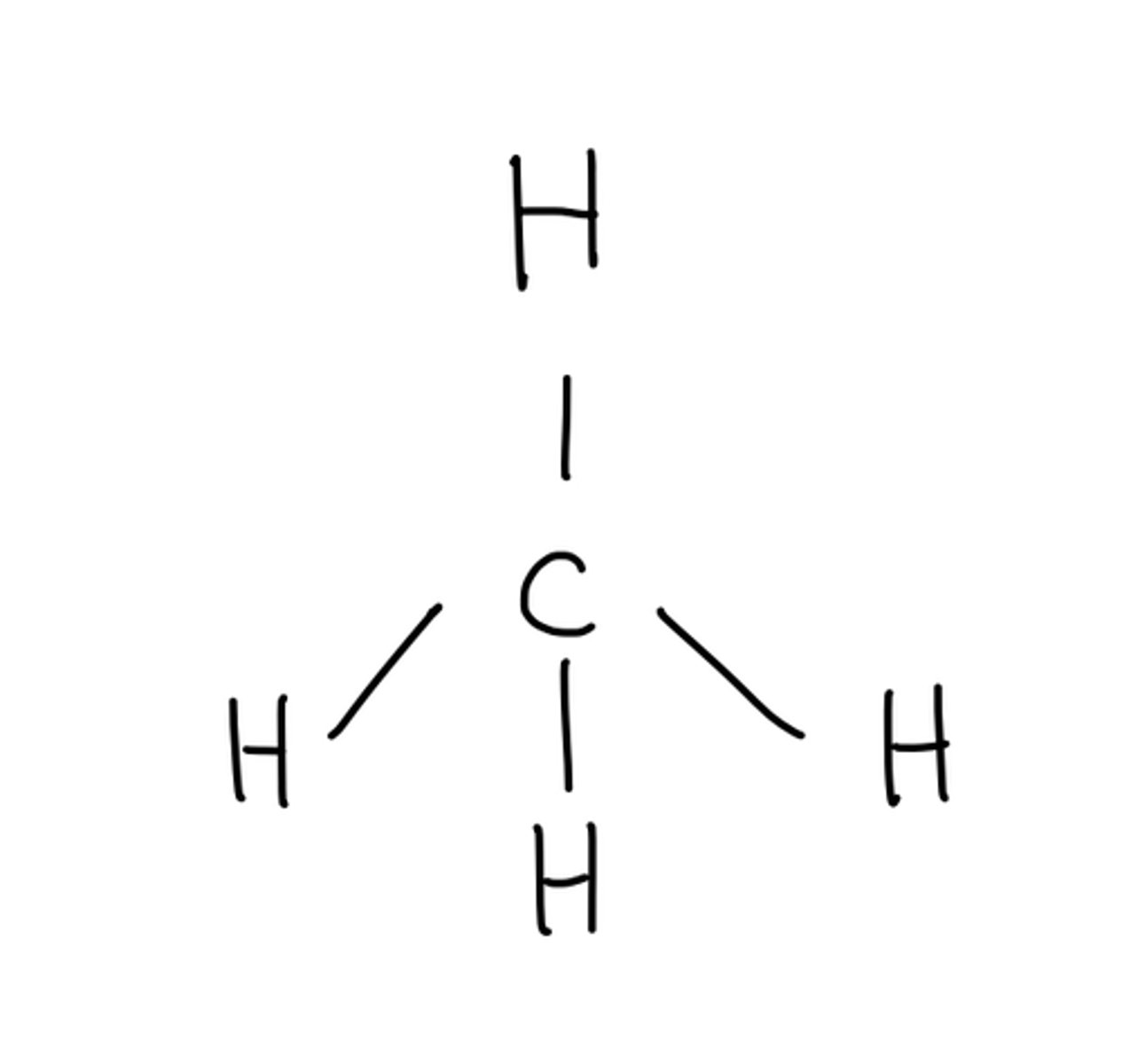
Molecular geometry for: CH4
Tetrahedral
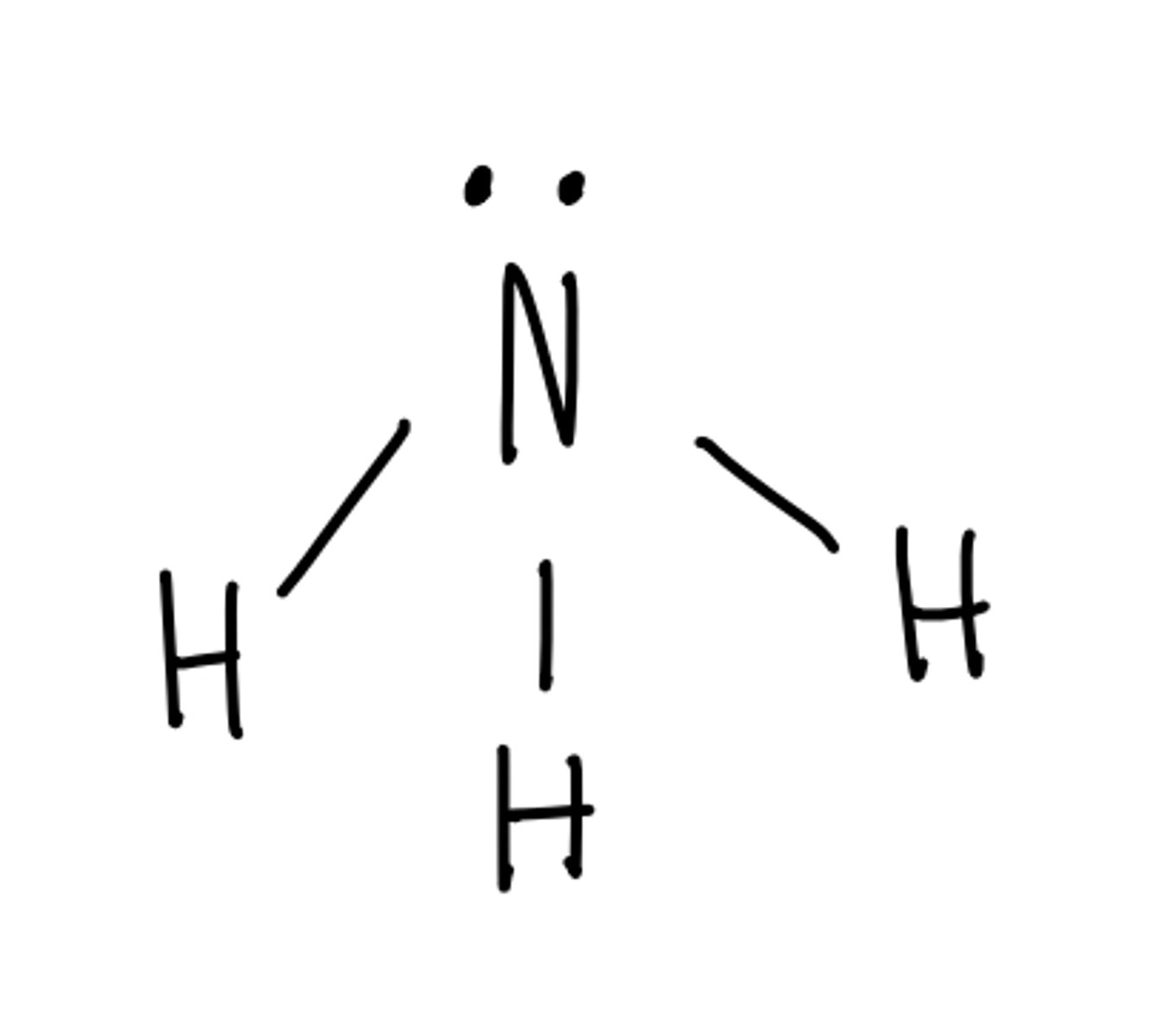
Molecular geometry for: NH3
Trigonal pyramidal
Trigonal pyramidal
1 lone pair, tetrahedral, 4 electron dense areas
Linear
0 lone pairs, linear, 2 electron dense areas
Bent
1 lone pair, trigonal planar, 3 electron dense areas or
2 lone pairs, tetrahedral, 4 electron dense areas
Tetrahedral
0 lone pairs, tetrahedral, 4 electron dense areas