Oxidative Phosphorylation
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
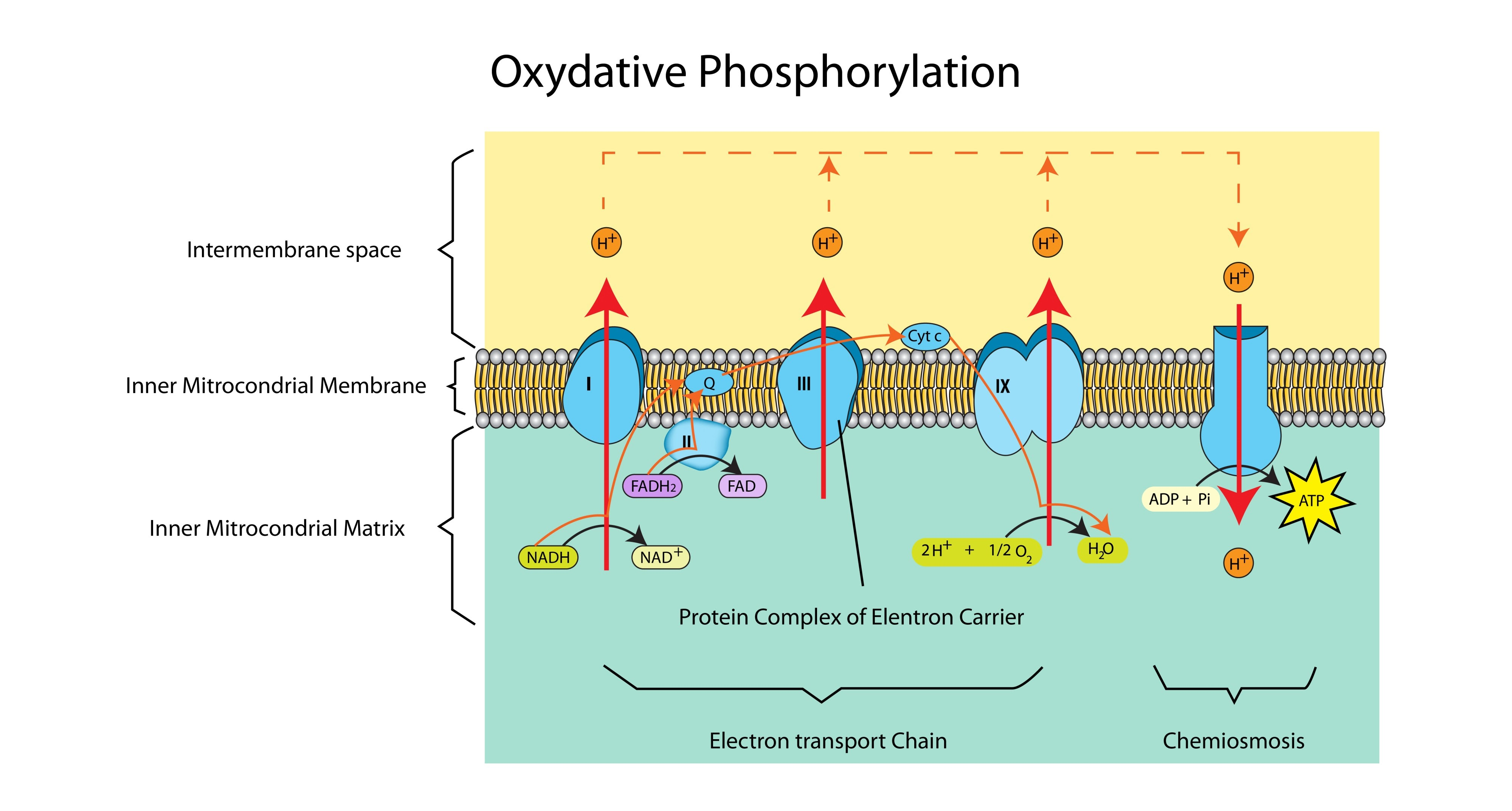
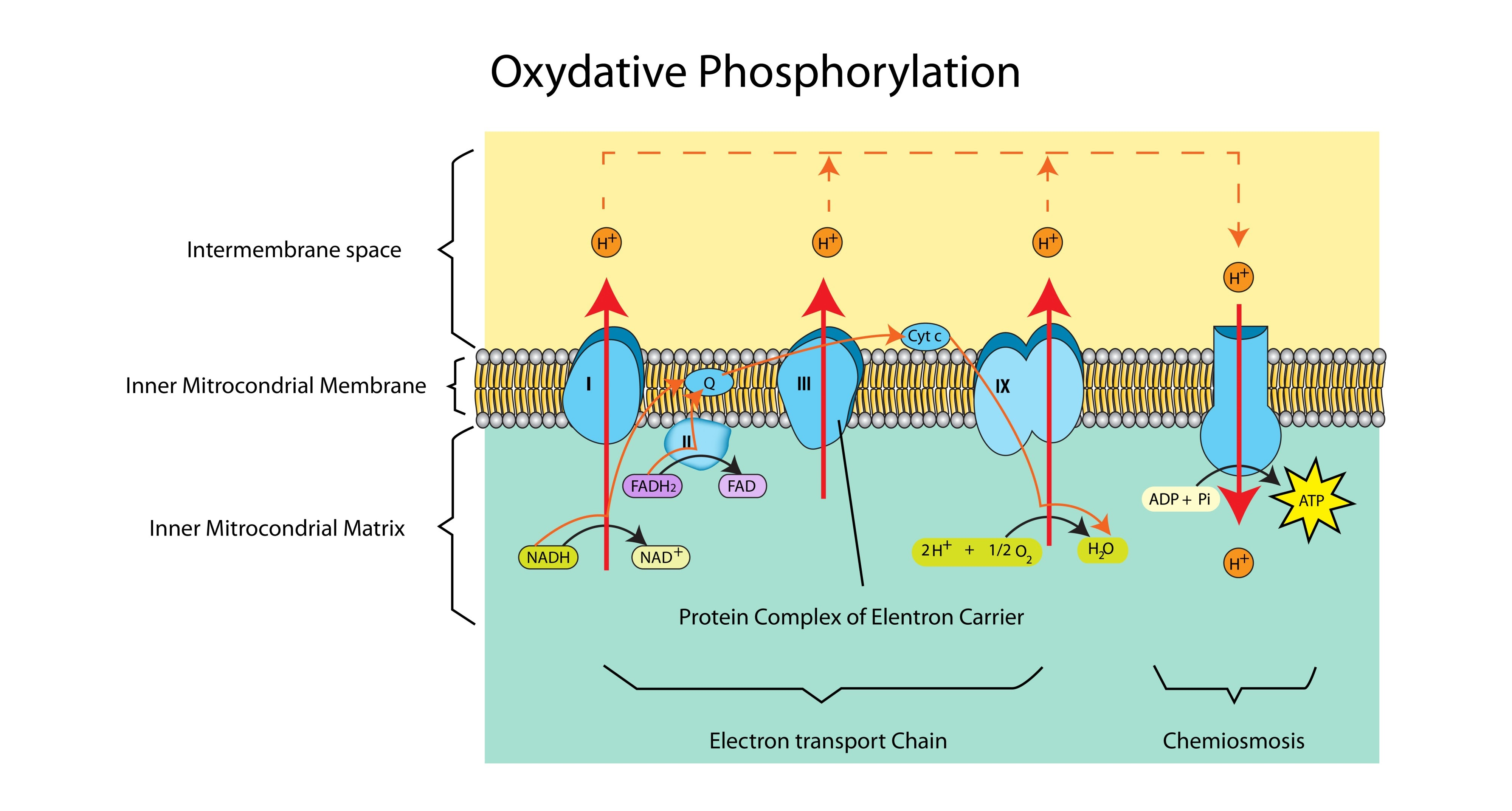
Where exactly does oxidative phosphorylation take place?
In the inner mitochondrial membrane (the cristae).
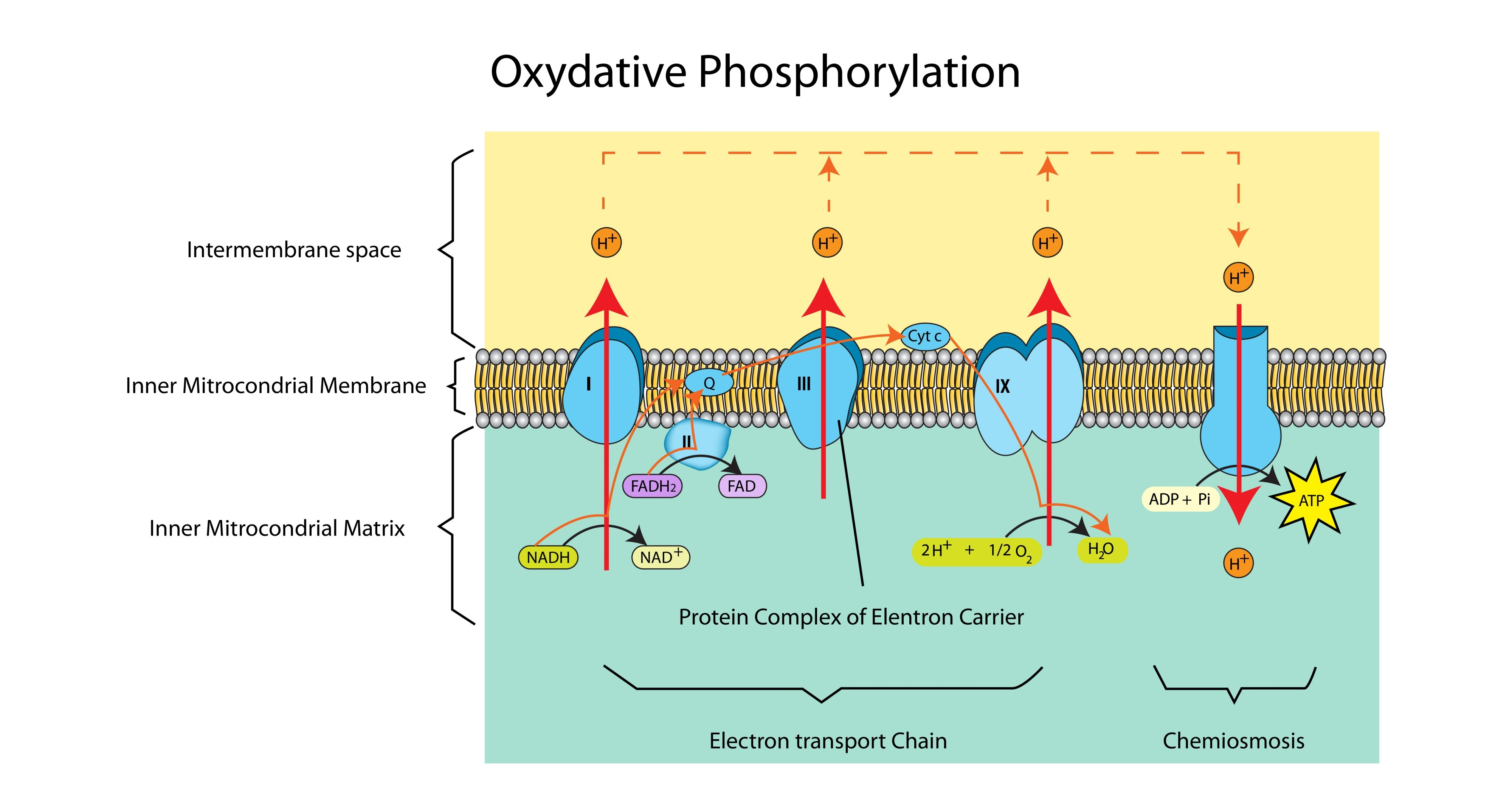
What is the source of electrons and protons for the ETC?
Reduced NAD and Reduced FAD (produced in Glycolysis, the Link Reaction, and the Krebs Cycle).
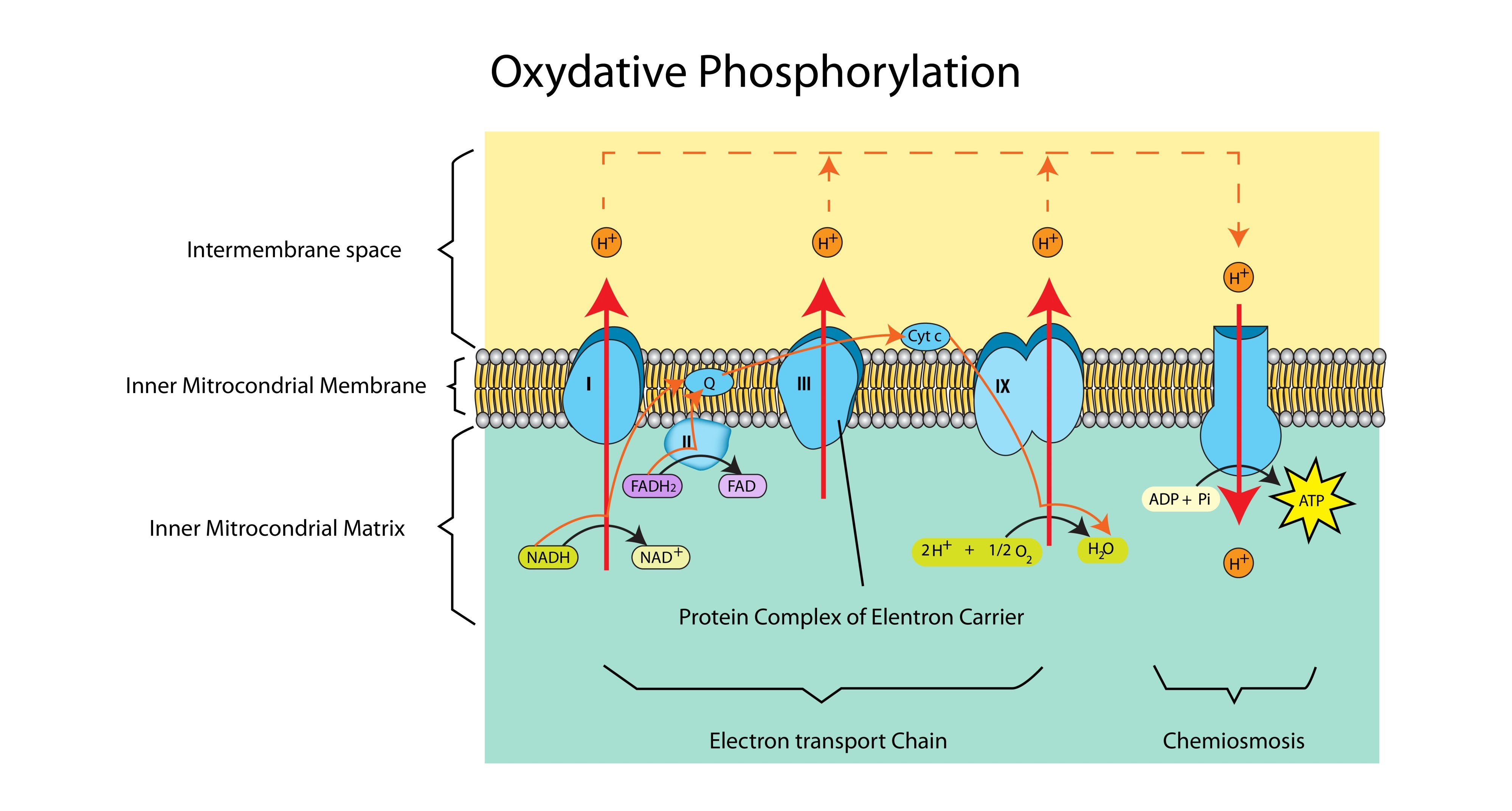
What happens to the energy released as electrons move down the ETC?
It is used to actively pump protons ($H^+$) from the matrix into the intermembrane space.
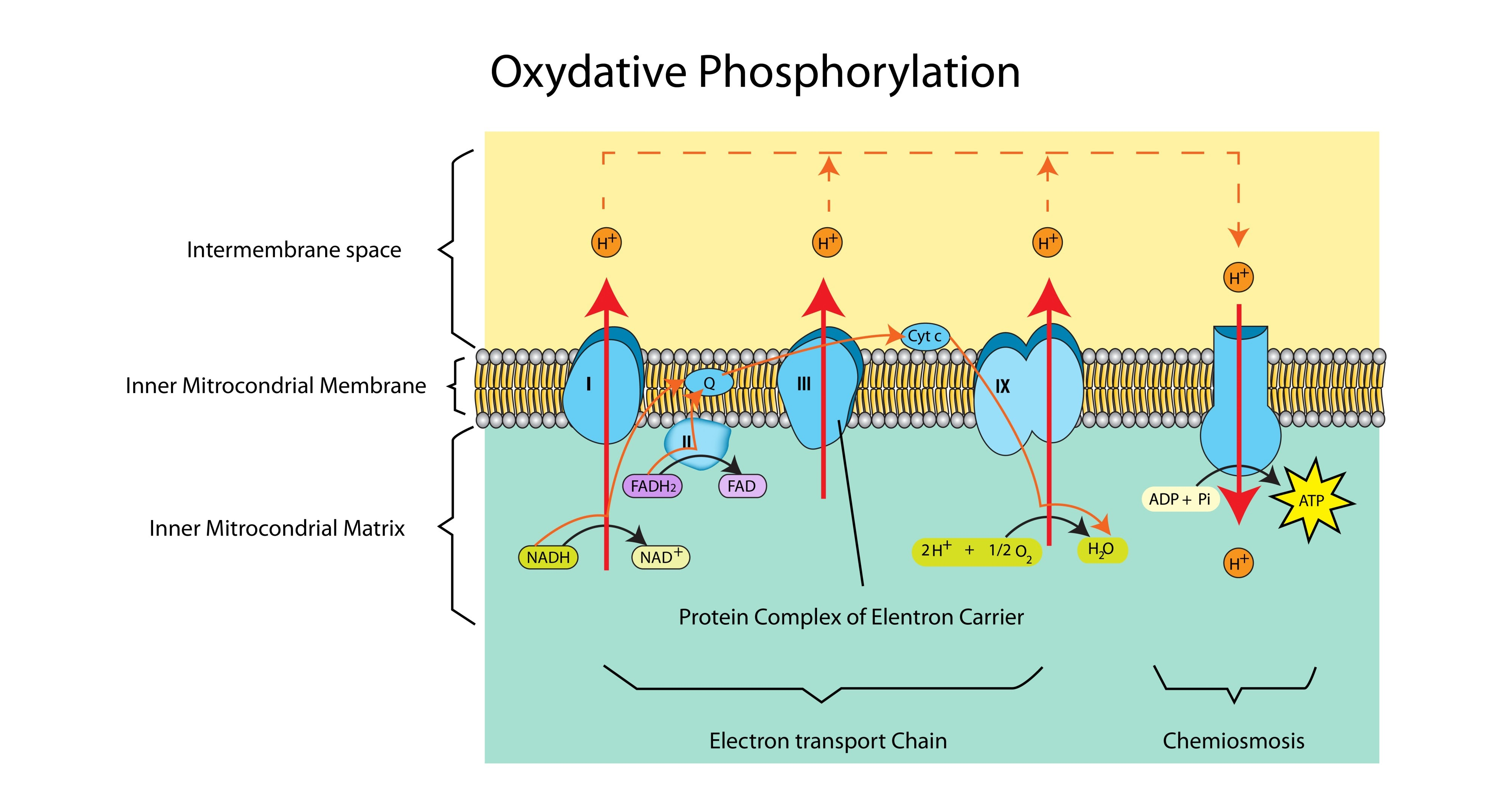
What is the specific role of Oxygen in oxidative phosphorylation?
It acts as the final electron acceptor. It combines with electrons and protons to form water.
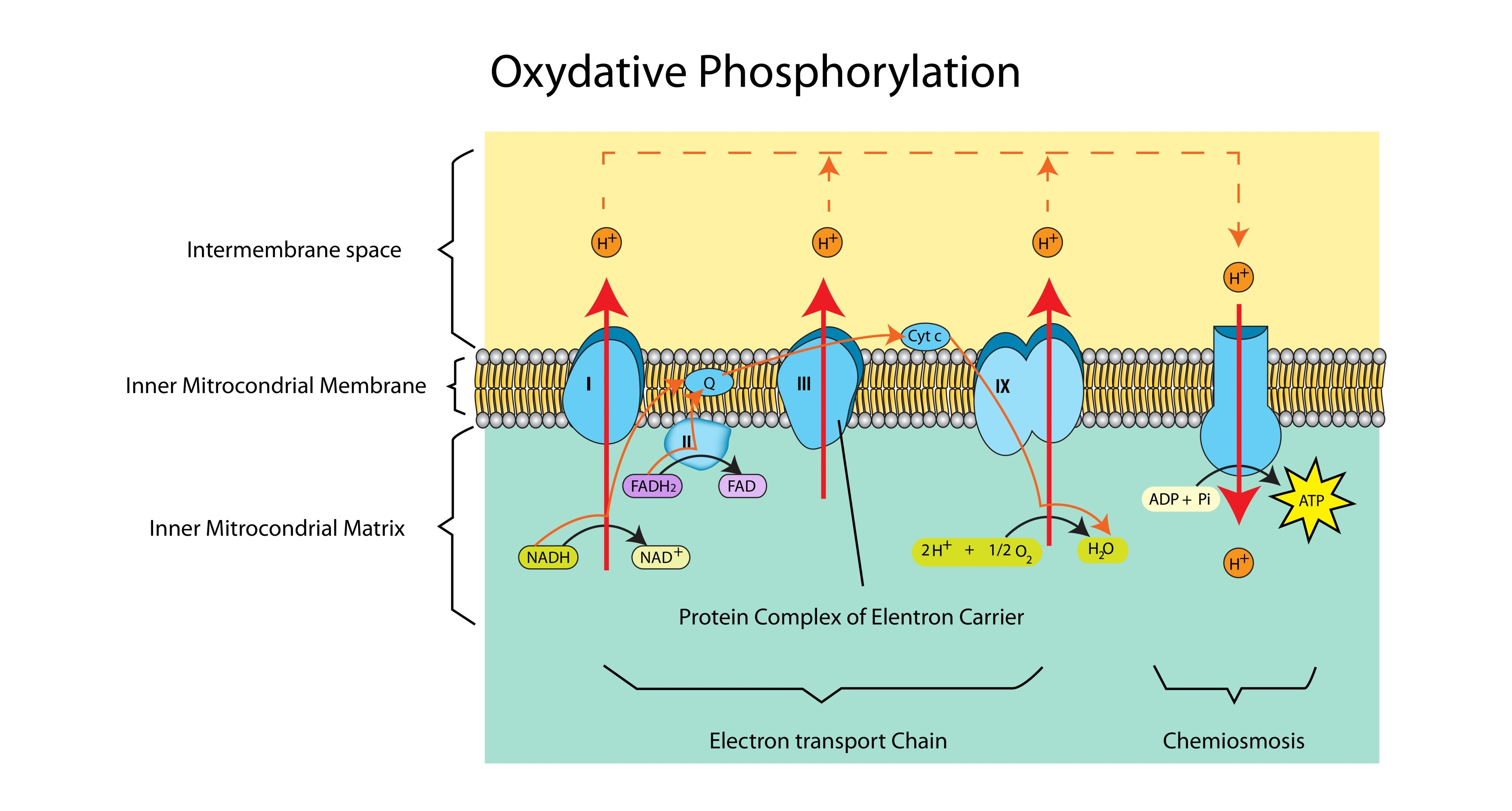
What would happen to the ETC if oxygen were not present?
Electrons would have nowhere to go and the chain would "back up." Protons would not be pumped, the proton gradient would collapse, and ATP synthesis would stop.
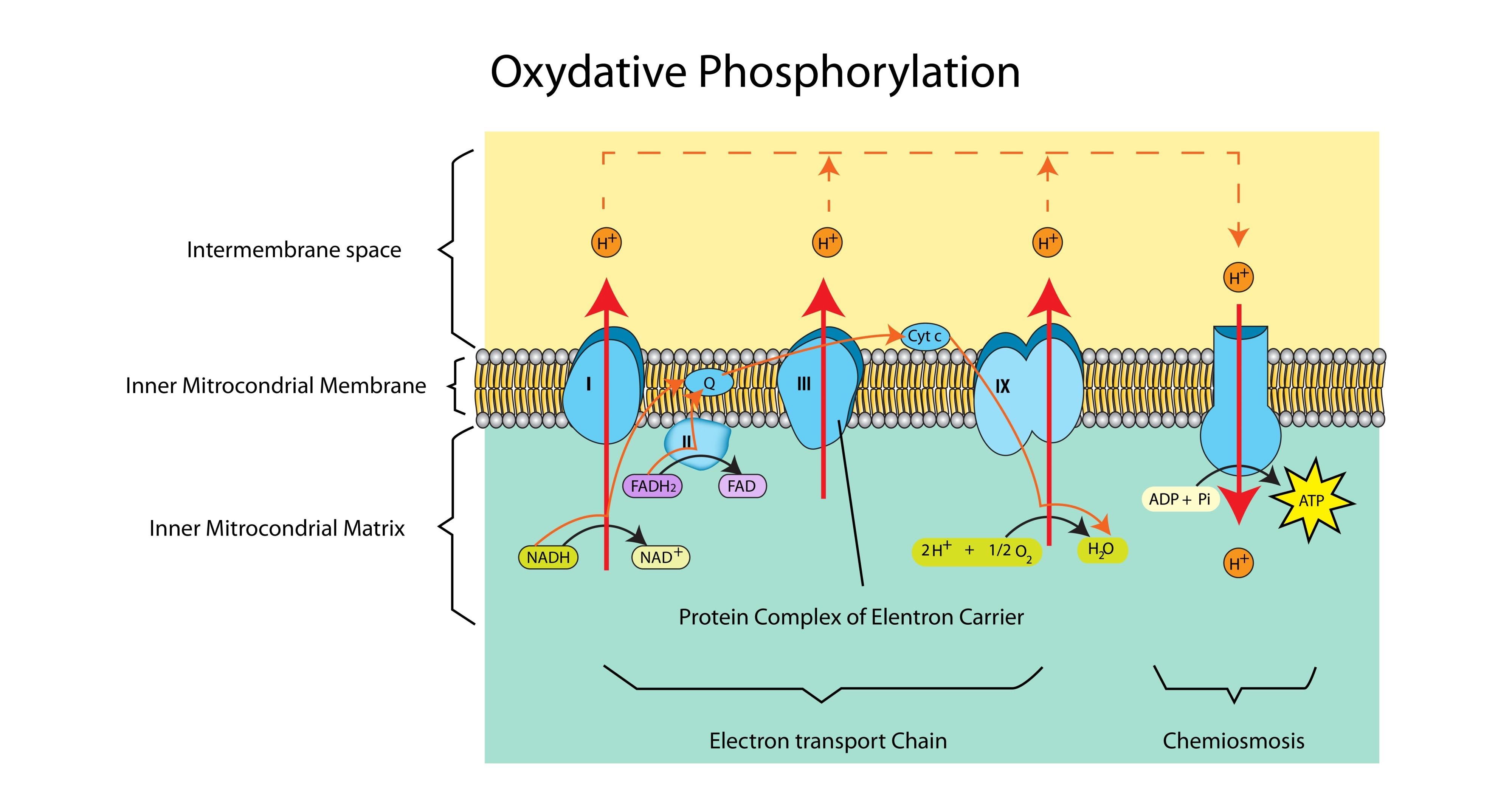
What is Chemiosmosis?
The synthesis of ATP driven by the flow of protons (H+) down an electrochemical gradient through the enzyme ATP synthase.
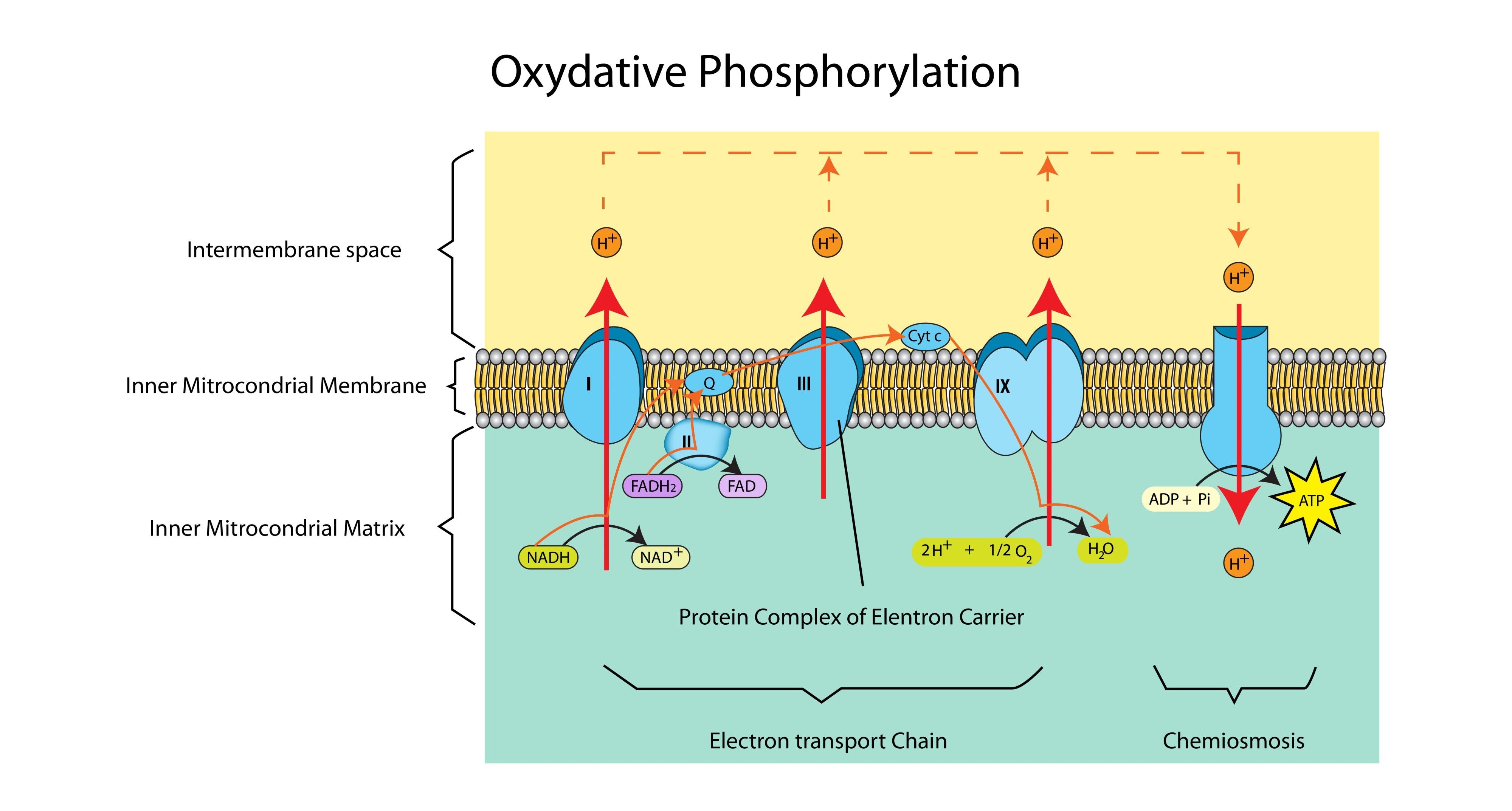
Why is the intermembrane space ideal for accumulating protons?
It is a very small space, so a high concentration of protons (low pH) can be built up quickly to establish a steep concentration gradient.
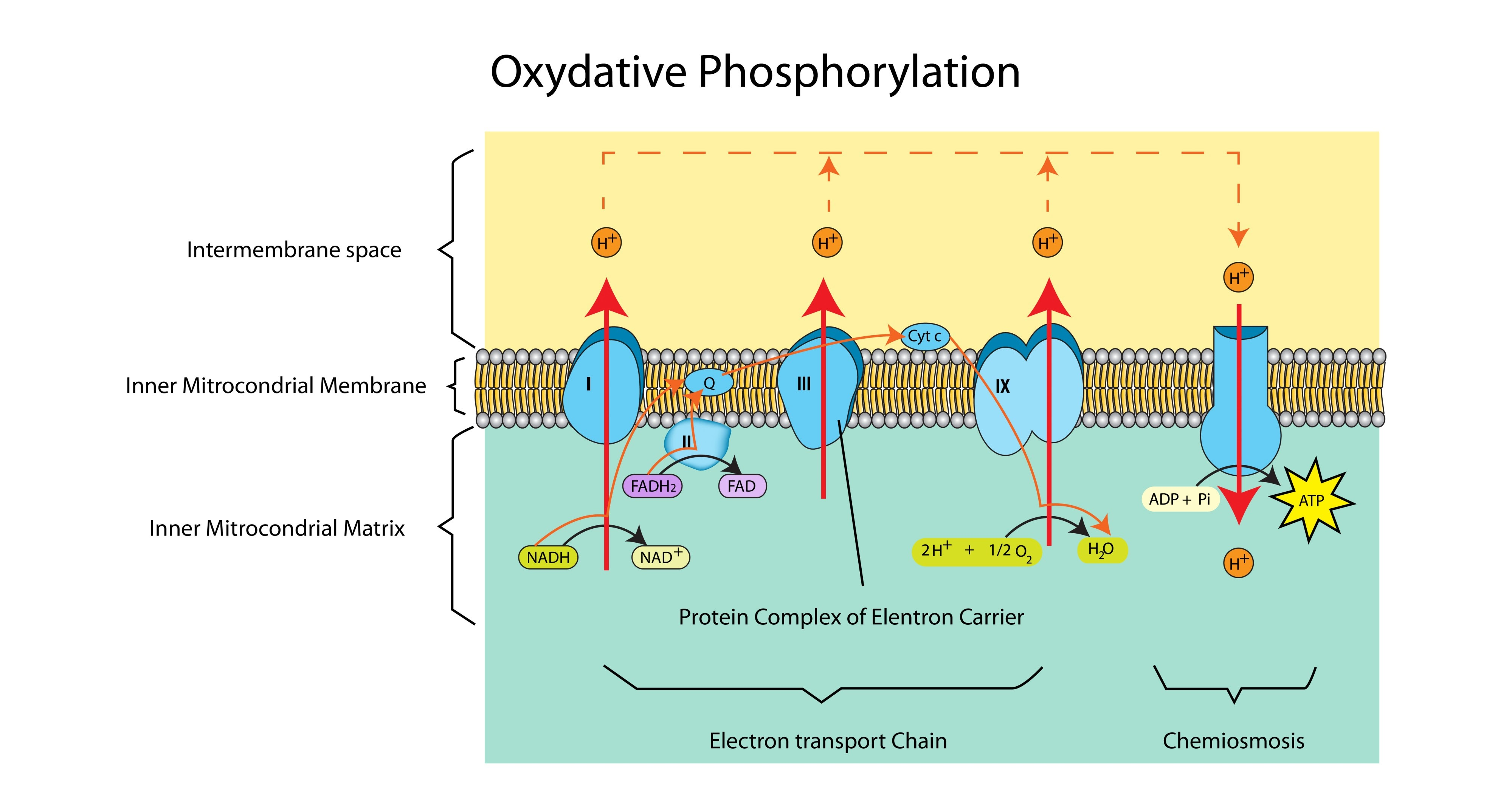
Which enzyme catalyses the formation of ATP?
ATP Synthase.
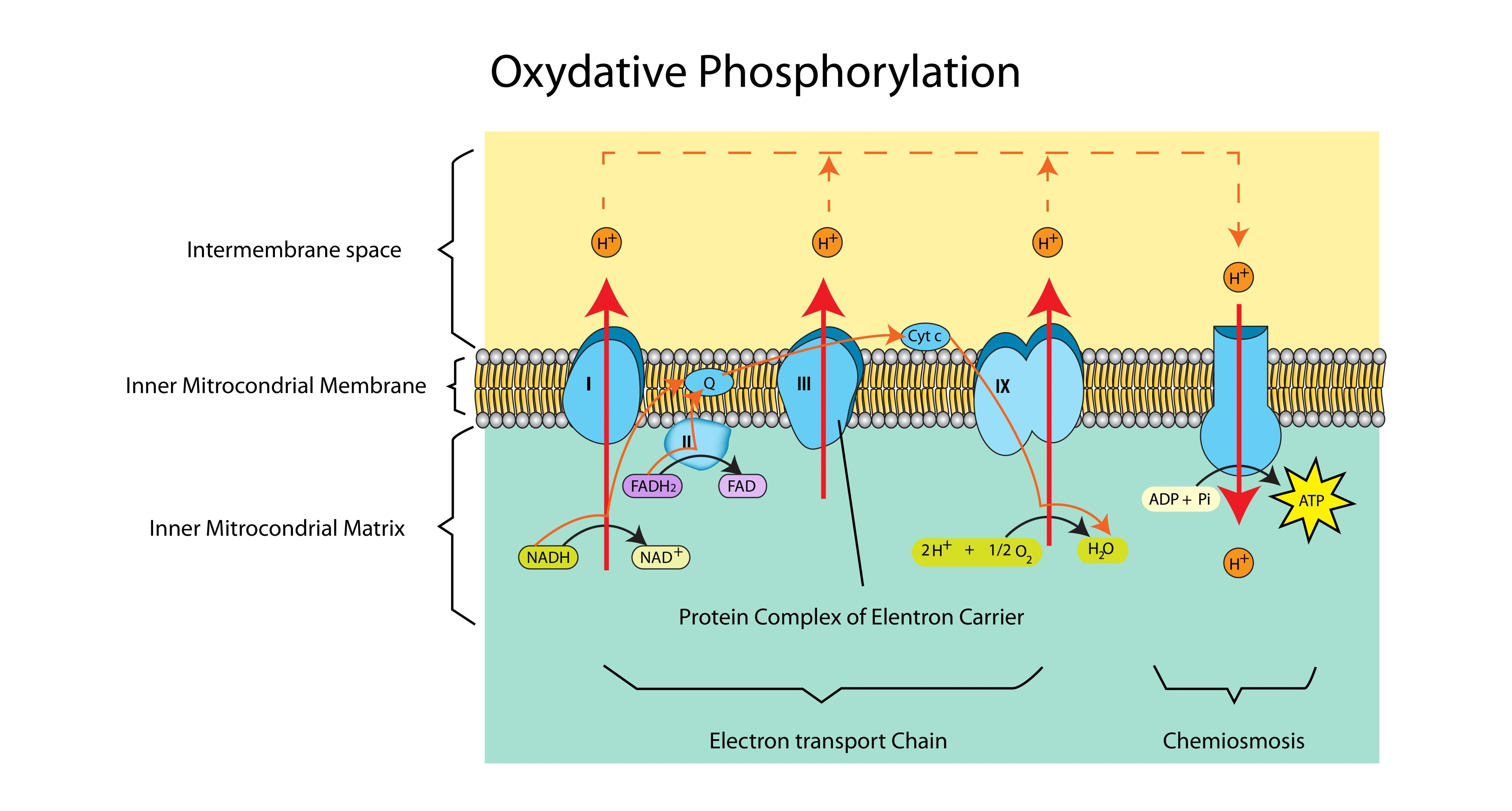
How many ATP molecules are theoretically made from one NADH vs one FADH?
NADH: approx 2.5 - 3 ATP (enters start of chain).
FADH: approx 1.5 - 2 ATP (enters later in the chain).
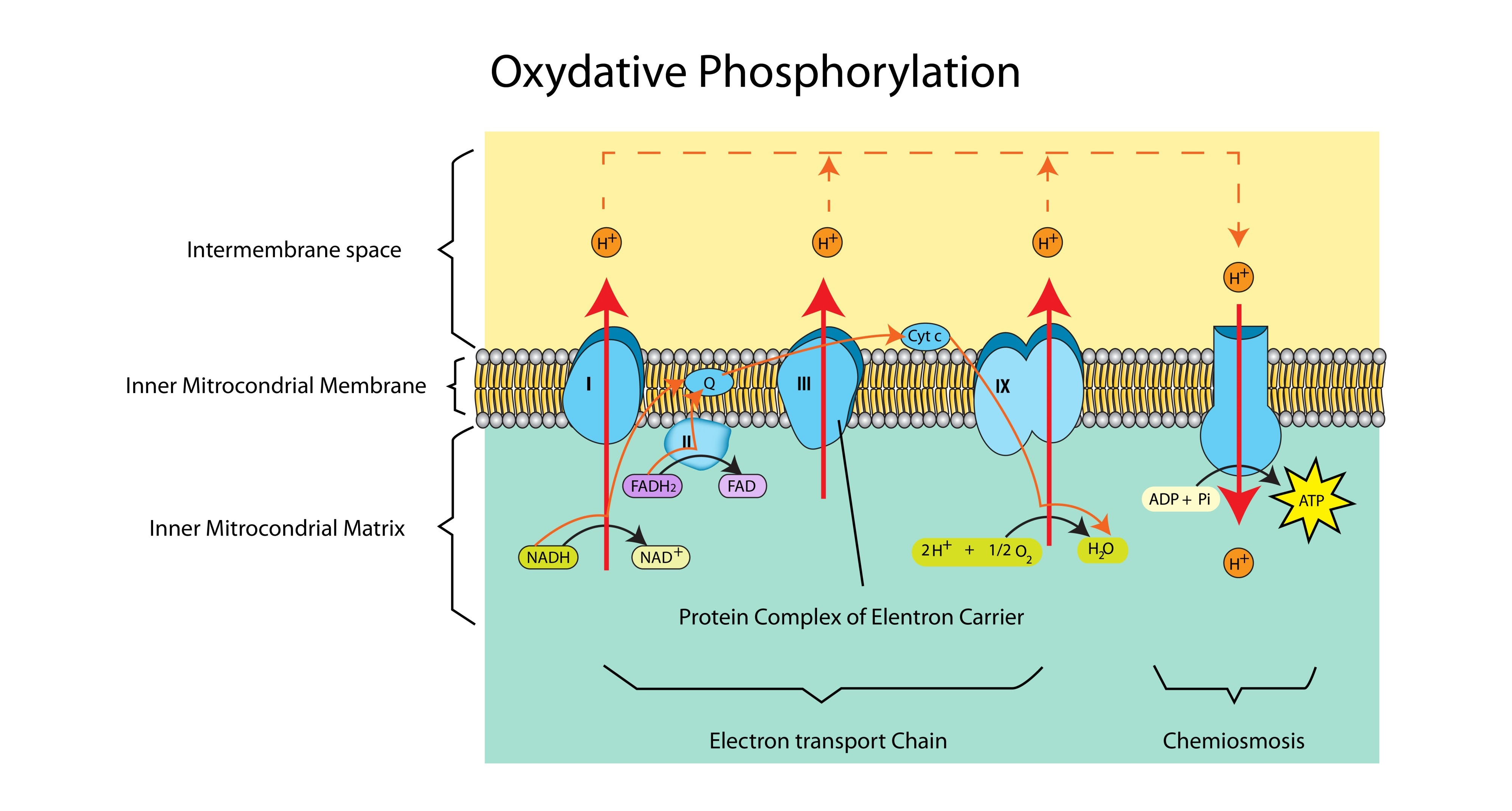
What is the total theoretical yield of ATP from one molecule of glucose during aerobic respiration?
32 ATP (though practically it is often closer to 30 due to leakage).
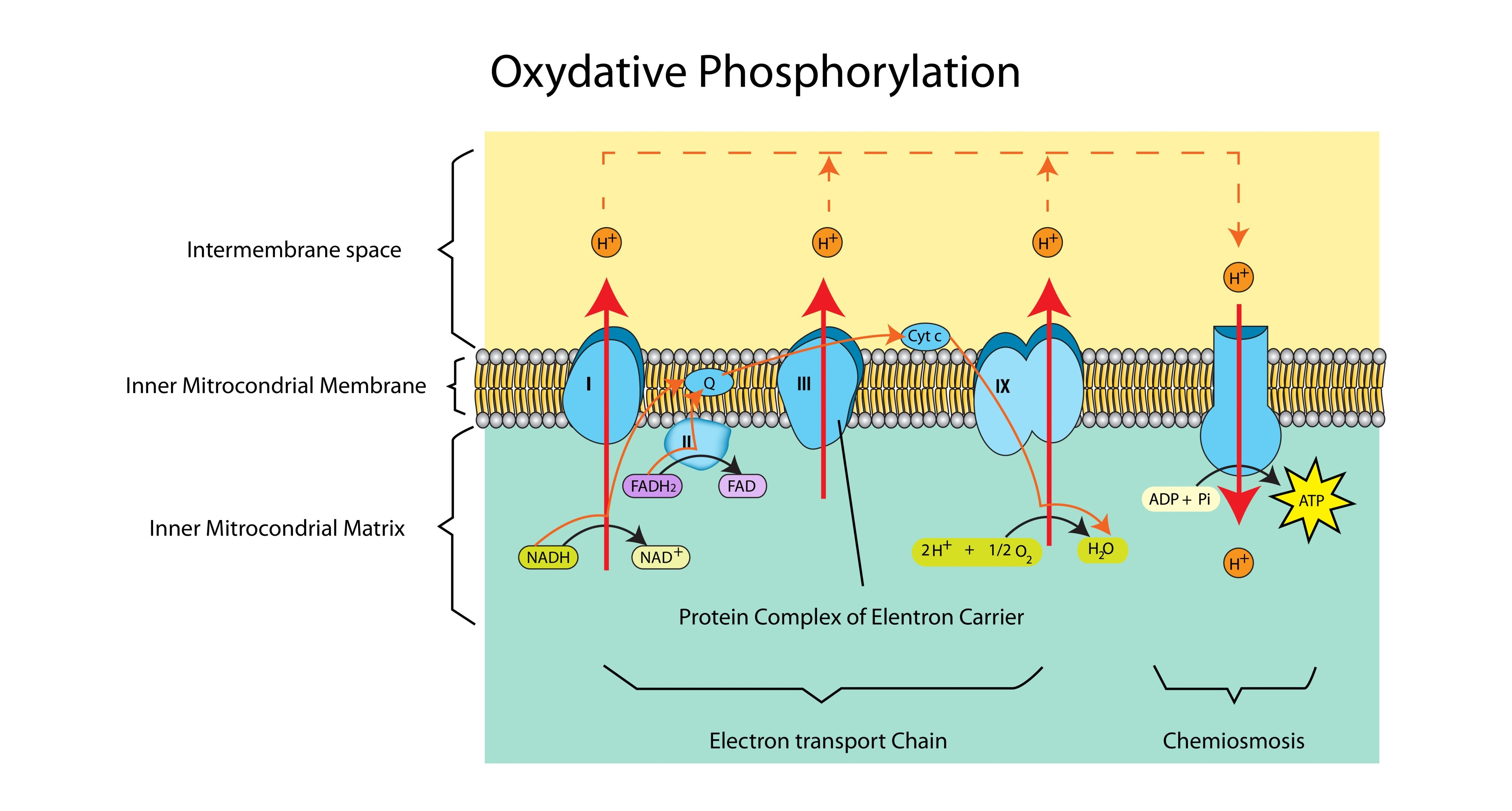
Why does FAD produce less ATP than NAD?
Reduced FAD donates its electrons to a carrier further down the chain than NAD. This means fewer protons are pumped across the membrane, so less ATP is generated.
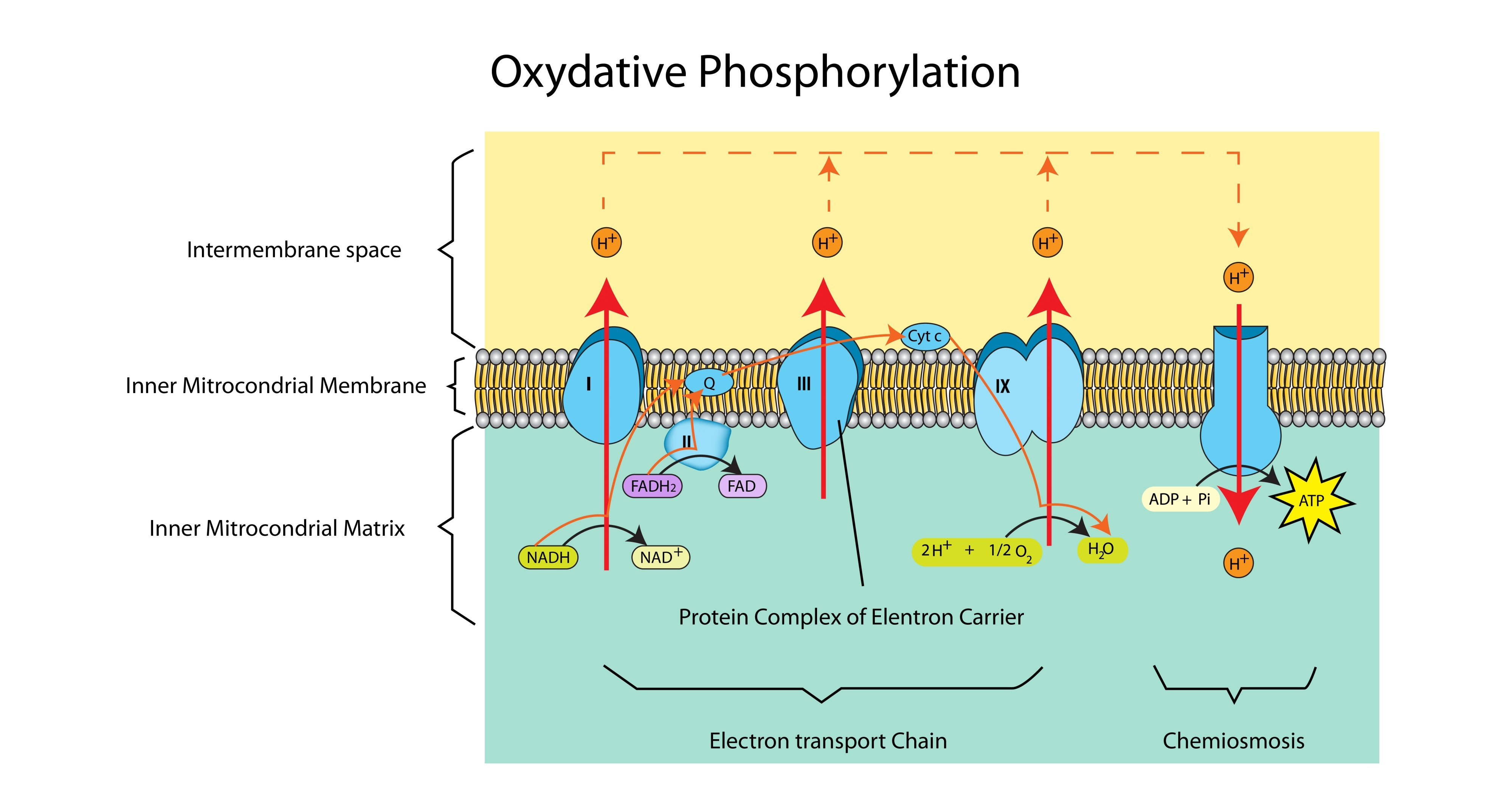
What is the final product formed when oxygen accepts electrons?
Water.
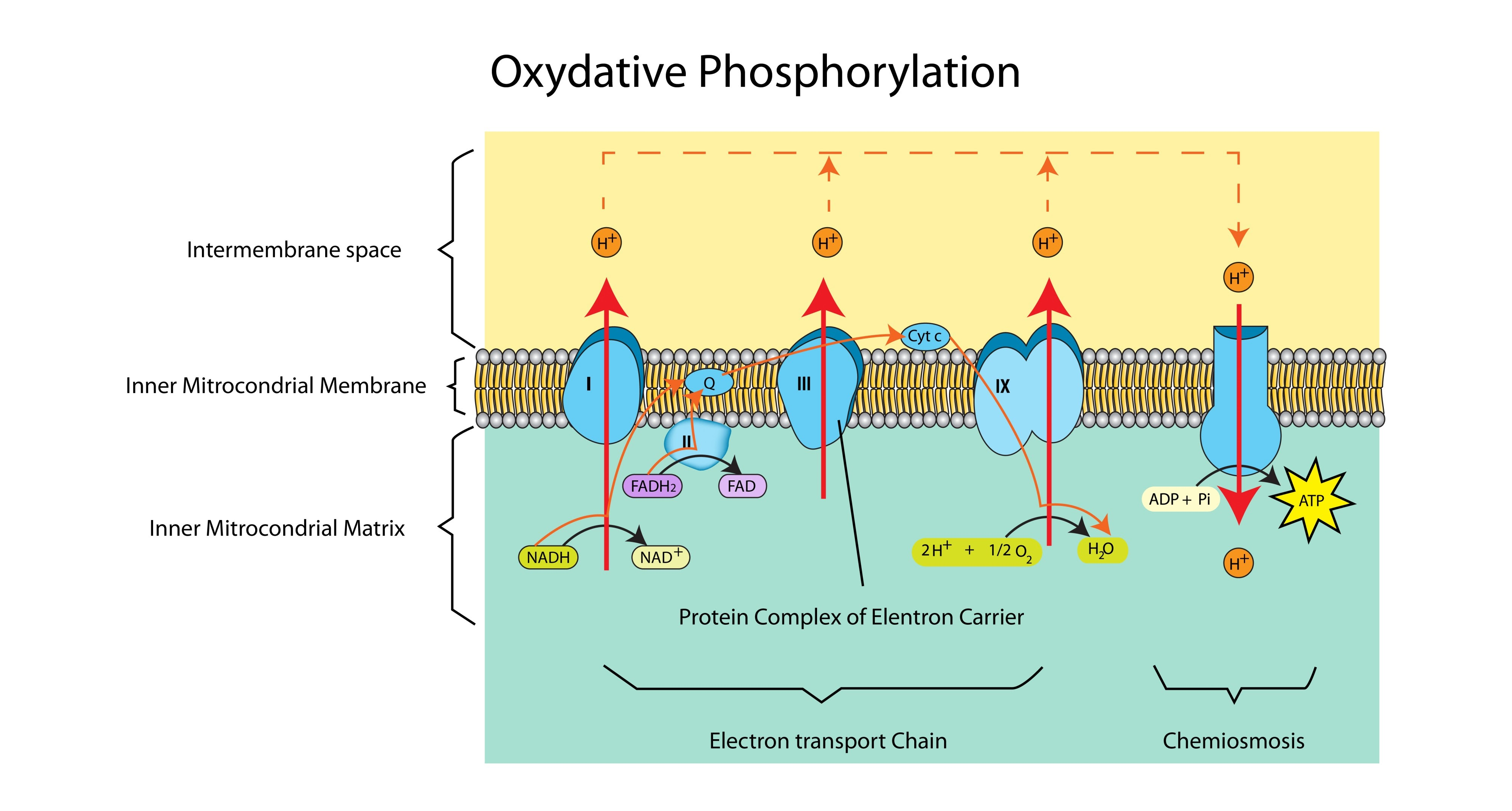
Describe the process of chemiosmosis in the mitochondria. (5 marks)
Protons (H+) accumulate in the intermembrane space due to active transport by the ETC (1).
This creates a proton/electrochemical gradient (higher concentration in intermembrane space than matrix) (1).
Protons diffuse back into the matrix through ATP Synthase (1).
This flow causes a conformational change in ATP Synthase (1).
Which catalyses the reaction: ADP + Pi → ATP (1).
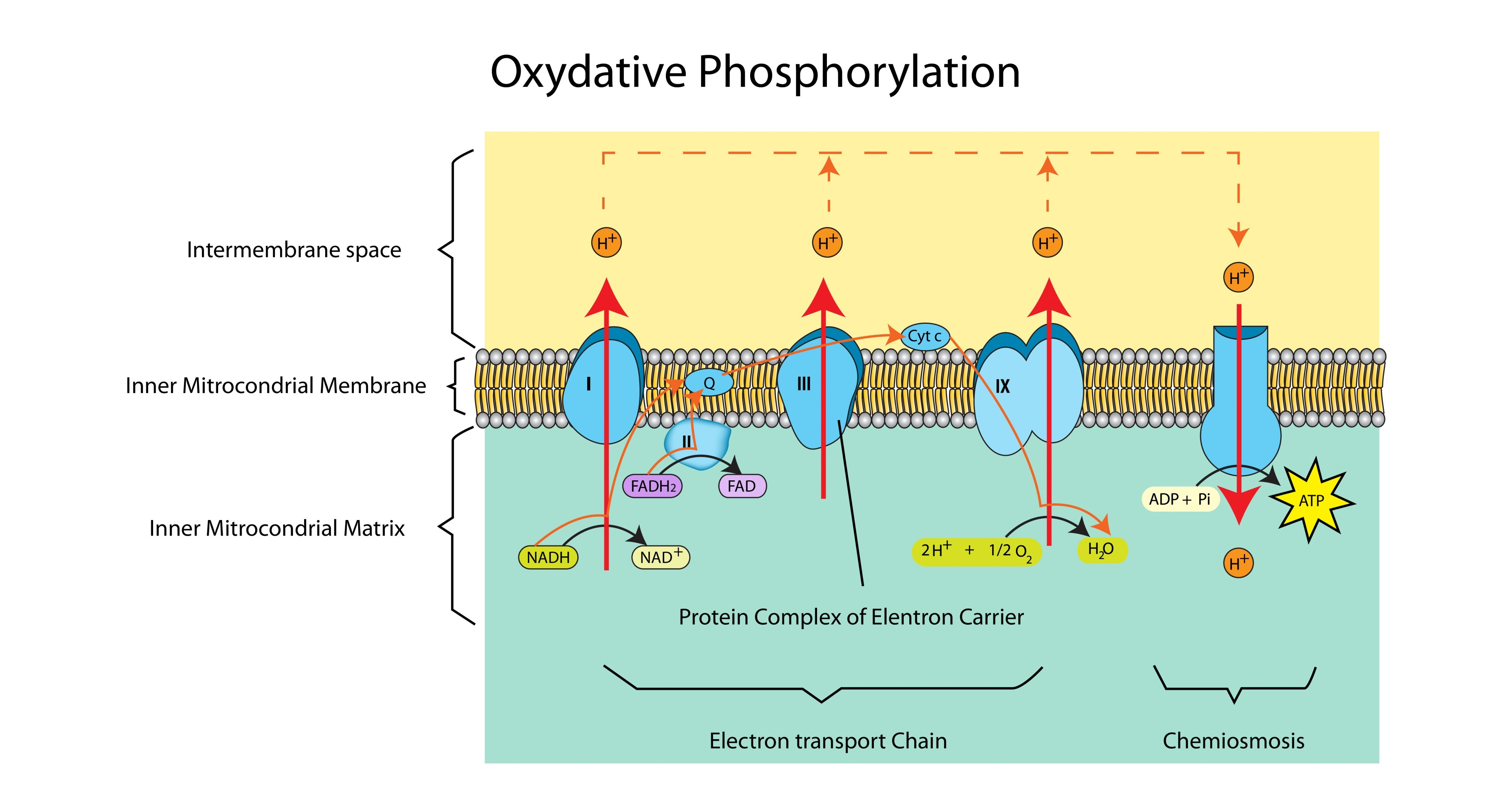
Explain the role of the electron transport chain in the generation of ATP. (3 marks)
It accepts electrons from Reduced NAD/FAD (1).
As electrons pass along the chain, they release energy (1).
This energy is used to pump protons across the membrane to create the gradient required for ATP synthesis (1).
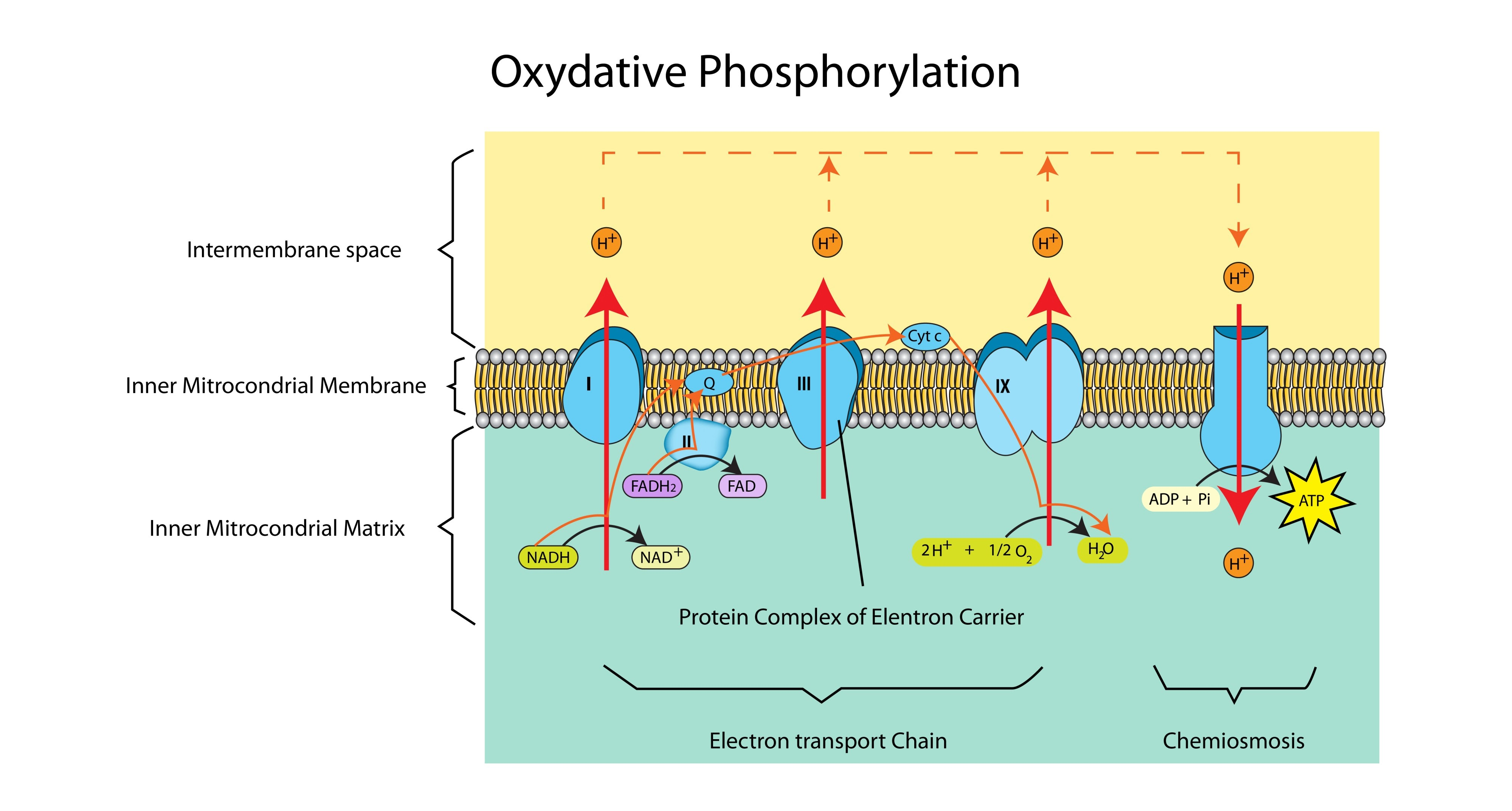
Cyanide is a poison that inhibits the final electron carrier in the ETC. Explain how cyanide stops ATP production. (2 marks)
Cyanide prevents electrons from being passed to oxygen (1).
The movement of electrons stops, so no protons are pumped (1).
The proton gradient dissipates, so ATP synthase stops working.
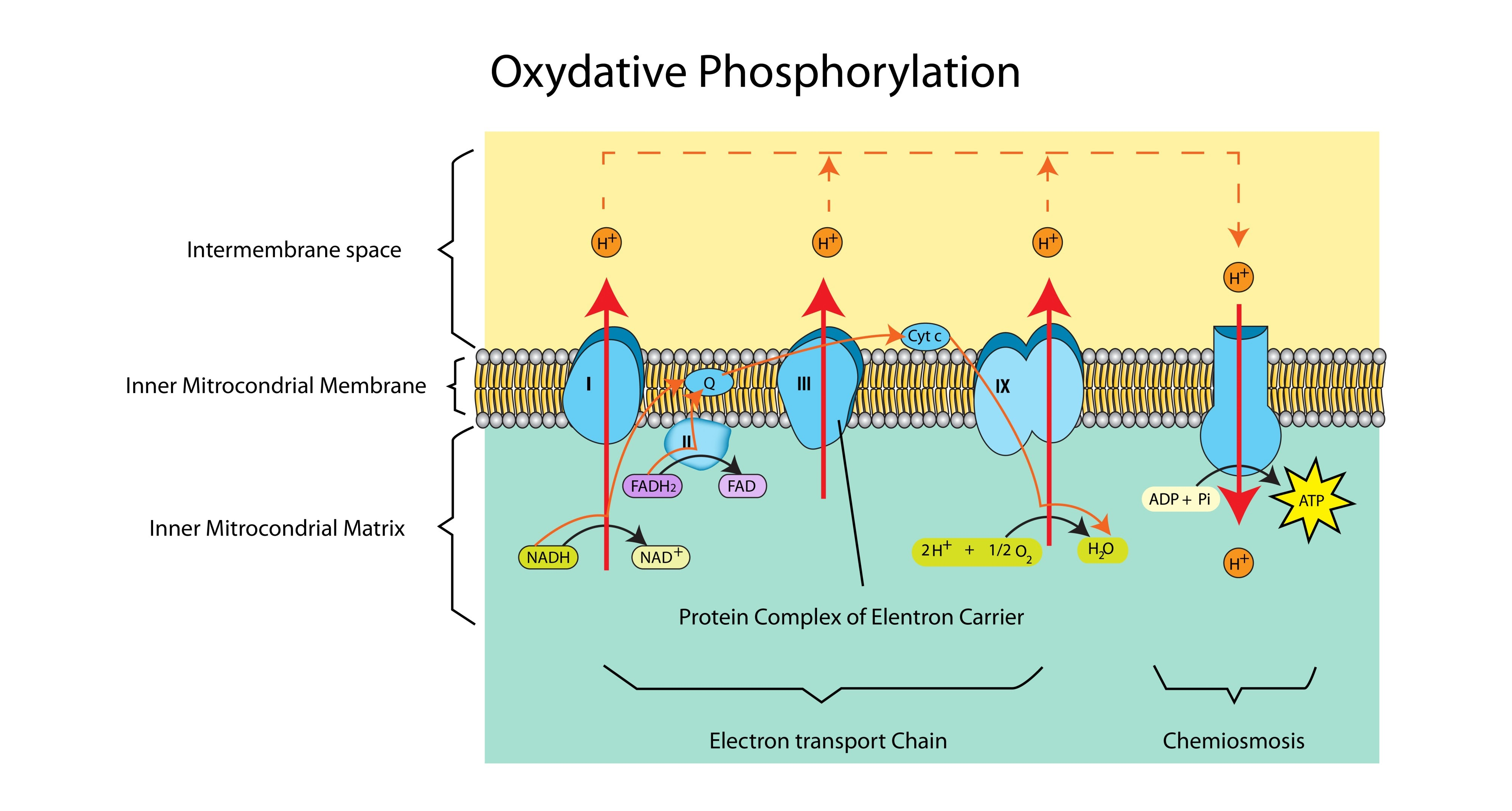
Why is the inner mitochondrial membrane folded into cristae? (2 marks)
To provide a large surface area (1).
To hold more electron transport chains and ATP synthase enzymes, maximizing the rate of ATP production (1).
Oxygen is the "final electron acceptor." Explain what this means and why it is essential. (3 marks)
Oxygen sits at the end of the ETC and removes electrons (and protons) to form water (1).
Without this removal, electrons would accumulate, and the carriers would remain reduced (1).
This would stop the flow of electrons, halting the pumping of protons and stopping aerobic respiration (1).