exam 2 common names
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
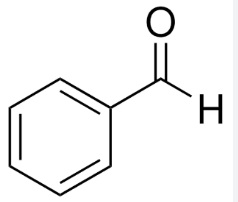
benzaldehyde
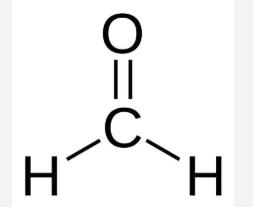
formaldehyde
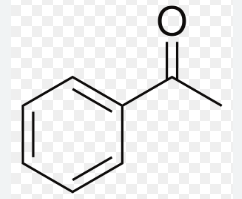
acetophenone
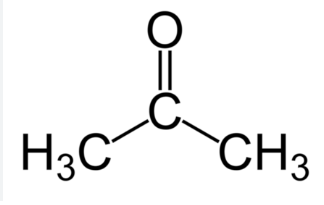
acetone
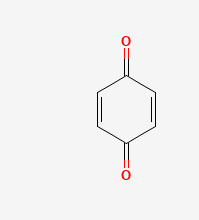
quinone
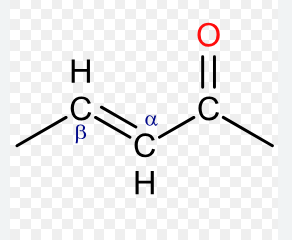
a,b-unsaturated ketone
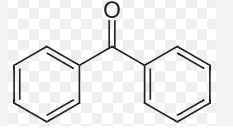
benzophenone
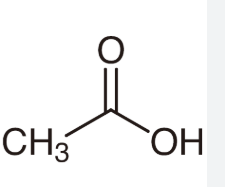
acetic acid
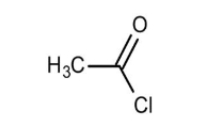
acetyl chloride
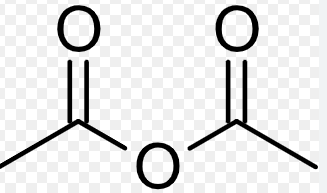
acetic anhydride
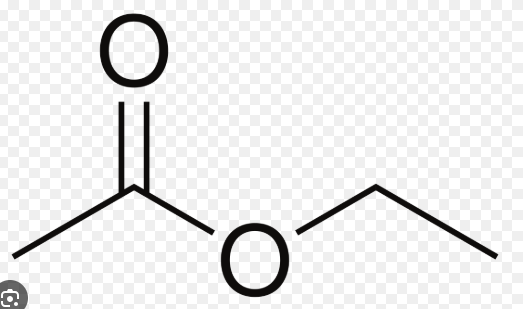
ethyl acetate
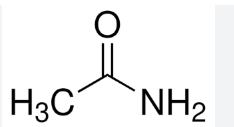
acetamide
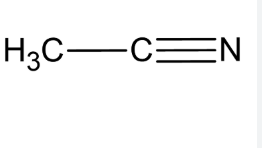
acetonitrile
naming ester
name as acid= drop -ic acid, add -ate
R=O-OR’—> R’ goes in front as a separate word
naming amides
name as acid
drop -oic acid, add -amide
and R groups on N, then add as N-alkyl to prefix
name nitriles
name as acid
drop -ic acid, add nitrile
order of acidity
acid chloride, anhydride, ester (RCOOR’), amide (RCONR2, R-NH or NH2), salt (RCOO-)
This order is based on the stability of the leaving group and the ease of nucleophilic attack.
Acid Chlorides are most reactive due to the highly electronegative chlorine atom, which makes it a good leaving group and facilitates nucleophilic attack.
Acid anhyrdide are moderately reactive, with the leaving group being an acyl group (RCO-).
Esters are less reactive than anhydrides, as the leaving group is an alkoxide (RO-) which is a weaker base than an acyl group.
Amides are the least reactive, with the leaving group being an amine (NH2) which is a poor leaving group due to its basic nature.
functional group priority
c. acid
c. anhydride
ester
acid chloride
amide
nitrile
aldehyde
ketone
alochol
thiol
amine