AP Chem Unit 6
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
48 Terms

Which of the following is the best claim and justification based on the students observation?
D
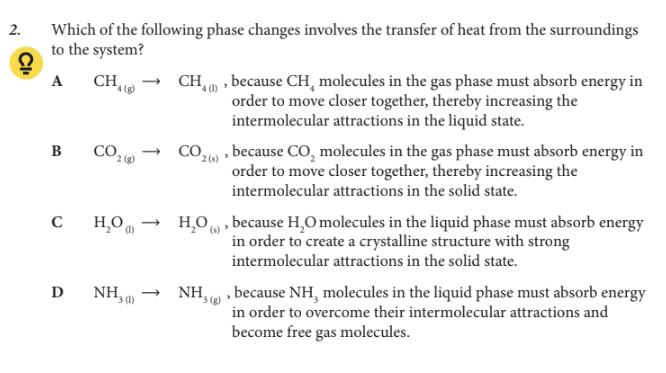
Which of the following phase changes involves the transfer of heat from the surroundings to the system?
D

Which of the following is a correct scientific justification for spraying water on the blossoms to protect them from temperatures below -2 °C ?
D
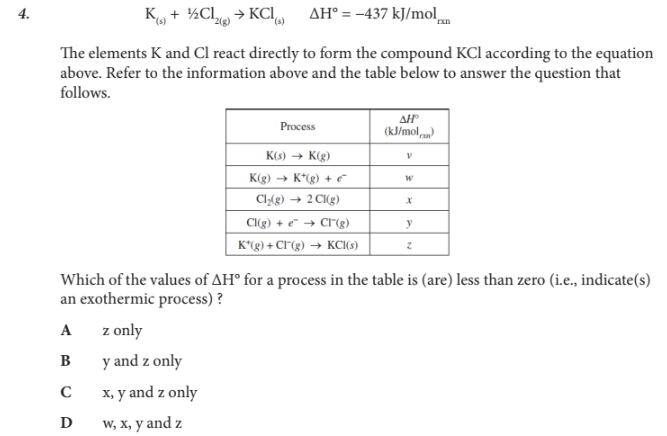
Which of the values of ΔH° for a process in the table is (are) less than zero (i.e., indicate(s) an exothermic process) ?
B
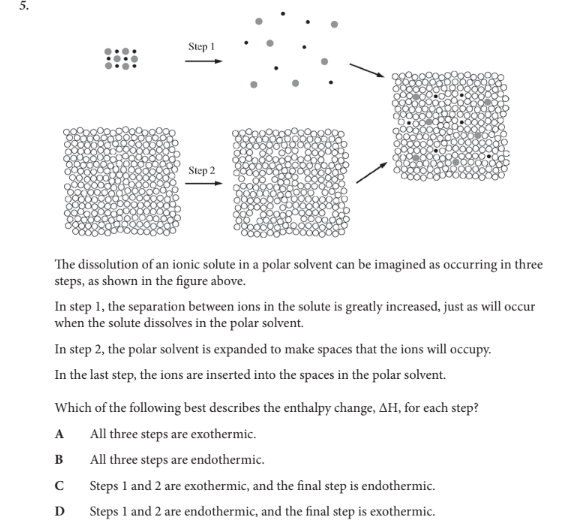
Which of the following best describes the enthalpy change, ΔH, for each step?
D

Which of the following best helps to explain why the value of ΔH° for the dissolving of CaF2 in water is positive?
C
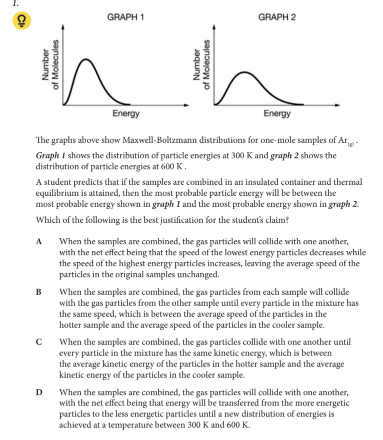
Which of the following is the best justification for the student’s claim?
A
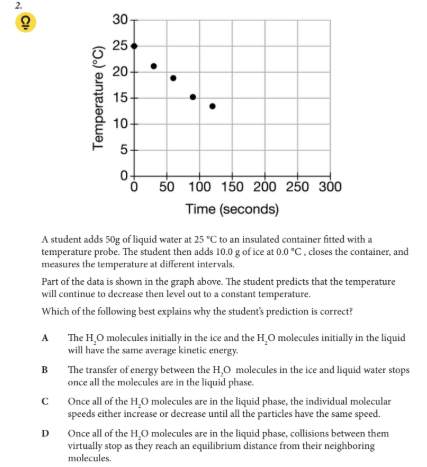
Which of the following best explains why the student’s prediction is correct?
A
Which of the following best explains why the student’s prediction is incorrect?
D

Which of the following can be concluded?
B
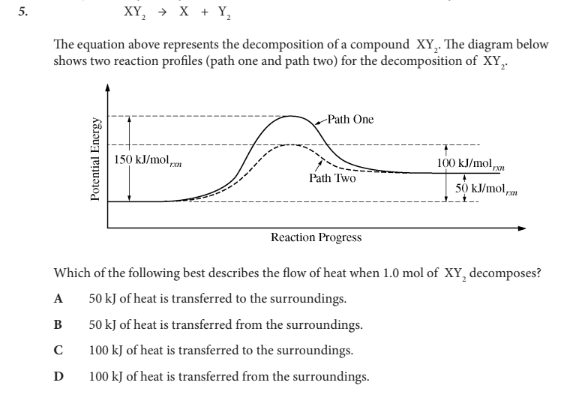
Which of the following best describes the flow of heat when 1.0 mol of XY2 decomposes?
B
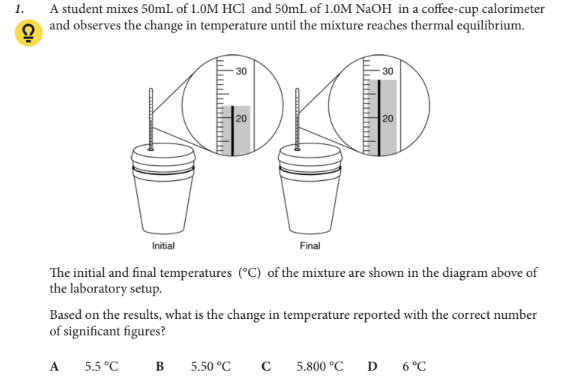
Based on the results, what is the change in temperature reported with the correct number of significant figures?
A
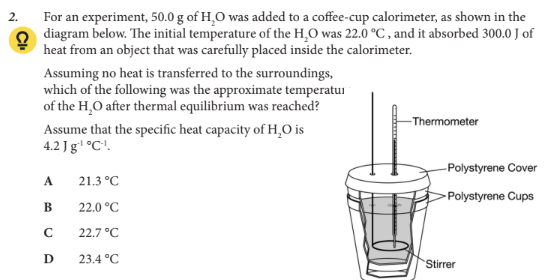
Assume that the specific heat capacity of H2O is 4.2 J g-1 °C-1.
D
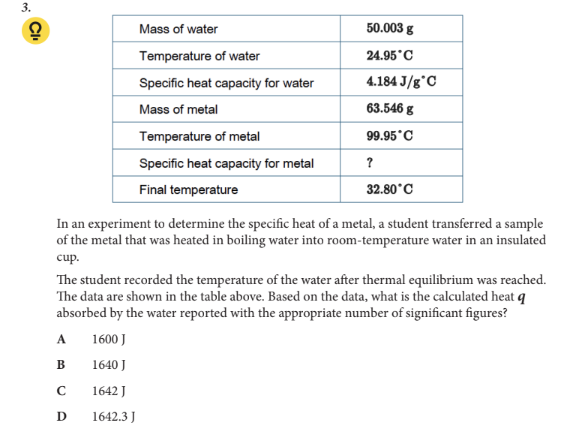
Based on the data, what is the calculated heat q absorbed by the water reported with the appropriate number of significant figures?
B

The final temperature of the mixture is closest to
D
Based on the data, which of the following can be concluded about the energy content for 1.0 g of each of the two substances? (The specific heat of water is 4.2 J/(g.°C).)
C
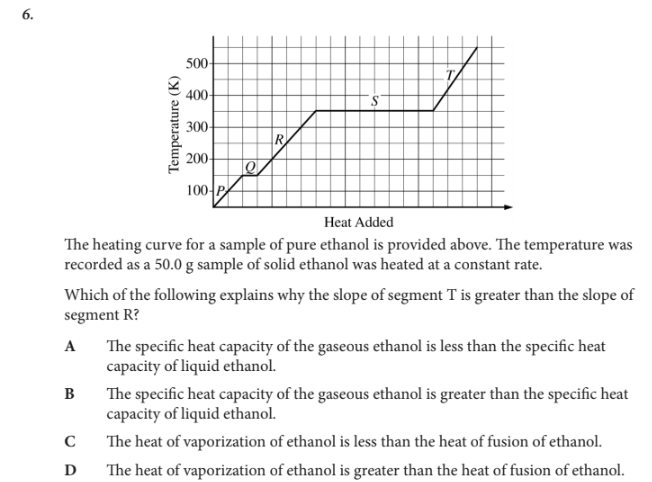
Which of the following explains why the slope of segment T is greater than the slope of segment R?
A
A hot iron ball is dropped into a 200. g sample of water initially at 50 °C. If 8.4 kJ of heat is transferred from the ball to the water, what is the final temperature of the water? (The specific heat of water is 4.2 J/(g·°C).)
C
If T1> T2, which of the following is true of the system when it has attained thermal equilibrium?
A
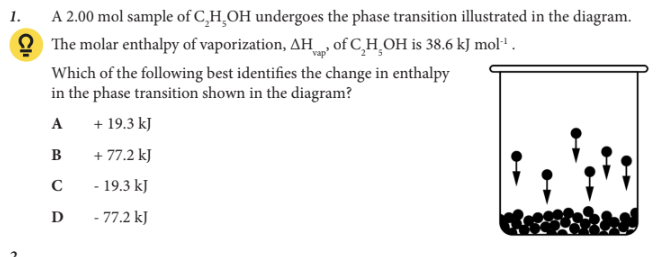
Which of the following best identifies the change in enthalpy in the phase transition shown in the diagram?
D
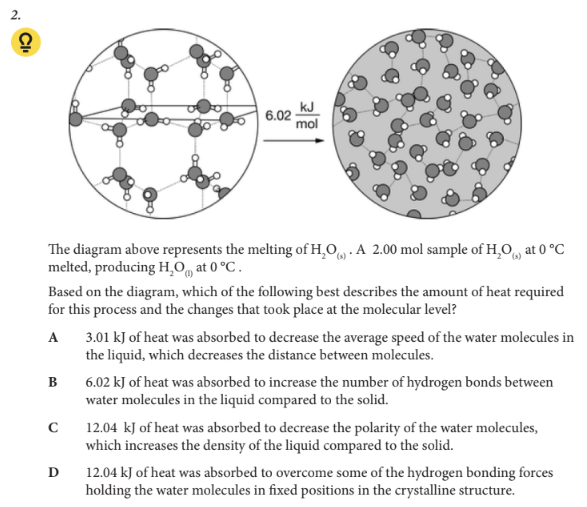
Based on the diagram, which of the following best describes the amount of heat required for this process and the changes that took place at the molecular level?
D
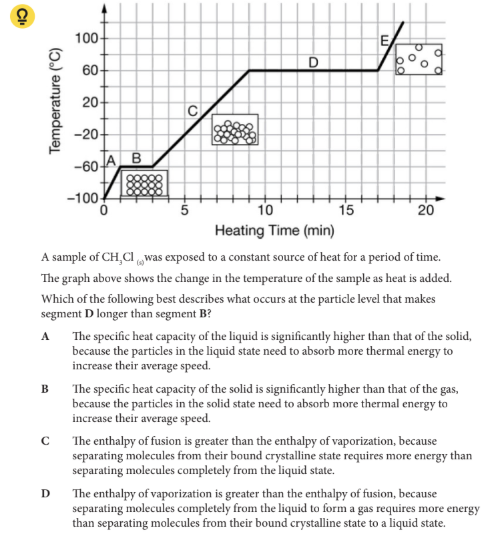
Which of the following best describes what occurs at the particle level that makes segment D longer than segment B?
D
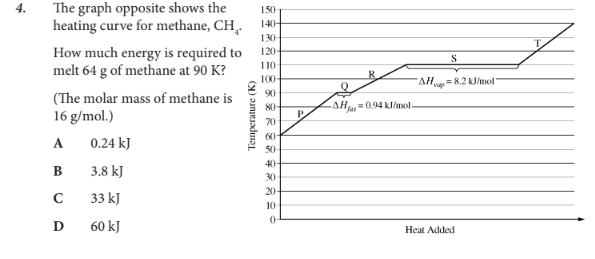
How much energy is required to melt 64 g of methane at 90 K?
B
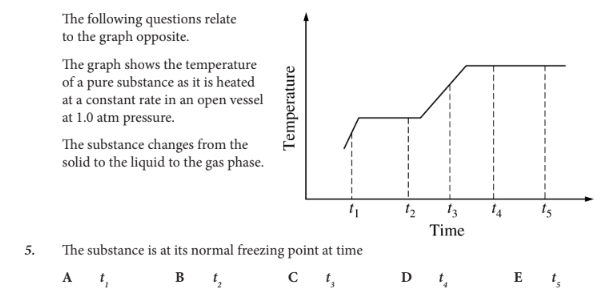
The substance is at its normal freezing point at time
B

Which of the following best describes what happens to the substance between t4 and t5?
A
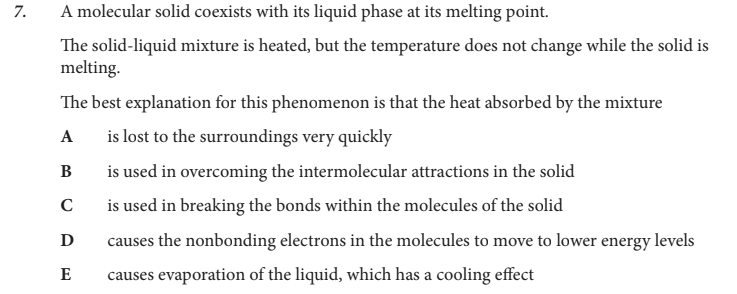
The best explanation for this phenomenon is that the heat absorbed by the mixture
B
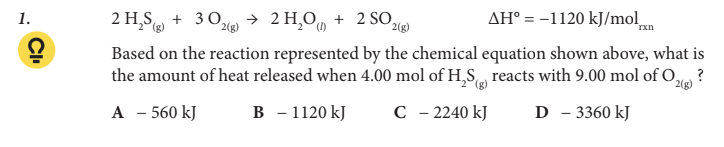
Based on the reaction represented by the chemical equation shown above, what is the amount of heat released when 4.00 mol of H2 S(g) reacts with 9.00 mol of O2(g) ?
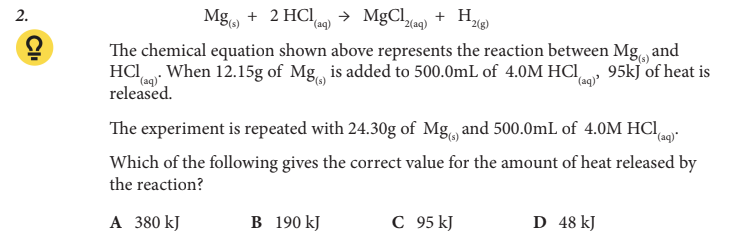
Which of the following gives the correct value for the amount of heat released by the reaction?
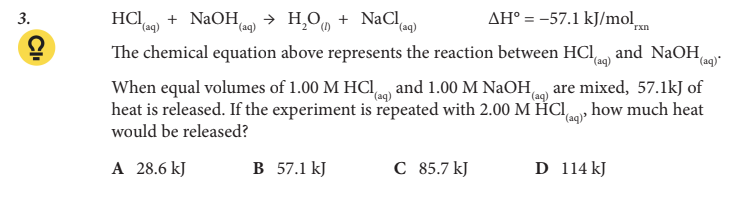
If the experiment is repeated with 2.00 M HCl(aq), how much heat would be released?
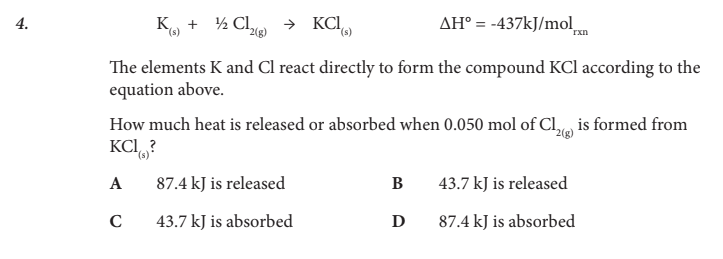
How much heat is released or absorbed when 0.050 mol of Cl2(g) is formed from KCl(s)?
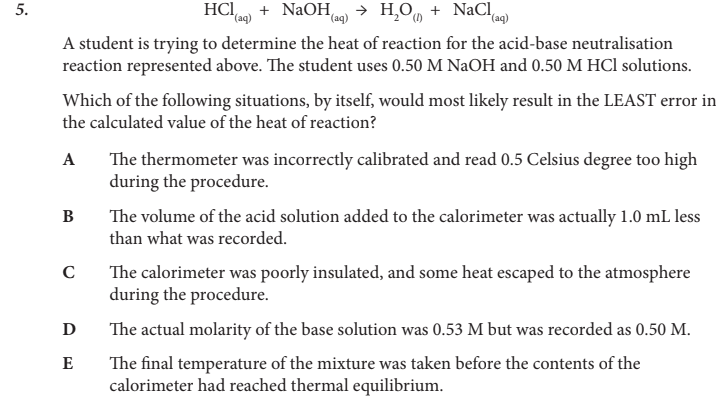
Which of the following situations, by itself, would most likely result in the LEAST error in the calculated value of the heat of reaction?
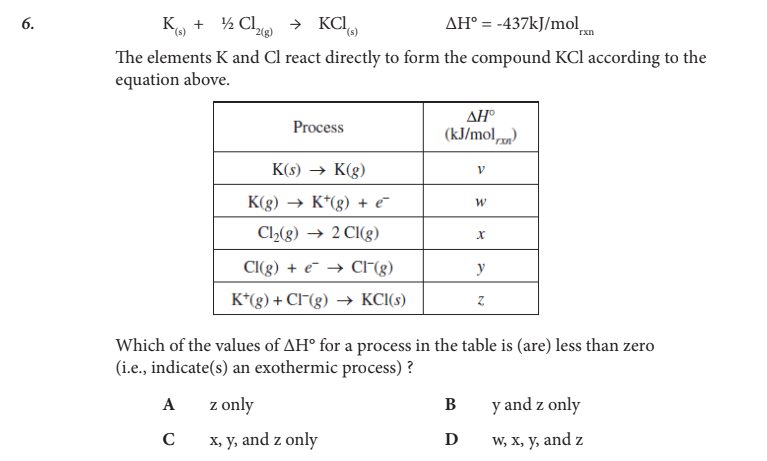
Which of the values of ΔH° for a process in the table is (are) less than zero (i.e., indicate(s) an exothermic process) ?
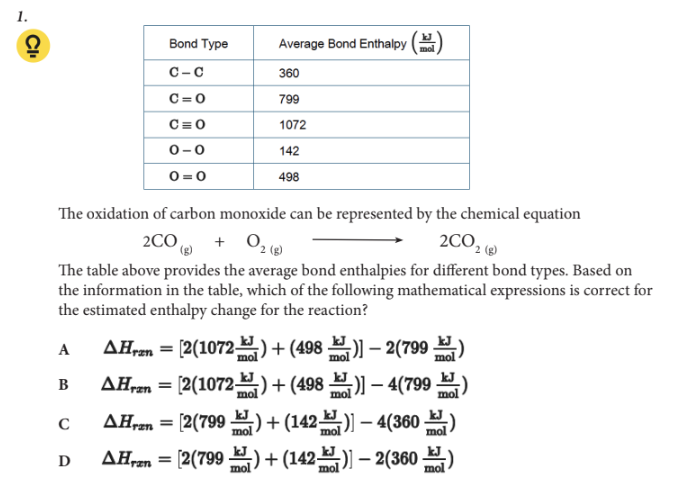
Based on the information in the table, which of the following mathematical expressions is correct for the estimated enthalpy change for the reaction?
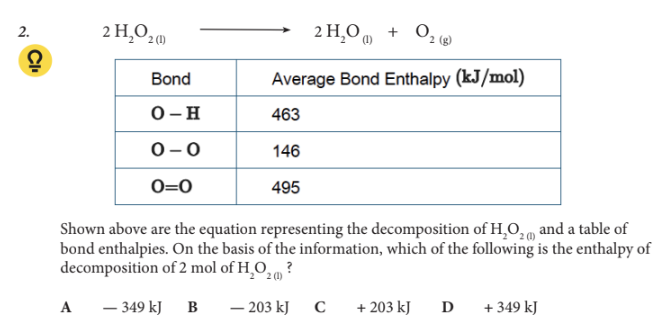
Which of the following is the enthalpy of decomposition of 2 mol of H2O2 (l) ?
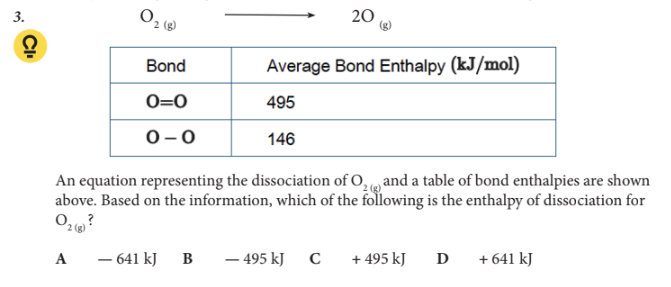
Based on the information, which of the following is the enthalpy of dissociation for O2 (g) ?
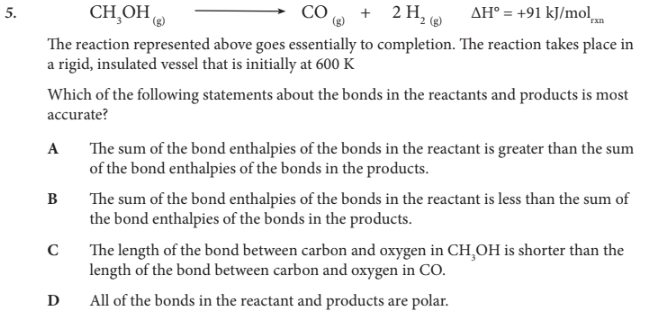
Which of the following statements about the bonds in the reactants and products is most accurate?
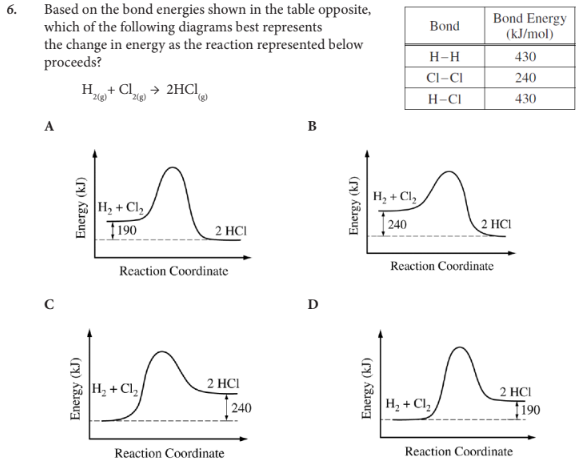
Based on the bond energies shown in the table opposite, which of the following diagrams best represents the change in energy as the reaction represented below proceeds?
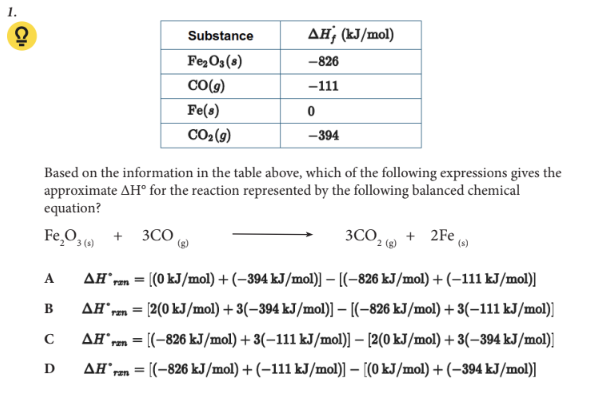
Based on the information in the table above, which of the following expressions gives the approximate ∆H° for the reaction represented by the following balanced chemical equation?
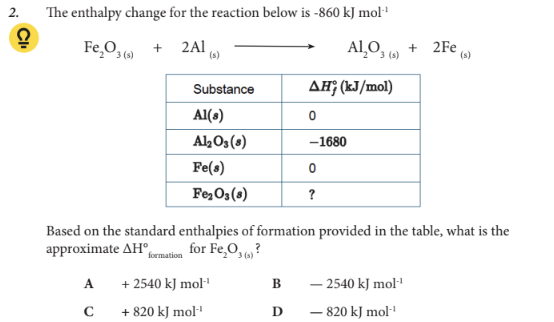
Based on the standard enthalpies of formation provided in the table, what is the approximate ∆H°formation for Fe2O3 (s) ?
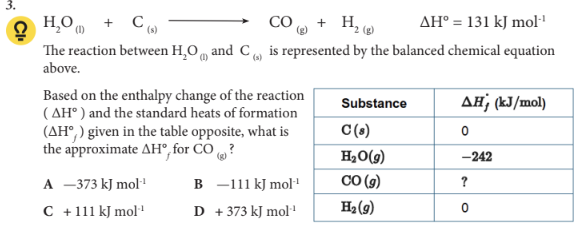
Based on the enthalpy change of the reaction (∆H°) and the standard heats of formation (∆H°f) given in the table opposite, what is the approximate ∆H°f for CO (g) ?
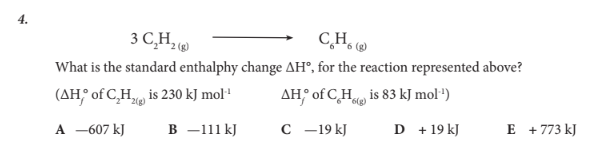
What is the standard enthalphy change ΔH°, for the reaction represented above?
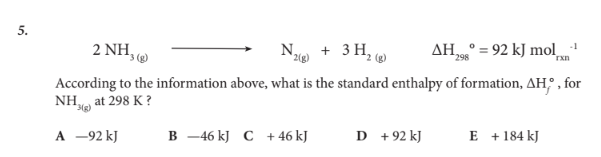
According to the information above, what is the standard enthalpy of formation, ΔHf° , for NH3(g) at 298 K ?
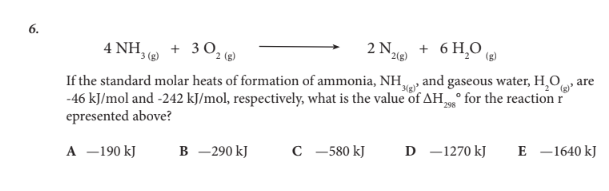
If the standard molar heats of formation of ammonia, NH3(g), and gaseous water, H2O(g), are -46 kJ/mol and -242 kJ/mol, respectively, what is the value of ΔH298° for the reaction represented above?
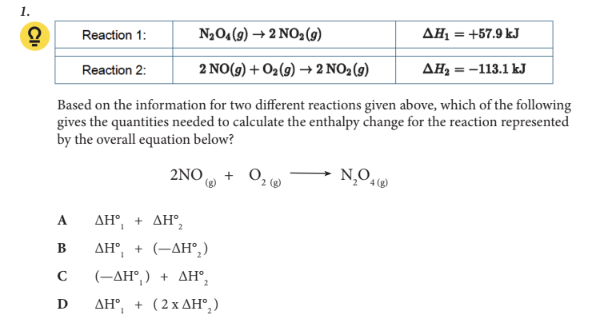
Based on the information for two different reactions given above, which of the following gives the quantities needed to calculate the enthalpy change for the reaction represented by the overall equation below?
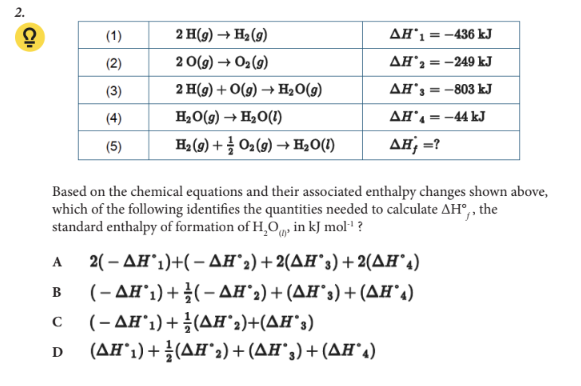
Based on the chemical equations and their associated enthalpy changes shown above, which of the following identifies the quantities needed to calculate ΔH°f, the standard enthalpy of formation of H2O(l), in kJ mol-1 ?
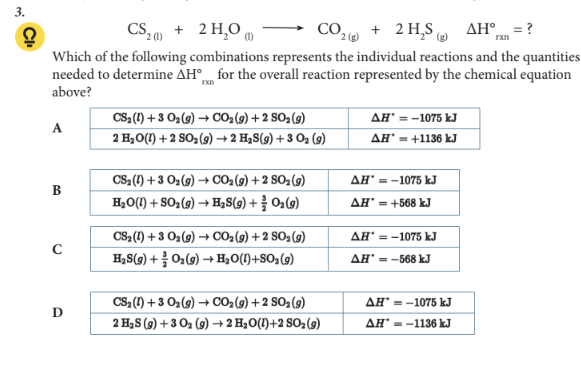
Which of the following combinations represents the individual reactions and the quantities needed to determine ΔH°rxn for the overall reaction represented by the chemical equation above?
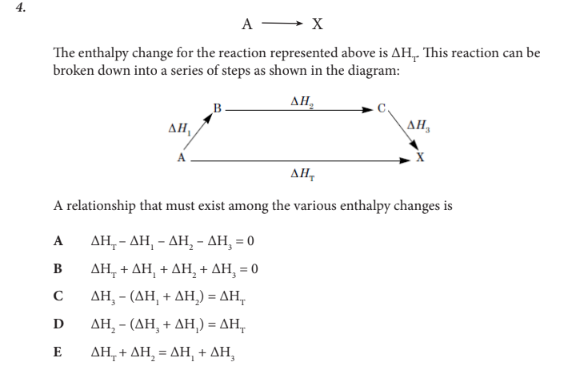
A relationship that must exist among the various enthalpy changes is
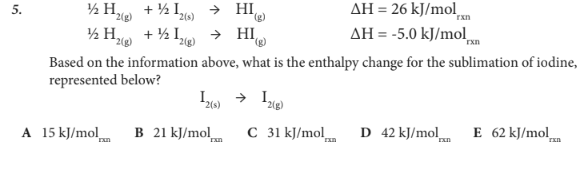
Based on the information above, what is the enthalpy change for the sublimation of iodine, represented below?