Gas Exchange and Transport: CO2 (Week 2, Mod 9)
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What happens when CO2 dissolves in an aqueous solution?
Makes the solution ACIDIC
** CA = carbonic anhydrase enzyme
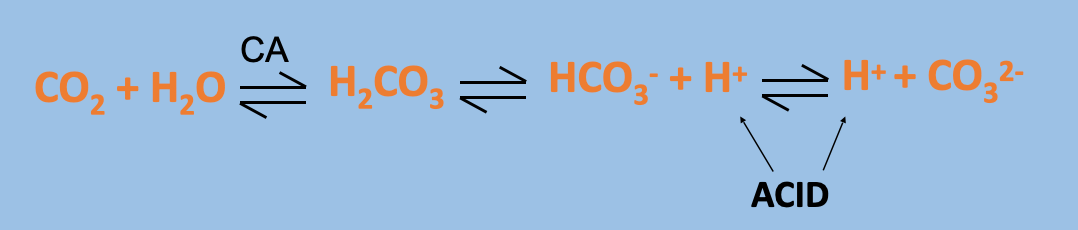
What is the total CO2 content of blood composed of?
Total CO2 content = [CO2 dissolved] + [H2CO3] + [HCO3-] + [CO32-]
If you can get rid of these ^ components, you can get rid of CO2
No specific carrier for CO2 in blood, so needs to be gotten rid of in other ways
What are the 3 ways that the body gets rid of CO2 in the blood? Which one is the primary way?
1) Dissolved CO2 (only 5%)
CO2 is much more soluble in blood than O2
5% of CO2 is transported unchanged, simply dissolving in the plasma
2) Bound to Hemoglobin (10%)
CO2 combines reversibly with AMINO GROUPS on Hb, NOT Fe SITE
Binds to oxyhemoglobin, converting it to carbaminohemoglobin
CARBAMINOHEMOGLOBIN IS BLUE TINGED
This is what gives deoxy blood its blue color in the first place
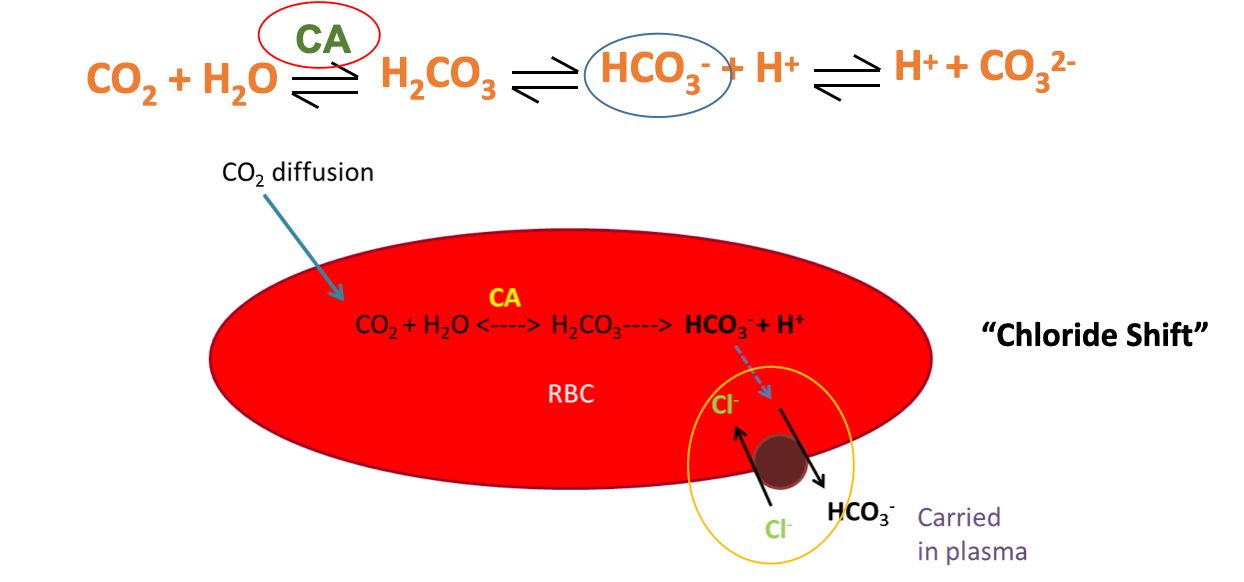
3) Bicarbonate ions (PRIMARY WAY: 85%)
Majority of CO2 is transported via the “chloride shift” in RBCs
CO2 diffuses into RBC, and is metabolized
Gets converted to bicarbonate
Chloride shift happens, bicarbonate switched out for chloride
This “gets rid” of CO2; is metabolized down to its components by RBCs
Is PUT BACK TOGETHER via the REVERSE of this process at the lungs; done to release CO2 via exhalation
Carbonic anhydrase can speed up this process
What is the Haldane Effect?
Basically the CO2 version of the Bohr effect
Affinity of CO2 for the hemoglobin amino acid
Hemoglobin has a HIGHER affinity for CO2 when deoxygenated (makes sense; when deoxygenated, needs to take up CO2 from freshly oxygenated tissues)
Has LOWER affinity for CO2 when it is OXYGENATED
To release it at the lungs
The Haldane Effect essentially says that at areas of high CO2 concentration (tissues), the hemoglobin displaces O2 in FAVOR of CO2 uptake
Vis versa at the lungs
What is a capnograph, and what is its purpose?
A device that measures CO2 concentrations in expired air (alveolar CO2, aka PACO2)
Mostly measured in intubated animals during anesthesia
Is measuring the RATIO between PaCO2 (ARTERIAL) and PACO2 (ALVEOLAR)
Normally similar unless there are respiratory or circulatory disturbances
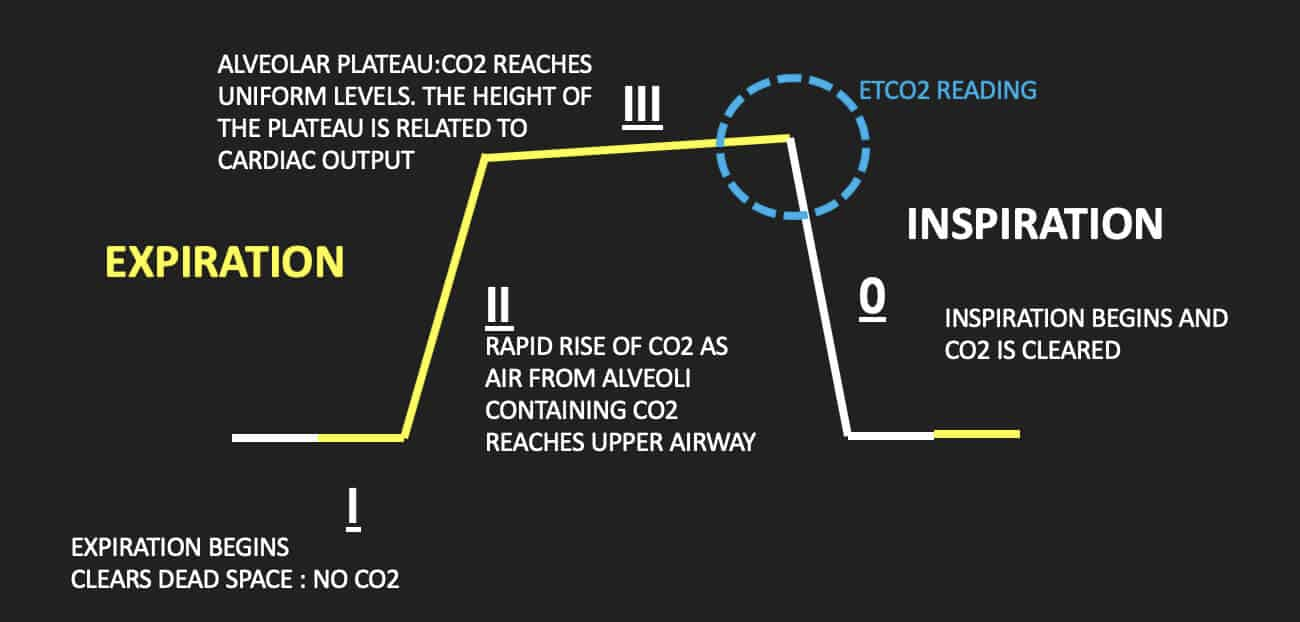
What is the ETCO2 value, and what is its significance in a capnograph reading?
ETCO2 = “End Tidal CO2”
Basically the max CO2 partial pressure before inhalation occurs
THIS is the value you’re reading during a capnograph - is at the end of the PACO2 plateau of the graph
ETCO2 should always land around 40
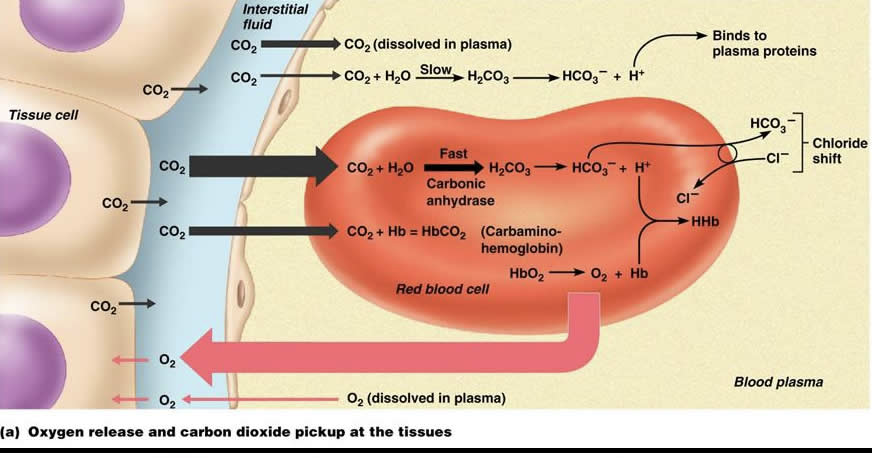
Summarize the 3 different gas exchange processes that are occurring here with CO2… remember, this is oxygenated blood at the tissues.
1) CO2 can be seen being dissolved in plasma, as well as being broken down metabolically for H+ ions to bind to plasma proteins…
Very small portion
2) CO2 from the tissues also is binding to the amino group of hemoglobin, turning it into carbaminohemoglobin
3) Most importantly, the CHLORIDE SHIFT can be seen
CO2 is taken up by the RBC and metabolized by carbonic anhydrase, which reduces the CO2 to bicarbonate (HCO3- ) and hydrogen
Bicarbonate in the RBC is then switched out for Cl from the plasma, releasing bicarbonate into the plasma
The REVERSE of this happens at the level of the alveoli… bicarbonate taken from plasma and switched out with chloride, then CO2 is reconstructed and released into the alveoli for exhalation
What are the 11 main things you NEED to remember about gas transport for O2 and CO2?
(Just remember these facts; use this notecard as a reminder)
O2 uptake and CO2 removal are both critical for normal physiological function
PO2 in the atmosphere is altered by several factors (inhaled CO2 = zero)
PO2 in alveoli (less than atmospheric PO2 due to presence of PCO2 and humidity) usually around ~105 mmHg.
PO2 in alveoli similar to PO2 in pulmonary vein and arterial blood (equilibrium)
If PO2 in alveoli decreases, PO2 will decrease in arterial blood
O2 carried in blood by Hb (mammals, birds) –Hb shows cooperativity, 4 binding sites for O2 , Hb gets saturated with O2, Affinity of Hb can be altered to enhance O2 delivery.
CO2 produced by respiring tissues dissociates to HCO3- and H+ (REMEMBER the equation- always associate high CO2 with low/acidic pH).
CO2 transported in 3 ways- main way is by HCO3- .
Haldane effect - Hb has higher affinity for CO2 when it is deoxygentated (CO2 diplaces O2 from Hb at tissues)
All mechanisms ensure O2 delivery to meet metabolic demand and CO2 removal (usually matched)
CO2 is important to monitor clinically (as it is a gaseous acid) can do this via capnography