Ochem Rxns Ch
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms

What reagents?
HCl, HBr, or HI

Regiochemistry?
Carbocation rearrangements

Type of Reaction?
Sn1

What types of reactants to use this on?
tertiary and secondary alcohols

Product?
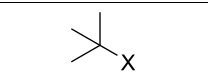

Product?
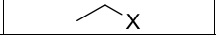

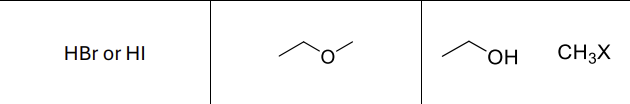
What reagents?
HBr or HI

What type of reaction?
Sn2

What type of reactants does this work best on?
primary alcohols

Product?
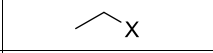

What type of Reaction?
Sn2

This reactions maintains or inverts stereochemistry?
Inverts

What type of reactants?
Primary and secondary alcohols ONLY

Product?
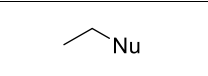

What reagents?
TsCl (or MsCl ot TfCl), Pyridine
NaNu
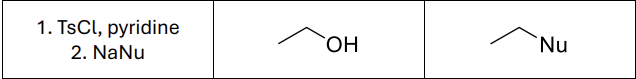
What type of reaction?
Sn2
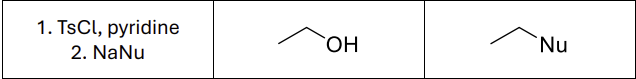
Does this reaction maintain or invert stereochemistry?
Invert
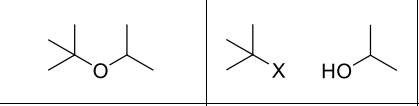
What reagents?
HBr or HI

What product?
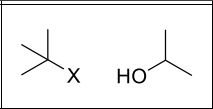
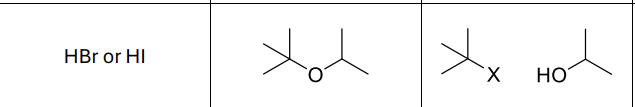
What type of reaction?
Sn1

Where will R’-X form?
Side that is more substituted
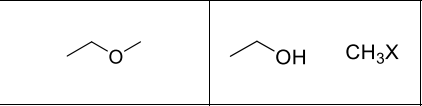
What reagents?
HBr or HI
What type of reactions is this?
Sn2

Where with R’-X form?
less substituted side

What reagents?
HCl, ROH

Where will the nucleophile add?
To the more substituted side

What type of reaction?
Sn1

What reagents?
RONa, ROH

Where will the nucleophile add?
less substituted side

What type of reaction?
Sn2

What are the reagents?
H2SO4, heat or POCl3, pyridine, 0 degrees celsius
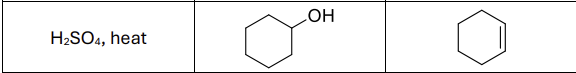
Regioselectivity?
Carbocation rearrangements possible
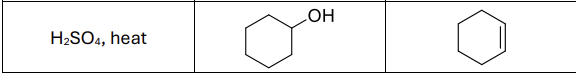
What type of reaction?
E1 rxn
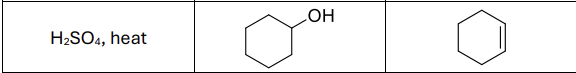
What type of reactants work best?
secondary and tertiary alcohols
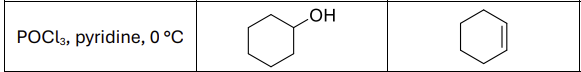
Any regioselectivity?
Always forms Zaitsev product
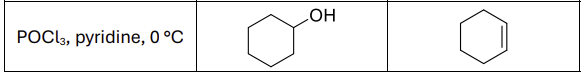
What type of reaction
E2
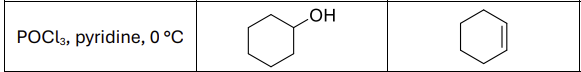
What type of reactants work best?
secondary and tertiary alcohols

What reagents?
H2Cro4 (strong oxidizer)

Product?
Carboxylic Acid
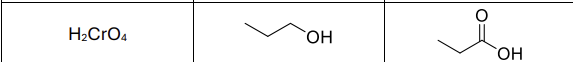
What type of reactant works with this?
Primary alcohols

What reagents?
PCC, CH2Cl2 or NaOCl, CH3COOH (mild oxidizer)
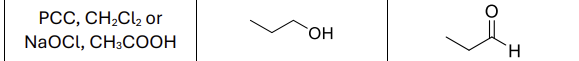
What type of reactant works with this reaction?
Primary Alcohol

What reagents?
H2CrO4 or PCC, CH2Cl2 or NaOCl, CH3COOH (strong or mild oxidizer)
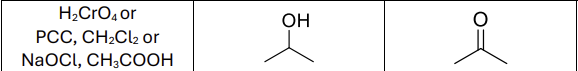
What type of reactants work?
secondary alcohols

What reagents?
OsO4
H2O2, H2O
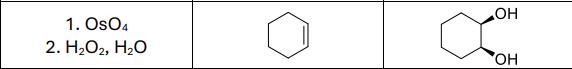
What stereoselectivity is there?
Syn Addition

What is the product called?
cis diol

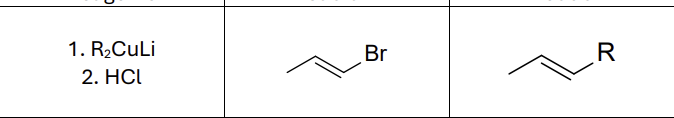
What reagents?
R2CuLi
HCl
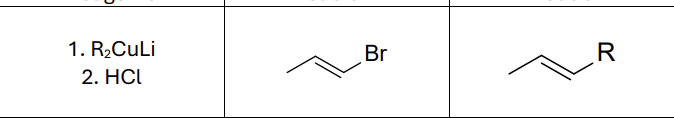
Does this reaction invert or maintain stereochemistry?
Maintain it
What reactants work with this reaction?
aryl and vinyl halides
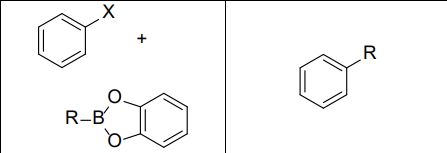
What reagents? (Suzuki Rxn)
PdL2, NaOH
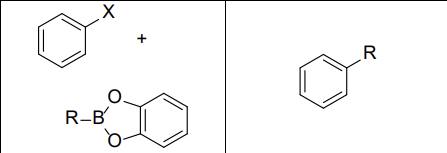
Does this reaction invert or maintain stereochemistry?
Maintain it
What type of mechanism does the Suzuki Reaction use?
Palladium-catalyzed cross-coupling
What reactants can be used with the Suzuki Reaction?
Vinyl or Aryl Halides
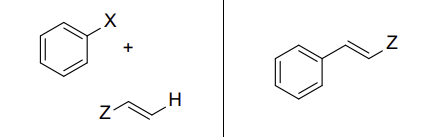
What reaction is this and what are the reagents?
Heck Reaction
PdL2, (CH3CH2)3N
What is the product of a Heck Reaction
Trans alkene
What type of mechanism does the Heck Reaction use?
Palladium-catalyzed cross-coupling with Z being any functional group or alkyl chain
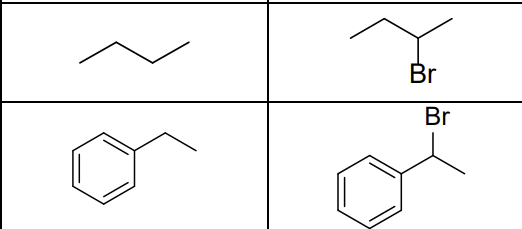
What are the reactants?
Br2, hv (light)
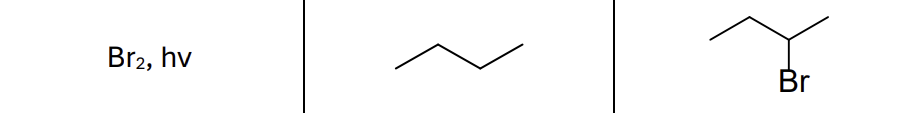
What type of reaction?
Radical reaction
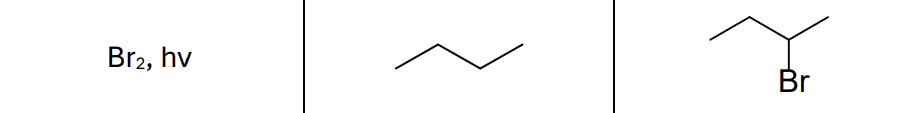
Where does the radical form?
Most stable position
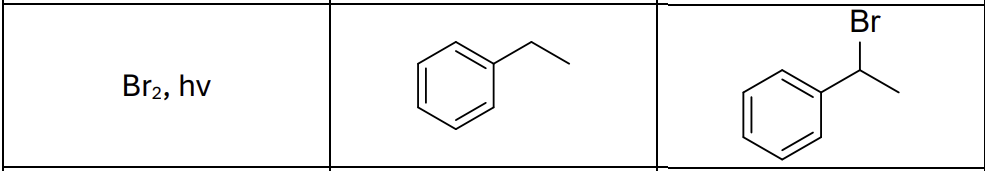
What type of reaction?
Radical reaction
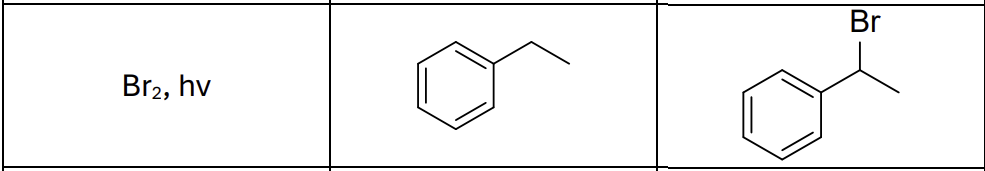
Where does the radical form?
benzylic position

What reagents?
NBS, Heat (delta) , peroxide
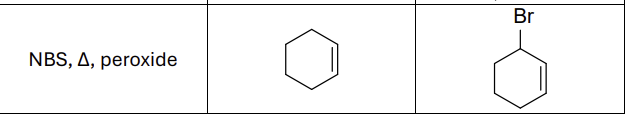
What type of reaction?
Radical reaction
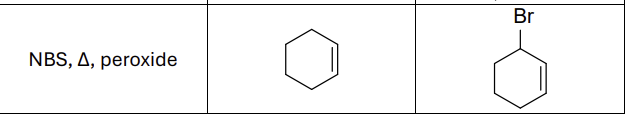
Where does the radical form?
allylic position