Cell Bio Mitochondrion and Aerobic Respiration
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
modern perspective of mitochondria
dynamic and multifunctional
mitochondria roles beyond ATP
apoptosis, signaling, heat generation
mitochondria
- double membrane organelles in nearly all eukaryotic cells
- generate most of the cell's ATP through aerobic respiration
- evolved from symbiotic bacteria
how is mitochondria distributed in cells?
- location matches energy needs
- supports local ATP supply
- clustered near contractile machinery, synapses, or ion pumps
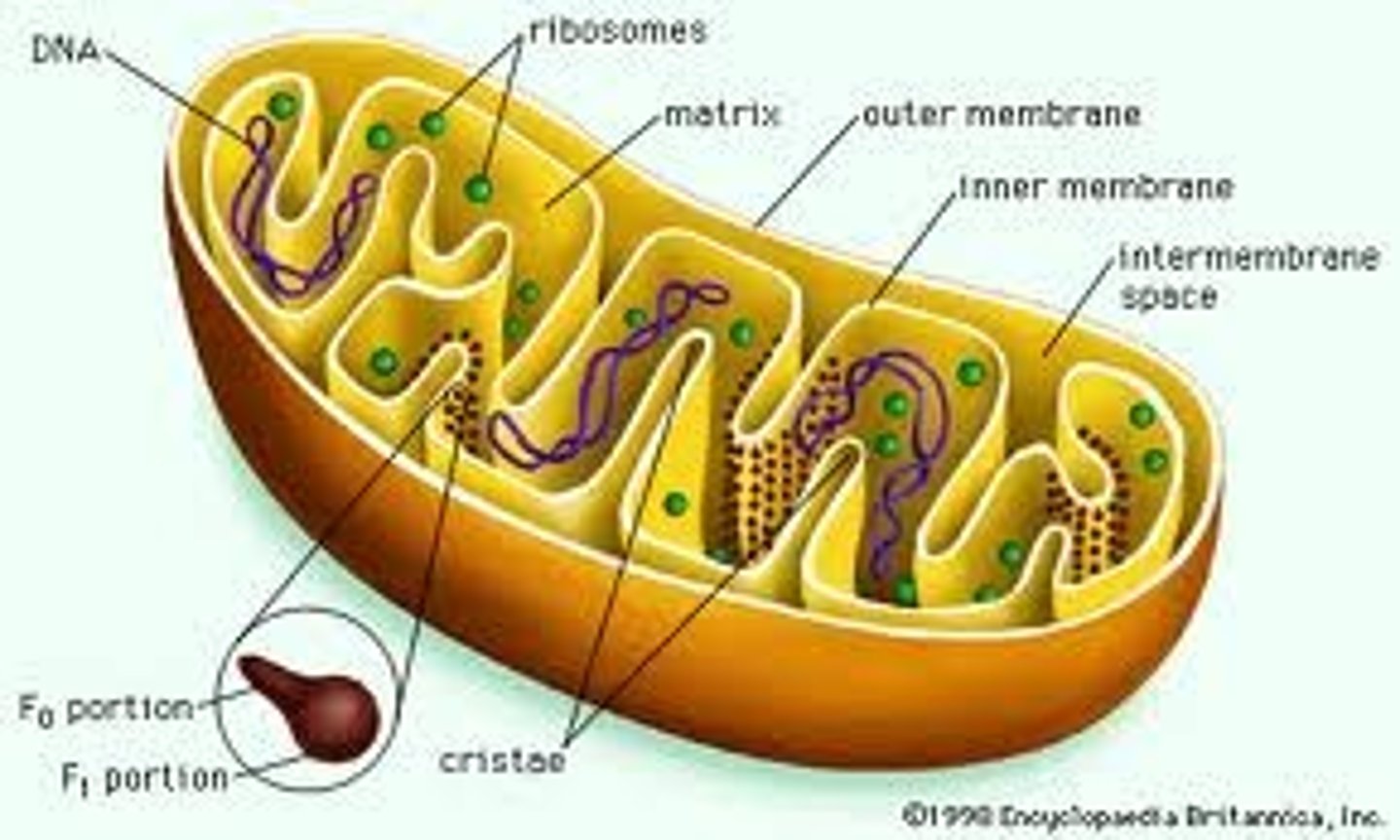
mitochondria outer membrane
permeable to small molecules
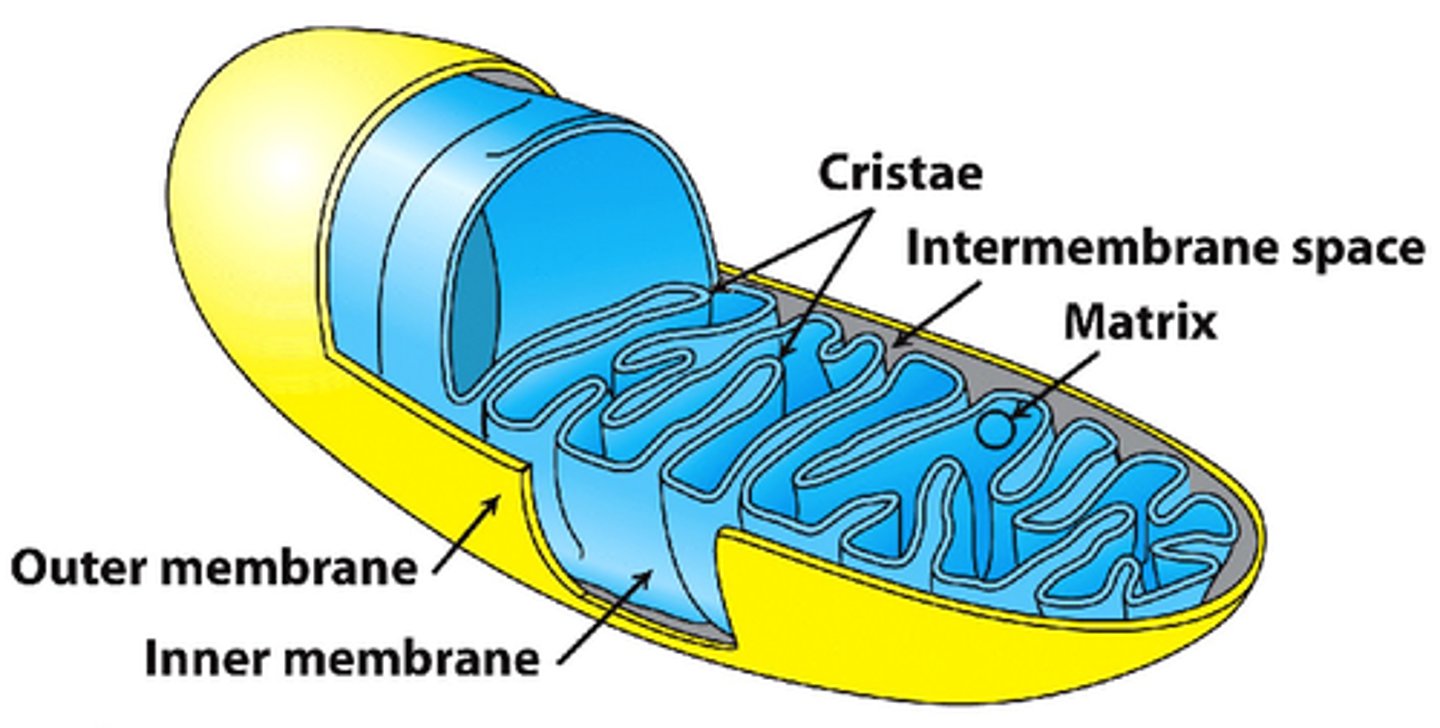
mitochondria inner membrane
impermeable, folded into cristae
mitochondrial compartmentalization
- intermembrane space vs matrix
- compartmentalization enables function
cristae
structural adaptation of mitochondria
- inner membrane folded into cristae
- increases surface area for electron transport and ATP synthesis
- cristae morphology varies with cell type and energy demand
cristae morphology vs energy demand
- cristae density correlates with ATP demand
- more folds = larger surface area for ETC
- tissue-specific variations (heart vs liver)
cardiac muscle mitochondria
- dense cristae
- high mitochondrial density
liver cells
- fewer cristae
- more metabolic versatility
ranking of energy demand (number of mitochondria) of different types of tissues
muscle cells > neurons > liver cells > stomach cells > red blood cells
mitochondria in muscle
- high mitochondrial density, dense cristae
- structure directly supports continuous contraction
- helps muscle repair
mitochondria unique features
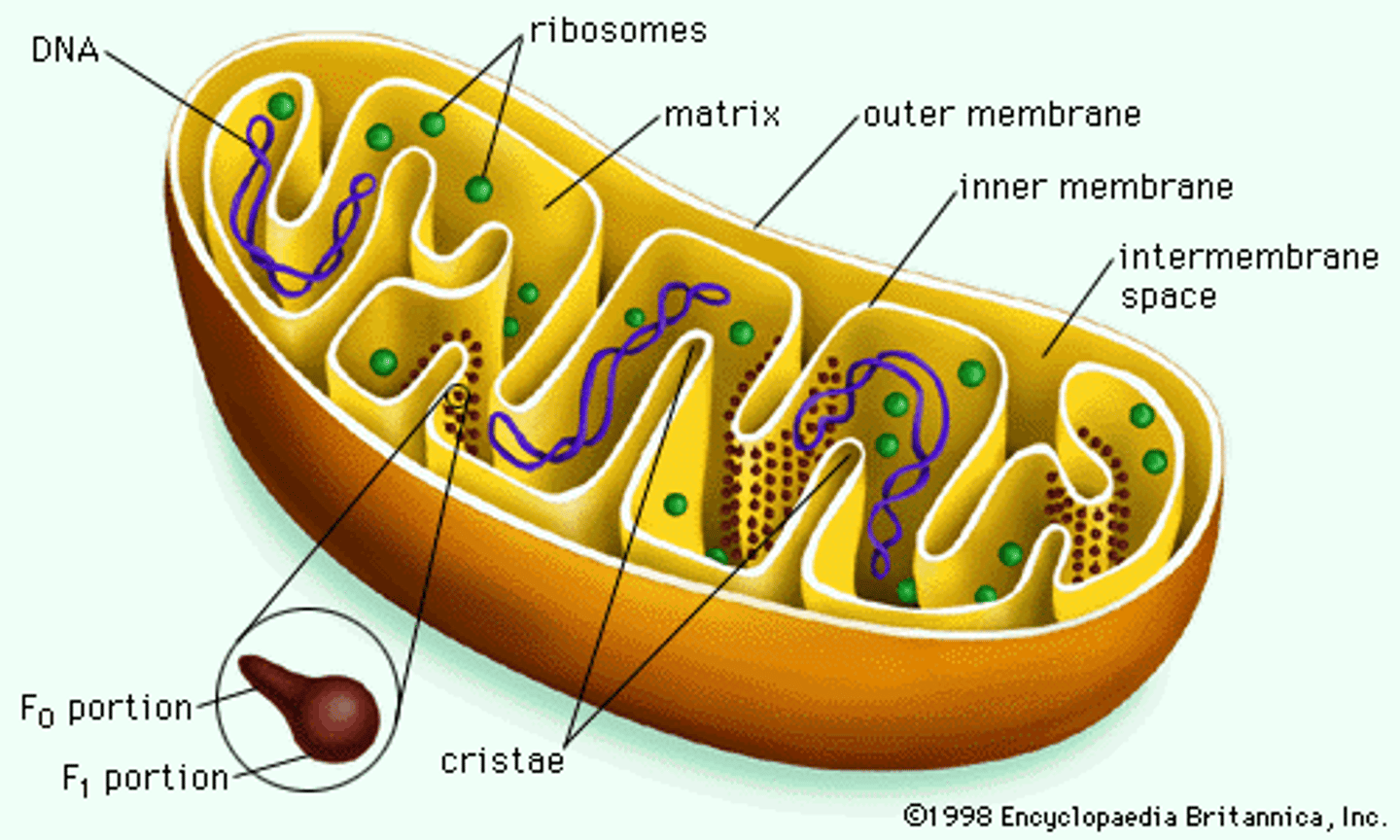
semi-autonomous
- own DNA (circular, bacterial-like)
- own ribosomes (70S, similar to bacteria)
- still rely on nuclear-encoded proteins from the cell
- replicate by fission, independent of cell cycle
mitochondrial dynamics
- constantly change via fusion and fission
- regulate mitochondrial shape, number, and function
- balance between repair and quality control
- can sacrifice ATP efficiency for heat production to adapt to needs
overview of metabolite transport
- inner membrane = highly sensitive
- transport proteins required
- regulates metabolic entry
pyruvate
glycolysis product from outside the mitochondria
pyruvate is imported by _____
mitochondrial pyruvate carrier (MPC)
pyruvate import
- once inside, converted to acetyl-CoA by pyruvate dehydrogenase (PDH) or Oxaloacetate by pyruvate carboxylase (PC)
- directly into TCA cycle which generates electron carriers for the ETC->ATP
pyruvate converts to lactate ->
lactic acidosis
lactic acidosis
- pyruvate reduced to lactate by lactate dehydrogenase
- occurs when mitochondria cant oxidize pyruvate
- lactate buildup -> decrease blood pH (lactic acidosis)
fatty acid import pathway through IMM
- fatty acids -> fatty acyl-CoA
- requires ATP investment
- first committed step
- fatty acyl-CoA requires shuttle
carnitine palymitolytransferase I (CPT1): step 1
- CPT1 on outer membrane
- transfers acyl group to carnitine
- produces acyl-carnitine, releasing Coenzyme A
carnitine/acylcarnitine translocase (CAT or CACT): step 2
- located in the inner mitochondrial membrane
- acyl-carnitine imported
- carnitine exported
- translocase=exchanger
carnitine palymitolytransferase II (CPT II): step 3
- CPT II inside matrix
- transfers acyl group back to CoA
- produces fatty acyl-CoA
carnitine deficiency
- defective shuttle -> energy failure
- symptoms: weakness, hypoglycemia
- CPT I/II or translocase defects
citric acid cycle (TCA/Krebs) overview
- central hub of metabolism
- oxidizes acetyl-CoA to CO2
- produces per Acetyl-CoA
- entry into TCA
citric acid cycle (TCA/Krebs) produces per Acetyl-CoA
- 1 GTP/ATP per cycle
- 3 NADH per cycle
- 1 FADH2 per cycle
citric acid cycle (TCA/Krebs) entry into TCA
- glycolysis: 1 glucose (3C) -> 2 pyruvate (3C)
- pyruvate DH: 2 pyruvate -> 2 Acetyl-CoA
-> twice as many products as above per glucose
TCA step 1:
Acetyl-CoA (2C) + oxaloacetate (4C) -> citrate (6C)
TCA step 2:
citrate (6C) isomerized or reorganized to isocitrate (6C)
TCA steps 1 and 2: purpose
- entry and rearrangement
- prepares substrates for oxidation
TCA step 3:
decarboxylation of isocitrate (6C) -> α-ketoglutarate (5C)
TCA step 3: purpose
- produces NADH and CO2
- major control point- energy harvesting reaction
TCA step 4:
decarboxylation of α-ketoglutarate (5C) -> succinyl-CoA (4C)
TCA step 4: purpose
- produces another NADH and a second CO2 is released
- multi-enzyme complex
TCA step 5:
succinyl-CoA -> succinate
TCA step 5: purpose
- produces GTP (or ATP)
- example of substrate-level phosphorylation
- captures energy directly
TCA step 6:
succinate -> fumarate (FADH2)
TCA step 7:
fumarate -> malate (hydration)
TCA step 8:
malate -> oxaloacetate (NADH)
total yields of TCA cycle per acetyl-CoA
- 3 NADH
- 1 FADH2
- 1 GTP/ATP
total yields of TCA cycle
- 2 CO2 released
- key source of reducing power
regulation points of TCA cycle: key enzymes
- citrate synthase
- isocitrate DH
- α-KG DH
- (steps 1, 3, 4)
regulation points of TCA cycle: inhibition and activation
- inhibited by ATP/NADH
- activated by ADP/Ca2+
- allosteric regulation
regulation points of TCA cycle
balances energy demand with fuel supply
amphibolic nature of TCA cycle
- catabolic and anabolic
- central metabolic hub/crossroads that impacts many cellular functions
catabolic nature of TCA cycle
oxidizes acetyl-CoA -> CO2
anabolic nature of TCA cycle
provides precursors for biosynthesis
the Warburg Effect
- cancer cells favor aerobic glycolysis -> formation of lactate, even with oxygen
- reduced reliance on TCA/oxidative phosphorylation in mitochondria
- fuels rapid cell prolifieration
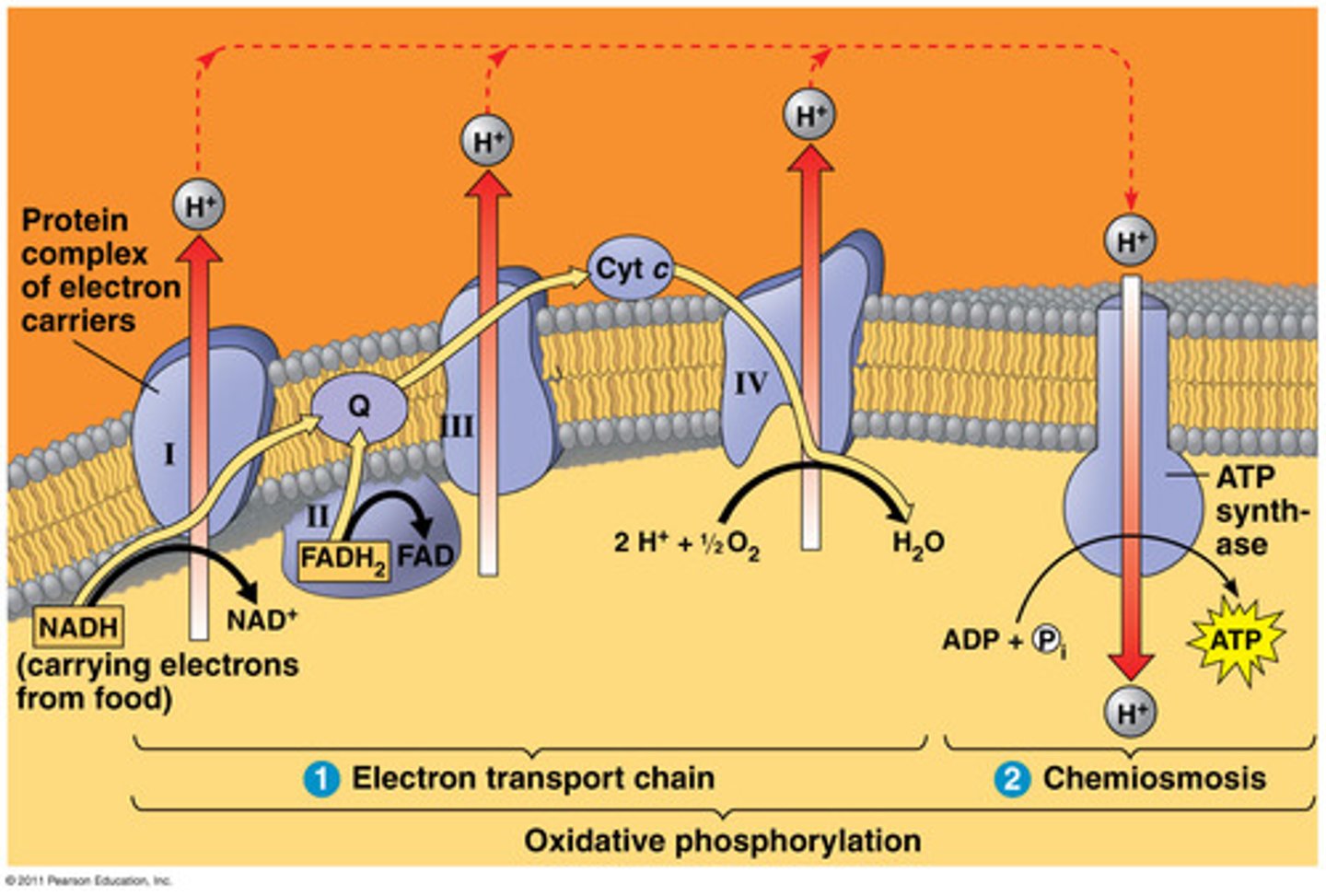
role of NADH and FADH2 in respiration
- NADH and FADH2 = key electron donors
- carry high energy electrons to ETC
- drive proton pumping and ATP synthesis
reduction
gaining electrons
oxidation
loss of electrons
reducing power
ability to donate electrons in redox
reducing power: redox (overview)
- defined by reduction potential (ΔE°')
- difference in potentials can be converted to free energy change (Nernst equation)
- energy released drives proton pumping and ATP synthesis
reduction potential (ΔE°')
negative value is strong electron donor
Nernst equation
ΔG°' = -nFE°'
- difference in potentials converted to free energy change
NADH pathway features
- produced by glycolysis, PDH, TCA cycle
- delivers electrons to Complex I in ETC
- yields ~2.5 ATP per NADH
shuttles for cytosolic NADH
- NADH from glycolysis cannot cross IMM
- malate-aspartate shuttle (efficient)
- glycerol-3-phosphate shuttle (less efficient)
malate-aspartate shuttle does what?
transfers electrons through malate to get across IMM
FADH2 pathway features
- generated in TCA (succinate DH) and β-oxidation
- donates electrons via Complex II
- ~1.5 ATP per FADH2
electron carriers into ETC -> ATP