Enzyme mech walkthrough - LDH mechanism
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
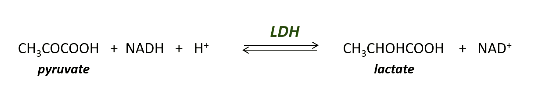
LDH is used as an enzyme to catalyse what reaction?
LDH has what structure, in terms of number of subunits used to make its full structure.
It is a tetrameric enzyme.
And LDH has a ________ conversion of pyruvate to -Lactate under _____ conditions
LDH has a stereospecific conversion of pyruvate to L-lactate under anaerobic conditions.
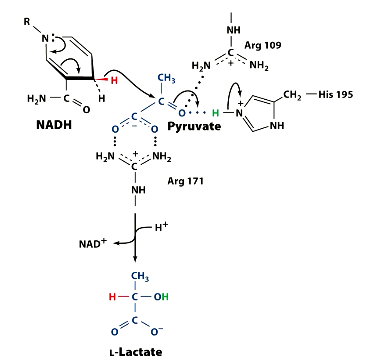
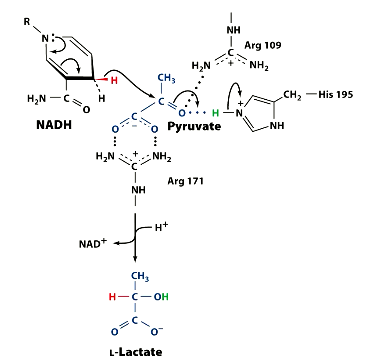
What amino acids are present in the LDH enzyme active site, which help catalyse the reaction?
His 195
Arg 109
Arg 171
Visualise the mechanism. Remember, the equation and recall which amino acids are present. There are 3.
Notice the Amino acids placements.
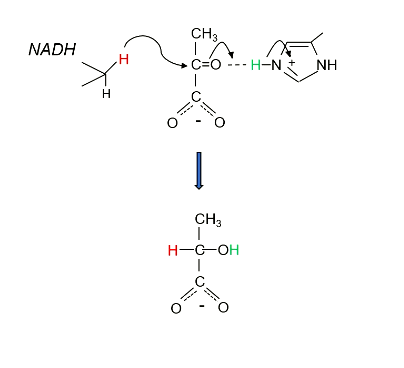
The first step of LDH catalysing this rection, is the formation of a _____ compex, in this order:
The first step of LDH catalysing this rection, is the formation of a ternary complex, in this order: Enzyme binds NADH, then pyruvate.
The second step is the NADH transfer.
What is unusual about the transfer NADH is making in the LDH mechanism??
It is making a hydride transfer (H-) to 2’ C
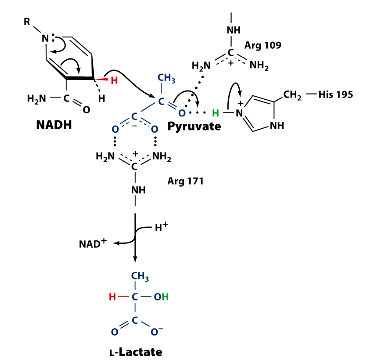
And why is this?
C=O is polarised. The positive C allows H- to attack.
Step 3: What is the result of this hydride transfer, in terms of pyruvate charge? How is this combatted?
Now there is a negative charge on the oxygen, as its double bond broke.
His 195 donates its proton, acting as a base.
What is produced in step 4?
L-lactate is produced
There is one more step, what is it?
Active site loop closure ensures no water enters the active site.
So what did arg ___ and arg ___ do?
Arg 109 - binds pyruvate carbonyl group
Arg 171 - binds pyruvate carboxylate
this orients pyruvate as it enters actoive site, ensuring stereo specificity.
sketch the mechanism.