UAMS Biochemistry Final Fall 2024, KAHOOT REVIEW
1/291
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
292 Terms
Hormone that helps regulate the stomach's production and secretion of hydrochloric acid (HCl) and gastric motility
Gastrin
PCO2 normal range is
35 - 44 mmHg
HCO3 normal range is
23 - 29 mmol/L
pH normal range is
7.35 - 7.45
>95% is the normal range for what blood gas measurement
Oxygen (O2) Saturation
Which of the following measurements is not included in blood gas panel?
pCO2
Total Protein
pO2
pH
Total Protein
Would the value of 37 mmol/L be normal for Total CO2 (venous)?
No, normal range is 22 - 33 mmol/L
Bicarbonate (HCO3-) normal range is
23 - 29 meq/L or mmol/L
Total Cholesterol equals
HDL + LDL + VLDL
Potassium has the exact same reference range as Creatinine
False.
Potassium (K): 3.5-5.1 mmol/L
Creatinine: 0.8-1.2 mg/dL
Normal range for Chloride is
98-107 mmol/L
35-44 mmHg is normal range for
pCO2 "partial pressure of carbon dioxide"
>80 mm/Hg is normal for
pO2 "partial pressure of oxygen"
BUN (Blood urea nitrogen) normal range is
8-20 mg/dL
Would haptoglobin result of 20 mg/dL be considered normal?
No, normal range is 30 - 200 mg/dL
Hgb A1C normal is
<5.7%
If a patients creatinine is 5.0 mg/dL, they are likely to have decreased kidney function.
True.
Creatinine normal range is 0.8-1.2 mg/dL
A potassium of 6.5 mmol/L indicates possible haemolysis.
True.
Potassium Normal Range is 3.5-5.1 meq/L or mmol/L
Rapid Osmolality Formula
Sodiumx2 + Glucose/18 + BUN/2.8
Rapid Anion Gap formula
(Na + K) - (Cl + HCO3)
Always verify Triglycerides are _____________ before using the Friedewald formula
Less than 400
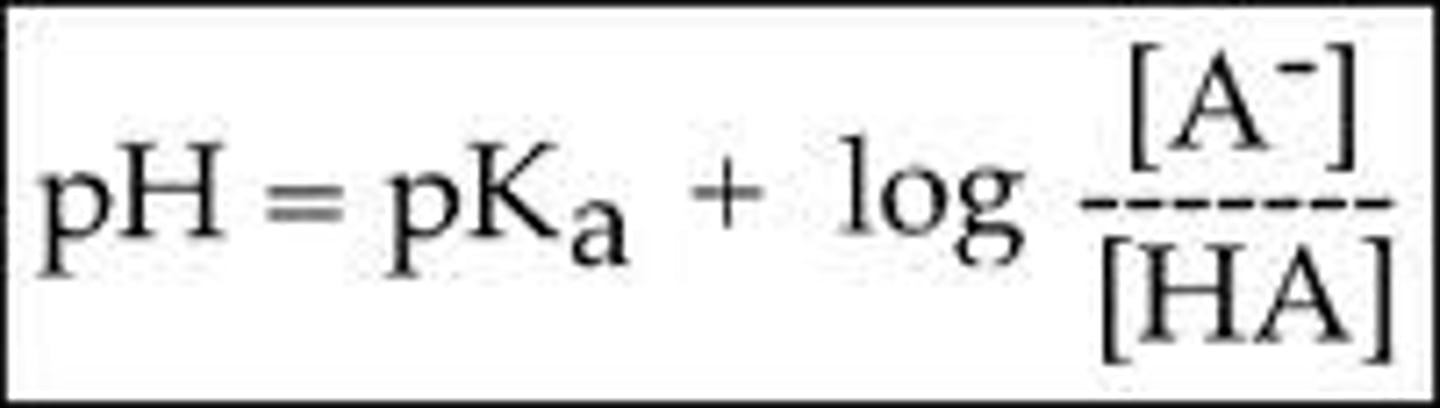
Henderson-Hasselbach
Sodium result of 500 mmol/L is abnormal and indicative of IV solution contamination requiring sample recollection.
True. Normal range is 136-145 mmol/L so something is likely wrong with the sample
Hyperuricemia with joint pain is indicative of ___________.
Gout
Creatinine Clearance
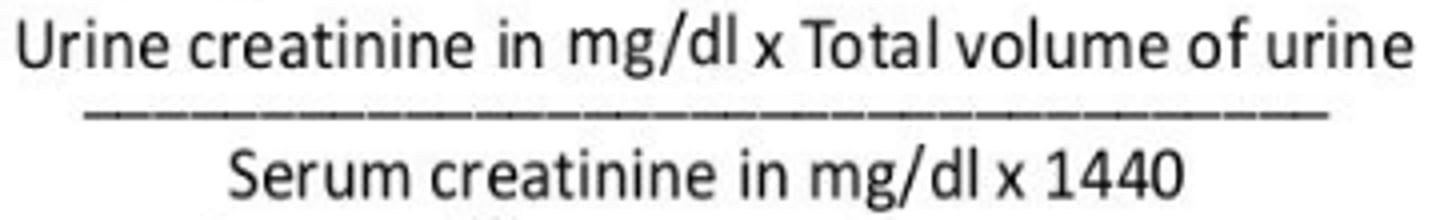
([Na+] + [K+]) - ([Cl-] + [HCO-3])
Anion Gap
Fe/TIBC x 100 is
% Transferrin Saturation
Coefficient of Variation (%CV)

Which equation below is known as the Friedewald formula?
LDL = Total Cholesterol - (HDL + Triglyceride/5)
Triglyceride/5 is
VLDL
Total Cholesterol - (HDL plus Triglyceride/5)
LDL
2 - log (%T)
Absorbance
Put names in order for A = abc
Absorbance
Molar absorptivity
Path length
Concentration
A = abc is know as
Beer's Law
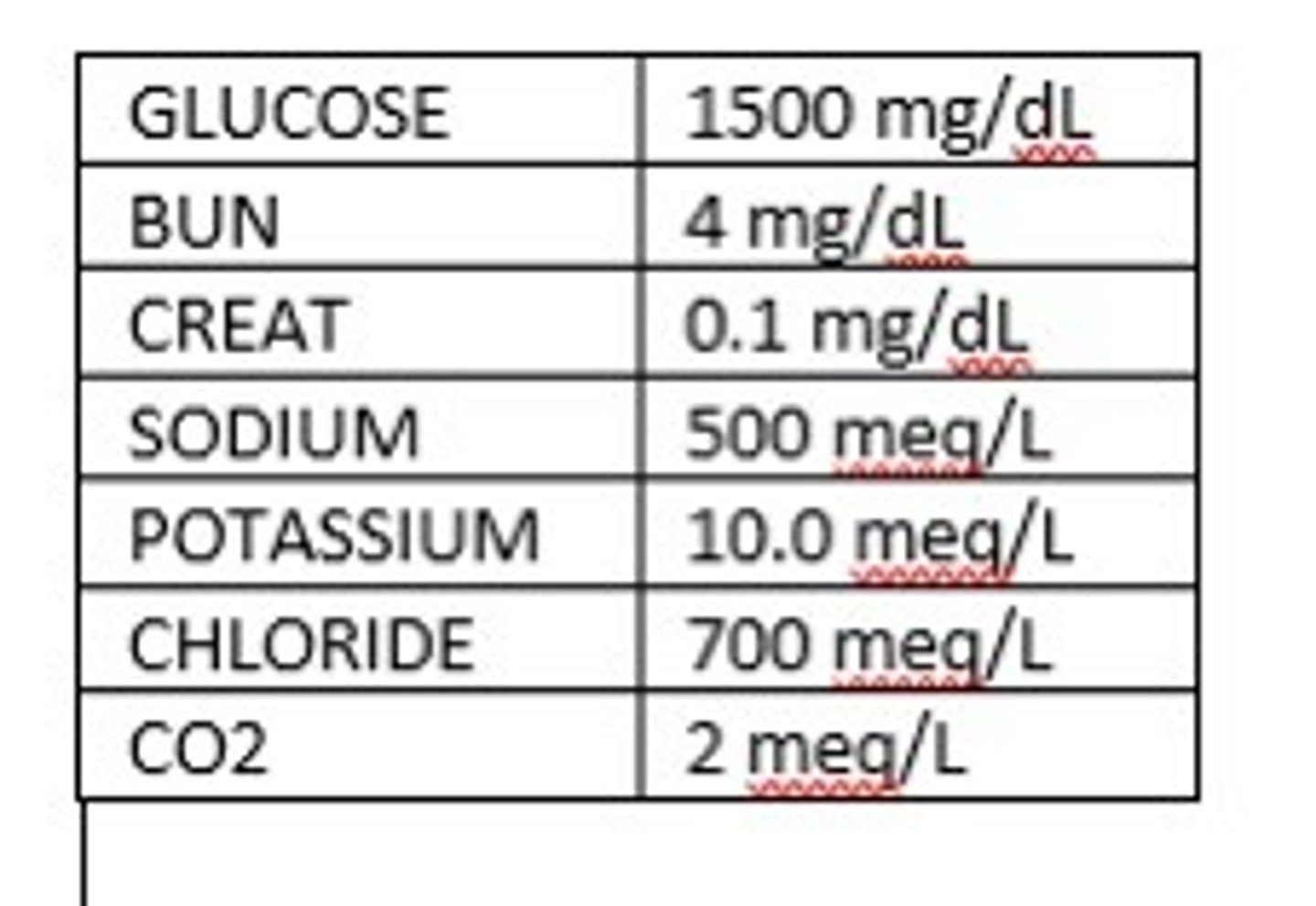
The following would not be compatible with life and would need to be redrawn.
True
Which of the following is a nonessential amino acid?
Tryptophan
Tyrosine
Threonine
Tysine
Tyrosine
This protein is decreased in patients with Wilson's disease.
Ceruloplasmin
Ceruloplasmin contains which of the following?
Copper
Zinc
Iron
Gold
Copper
Which protein test has mostly replaced the Erythrocyte Sedimentation Rate 'ESR' test for patients with suspected inflammation?
C-Reactive Protein
Nonprotein Nitrogen Compounds include?
Urea Nitrogen
Ammonia
Uric Acid
Creatinine
Smoking can increase _________ levels in blood.
Ammonia
_______ increase chemical reaction speeds by lowering activation energy requirements but don't actually cause reactions
Enzymes
Which of the choices represents an incorrect paring of CK isoenzyme & tissue location?
CK-MB and Heart
CK-MB and stomach
CK-BB and Brain
CK-MM and skeletal muscle
CK-MB and stomach
Which enzyme is likely deficient when a haemolytic anaemia develops due to consuming an oxidizing medication?
G-6-PD
Which enzyme is normally increased due to bone formation when the body is growing?
ALP "alkaline phosphatase"
Which is not a monosaccharide?
Lactose
Galactose
Fructose
Glucose
Lactose.
Disaccharide made of two simple sugars: galactose and glucose
Diabetes is a hormone imbalance disease characterized by?
Hyperglycemia
Scientific word for decreased plasma glucose levels
Hypoglycemia
The most commonly detected glycosylated haemoglobin is
Haemoglobin A1C
Which Lipoprotein is not included in Total Cholesterol?
Chylomicrons
Which lipoprotein is considered "good" due to it's ability to remove excess cholesterol from cells?
High-Density Lipoprotein, "HDL"
Which of the following is required for readable colour conversion of cholesterol and triglycerides?
Peroxidase
What is the most abundant protein in plasma/serum?
Albumin
Which test are run to aid acute pancreatitis diagnosis?
Lipase
Amylase
Which diagnostic criteria meet the requirements for pre-diabetes?
Fasting glucose of 115 mg/dL
HbA1C 6.1%
Which of the following is NOT an electrolyte?
Mg
Na
H2O
Ca
H2O
Which electrolyte affects neurological transmission?
Magnesium
A combination of a weak acid and its salt is a
Buffer
Which organs regulates acid-base balance by metabolic activity?
Kidneys
High pH, Normal HCO3, Low pCO2 indicates
Respiratory Alkalosis
Respiratory Alkalosis can be caused by
Fever
Drugs
Hyperventilation/hysteria
ISEs are commonly used to measure
Electrolytes
Porphyrins do not contain the porphyrin ring.
False
Two sites of heme synthesis are
Bone Marrow
Liver
Respiratory acidosis is primarily caused by defective kidney(s)
False.
it is primarily caused by issues with lung function, where the body is unable to adequately remove carbon dioxide through breathing, leading to a buildup of acid in the blood
These patient results are indicative of what type of pH imbalance? pH=7.27, pco2 = 57, HCO3 = 23
Acidosis, respiratory
Is this patient uncompensated, partially compensated or fully compensated? pH = 7.44, PCO2 = 25, HCO3 = 15
Fully Compensated; pH has returned to normal via compensation
Is this patient uncompensated, partially compensated or fully compensated? pH = 7.40, PCO2 = 42, HCO3 = 26
Patient does not have an imbalance
Untreated Addison's can lead to __________ ___________.
Acidosis, "metabolic" non-respiratory
Severe diarrhoea can lead to __________ ____________.
Acidosis, "metabolic" non-respiratory
Sedative overdose can lead to __________ acidosis.
Respiratory
Milk-alkali syndrome, caused by intake of too much calcium, can cause __________ _____________.
Alkalosis "metabolic" non-respiratory
The acronym, VLDL, stands for what?
Very Low-Density Lipoprotein
Ionic copper is an effective cofactor for many enzymatic reactions.
True.
Copper is primarily involved in enzymes classified as oxidases, which catalyze oxidation-reduction reactions in various metabolic pathways
Salivary _________ helps breakdown complex carbohydrates.
Amylase
MM, MB, & BB are all ______ isoenzymes.
CK
Historically, _______ ________ was detected via a manual blood test using florescent dye viewed with a black light.
G-6-PD deficiency
Cytochrome P-450 enzymes are found primarily in the ________.
Liver
Structurally, a _________ sugar has six carbon atoms.
hexose
_______________ are carbohydrate chains made up of 3 to 10 simple sugars
Oligosaccharides
Insulin is produced in the islets of __________ tissue of the pancreas
Langerhans
Hexokinase enzymatic testing is commonly used to measure blood __________ concentrations.
Glucose
Glycosylated Haemoglobin testing, like A1C, can be used to monitor patient compliance with ____________ treatment(s).
Diabetes
The Jaffee reaction is a method for testing ___________ levels.
Creatinine
Triglycerides are composed primarily of amino acid peptide bonds.
False.
Triglyceride components: A triglyceride molecule consists of a glycerol backbone and three fatty acid tails.
Glycogenesis
Process of converting glucose into glycogen, a stored form of glucose.
Gluconeogenesis
Process by which the body makes glucose from non-carbohydrate sources.
_________________ are good markers of renal health.
Nonprotein nitrogen (NPN)
urea, amino acids, uric acid, creatinine, and ammonia.
Albumin is rarely elevated in blood samples, but can be easily decreased in cases of ____________.
Malnutrition
Albumin and globulin results are used to calculate _______ ratio.
A/G "albumin/globulin ratio"
_______________ may cause problems with your enzyme results.
Inhibitors
Hypernatremia can be caused by increased water retention.
False.
it is actually caused by either too much sodium intake or too much water loss, leading to a relative increase in sodium concentration in the body.
Hyperkalemia can be seen in cases of ___________.
Acidosis
The ________________ equation is used for determining ____________.
Henderson-Hasselbach ; pH
When testing for renal function, NPN waste products found in urine include.
Urea
Uric Acid
Creatinine
Hyperemesis Gravidarum is characterized by _______________.
nausea & vomiting
Chemical used to induce sweat during the sweat electrolyte test.
Pyelocarbamezapine
Pancreatic function tests include
Sweat Electrolyte Determinations
Amylase & Lipase
Insulin
Glucagon
The Islets of Langerhans in the Pancreas secrete which hormones?
Islets of Langerhans:
Beta cells: Produce insulin
Alpha cells: Produce glucagon
Delta cells: Produce somatostatin
D-Dimer levels are abnormal in 90% of pulmonary embolism patients.
True