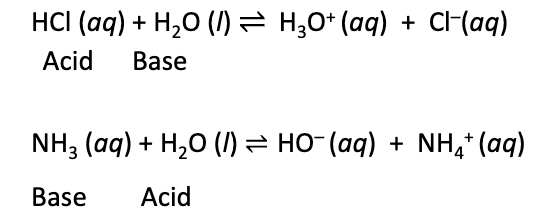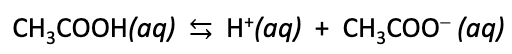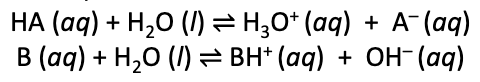CHEM 1112 Acid Base Equilibria
1/33
Earn XP
Description and Tags
Part I
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Aqueous species
species “dissolve” in water
in some cases molecules remain intact, and interact with water through non-covalent interactions
in some cases, the molecules no longer associate with each other and are surrounded by water molecules “hydration shell”
Ionic reactions
ionic species dissociate completley in aqueous solution, and are therefore more realistically represented as dissociated ions
consider the reaction between KOH and HCl in water.
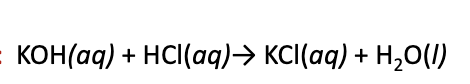
this is not the proper form, rather…
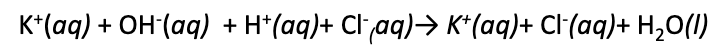
(above is before the spectator ions are eliminated)
Spectator Ions
ions whose presence is required to maintain charge neutrality
neither chemically nor physically changed by the progress of the reaction
identical form on both sides of the arrow
Net Ionic Equation
Complete ionic equation written out, with the spectator ions eliminated
easier to see which species are changed during the reaction
Solubility Rules
All common salts of group 1 elements are soluble
All ammonium ions (NH4+) are soluble
All common nitrates (NO3-) are soluble
Binary compounds of Group 17 (NOT F) with metals are soluble
Strong bases are the hydroxides of Group 1 and 2 metals (NOT Be)
Strong base
completley dissolves into ions when dissolved in water, releasing OH- ions
Hydroxides of Group 1 and 2 metals (NOT Be)
LiOH
NaOH
KOH
Ca(OH)2Sr(OH)2
Ba(OH)2
Strong acid
completely ionizes into ions when dissolved in water, releasing H+ ions
HClO4
HCl
HBr
HI
HNO3
H2SO4
Arrhenius definition (Acid)
a substance that produces H+ ions in aqueous solutions
a proton (H+ ion) donor
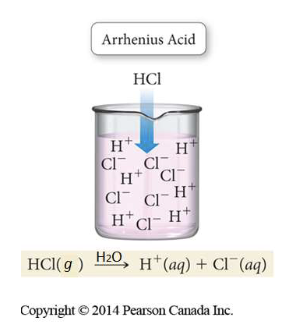
Arrhenius definition (base)
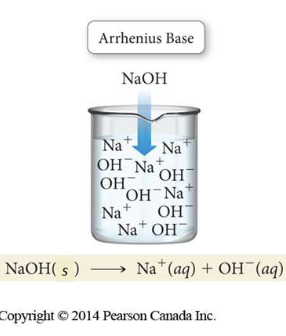
a substance that produces OH- ions in aqueous solutions
a proton (H+) acceptor
Lewis definition (acid)
electron pair acceptor
Lewis definition (base)
electron pair donor
Conjugate base of an acid
the base formed when an acid donates a single proton
H2O + HA ⇋ (H3O+) + A-
HA is the acid
A- is the conjugate base
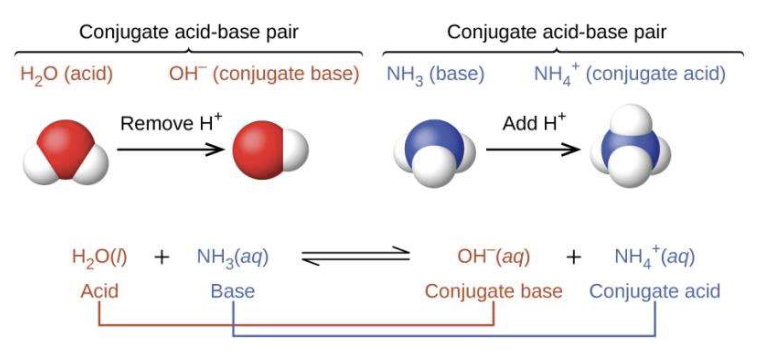
Conjugate acid of a base
the acid formed by the acceptance of a single proton by a base
(H3O+) + B- ⇋ HB + H2O
B- is the base
HB is the conjugate acid

Amphoteric
can act as either an acid or a base
water holds this property
Autoionize
molecule can spontaneously form ions without external influences
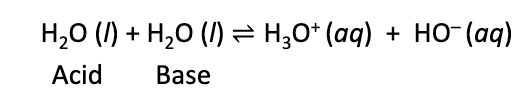
Ionization of water
water dissociates to form H+ and OH- ions
dissociation constant (Kw) for this 10-14 at 25oC
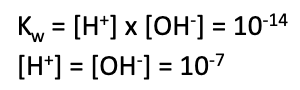
Monoprotic acid
some Bronsted acids are monoprotic, meaning they only have one acidic proton
consider acetic acid (CH3COOH), only one proton on the carboxyl group can be donated
Diprotic acid
some Bronsted acids are diprotic, meaning they have two acid protons.
consider sulfuric acid (H2SO4)…
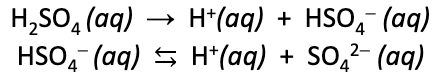
pH scale
used to express the hydronium ion concentration
the acidity of a solution is expressed as a pH value where pH= -log[H+]
neutral solution: [H+] = [OH-]
basic solution: [H+] < [OH-]
acidic solution: [H+] > [OH-]
more acidic the solution, the lower its pH
a change in pH of one unit reflects a tenfold change in the [H+]
Monoprotic strong acids
dissociate completely to donate their protons to water, so that there will be virtually no undissociated acid left in solution
HClO4
HCl
HBr
HI
HNO3
H2SO4
Strong bases
completely dissociate in solution to form hydroxide ions
LiOH
NaOH
KOH
Ca(OH)2
Sr(OH)2
Ba(OH)2
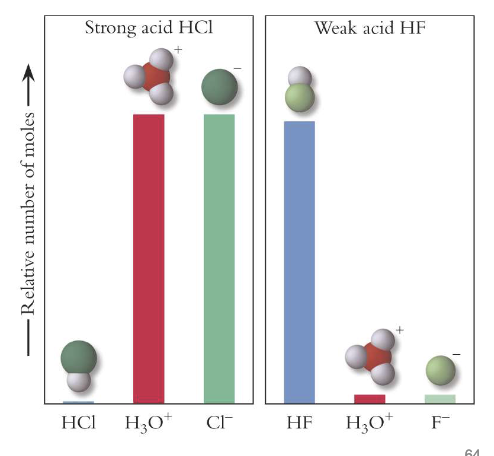
Strong vs weak acids
strong acids dissociate completely to donate their protons to water
the [H+] = initial acid concentration
weak acids do NOT dissociate completely to donate all of their protons to water.
the [H3O+] =/= weak acid concentration
Acid ionization constant (Ka)
Ka represents the equilibrium constant for the dissociate of an acid
indicates the strength of an acid
the stronger the acid, the larger the Ka value
Ka = [H3O+][A-] / [HA]
% ionization of a weak acid
% ionization = [H3O+]eqb’m / [HA]initial
Weak bases
do NOT dissociate completely in water
the concentration of HO- in a weak base solution is not equal to the concentration of the weak base
Kb = [BH+][HO-] / [B]
the stronger the base, the larger the value of Kb
Sources of H+ in solutions
Ionization of the acid/base
Autoionization of water
usually insignificant
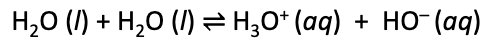
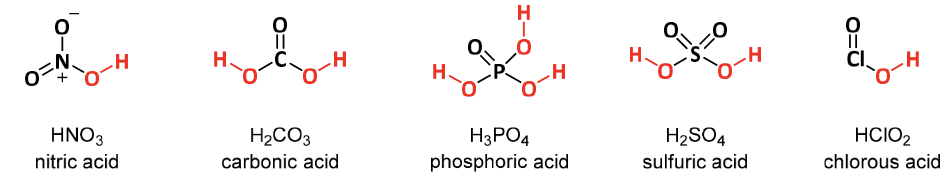
Oxyacids
acids that contains an inner atom bonded to a variable # of oxygen atoms and acidic OH groups
general formula: Y-OH
Amines
large group of weak bases
many other weak bases are derivatives of ammonia called amines, where some of the bonds to H have been replaced with bonds to other atoms
lone pair can accept H+
amines that have their free lone pair can act as a base (accept a proton)
Acid strength
recall: the stronger the acid, the larger the Ka
Ka is related to ∆Go
∆Go = -RTlnKa
∆G = ∆H - T∆S
qualitatively, would not expect big difference in ∆Sr for different HA
all have same change in # of species, all species are aq
differences must be due to different ∆H
the H+ is the same for all acid dissociates
qualitatively, the differences must be due to the relative differences between HA and A-
thus, qualitatively, we can understand the magnitudes of ∆G and thus Ka by considering the relative stabilities of the acid and its conjugate base
Factors affecting relative stabilities of HA and A-
electrostatic factors
ability to conjugate base to accomodate the negative charge
resonance
size
other EN groups
effect of charge (acid strength)
affects the ability to donate protons (opposite charges attract)
H3O is better able to donate proton to a base than H2O is; H3O is a stronger acid than H2O
HO- does not function as an acid in aq solutions
Acidity trends (across)
as you move across a period, and thus increase the electronegativity of a species, acidity increases
acid strength increases with increasing # of EN groups attached
increased polarity of species-H bond
increased stability of the product
Acidity trend (down)
increased size of the species as you move down the periodic table helps to better accomodate negative charge, and thus increases acidity
Carboxylic acids
oxyacids
organic (carbon) based
weak acids