Rate-determining step and Rate constants and temperature
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
The rate equation only includes what?
Reacting species involved in the rate determining step
The orders in the rate equation match the number of what?
Species involved in the rate-determining step
If a reactant isn’t present in the rate equation, what does this mean?
It isn’t present in the rate determining step
What is the Arrhenius equation?
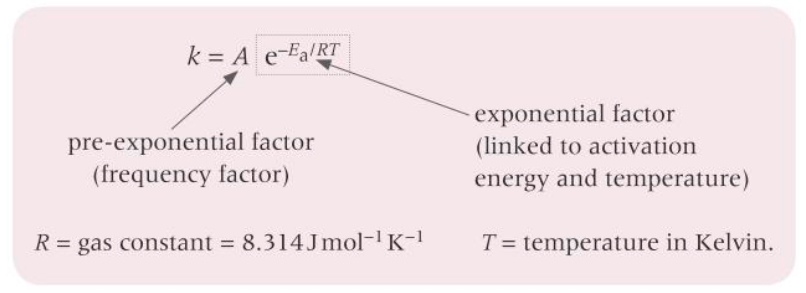
How can the Arrhenius equation be expressed as a logarithmic relationship?
Ln k = -Ea/RT + lnA
The logarithmic form of the Arrhenius equation enables what?
Ea and A to be determined graphically
A plot of lnk against 1/T gives a straight line graph
How can the Arrhenius equation be use to plot a graph?
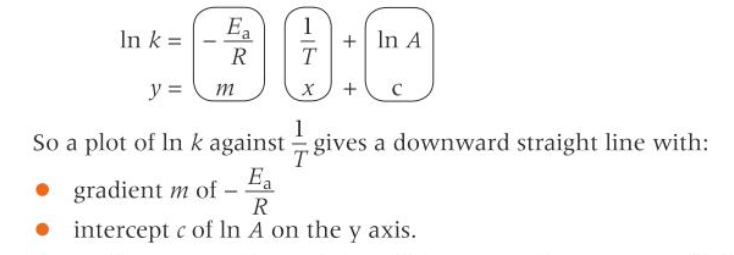
Does increasing the temperature increase the rate constant?
Yes, assuming the concentration of reactants remain unchanged
As the rate of reaction increases, the rate constant will increase