ch 12: Cocaine + stimulants
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
cocaine is derived from ______, a shrub native to _____ → ____ produces 2/3 of the world supply.
Erythroxylum coca
the Andes Mountains
COLOMBIA
forms of cocaine (3)
coca leaves: chewed → v. little abuse
powder cocaine (cocaine HCl) → oral, intranasal, IV, tropical
typically cut w other powders ie. levamisole
freebase: the base (cocaine) is separated (freed) from the HCl to increase lipid solubility → crosses BBB more easily
smoked → dangerously flammable

crack cocaine (2)
base freed by combining with baking soda and drying
smoked → more intense + rapid high than powder cocaine
metabolism of cocaine (3)
cytochrome P450 enzyme system in the liver
excreted in the urine, sweat, saliva, and breast milk
metabolites detectable in urine for 2-3 days after administration → up to 2 weeks in chronic users
eg. benzolecgonine
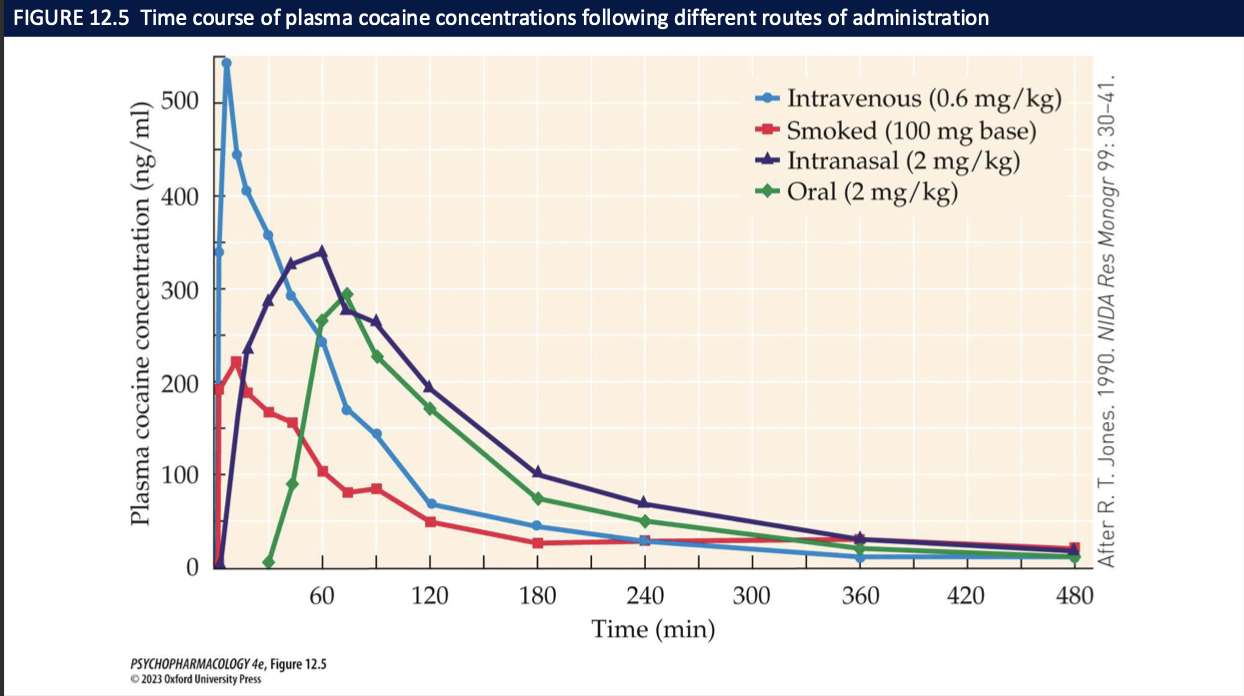
routes of administration of cocaine in order of fastest absorption (3)
smoking » injection > snorting > oral
once absorbed, cocaine is rapidly broken down + excreted
subjective high lasts ~30mins
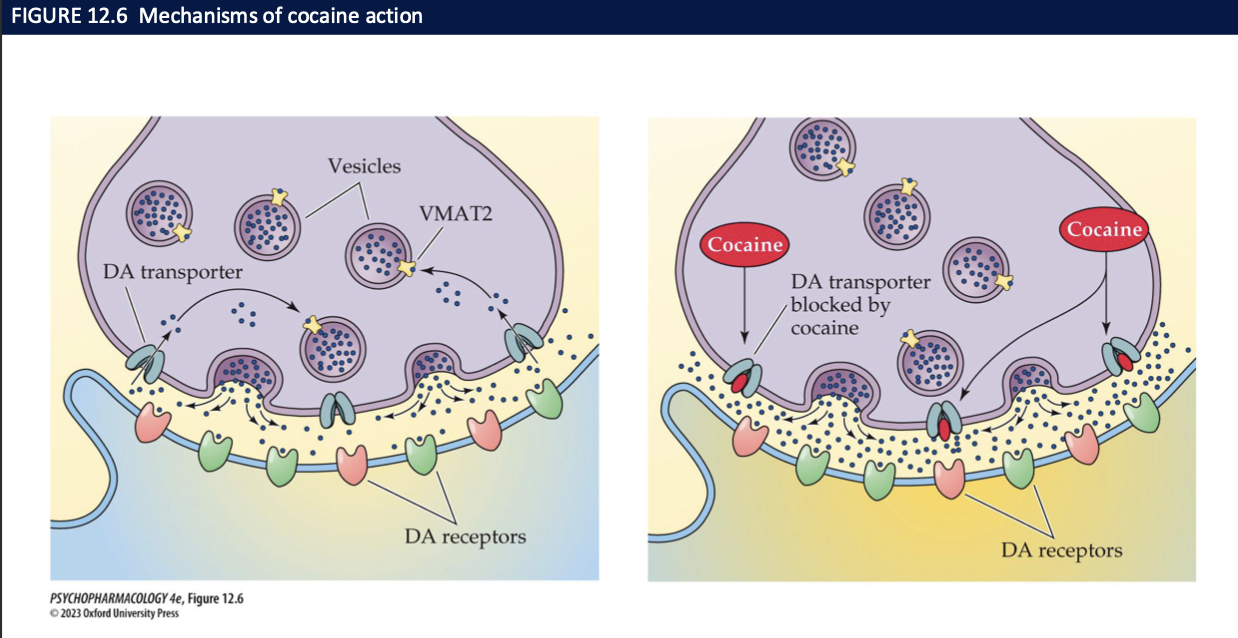
most of cocaine’s actions are due to _________ by inhibiting _______ → increase ____ concentrations of ___ increases the rate of _____
it’s ability to block reuptake of DA, NE, 5-HT
their membrane transporters
synaptic concentrations
transmitters
transmission
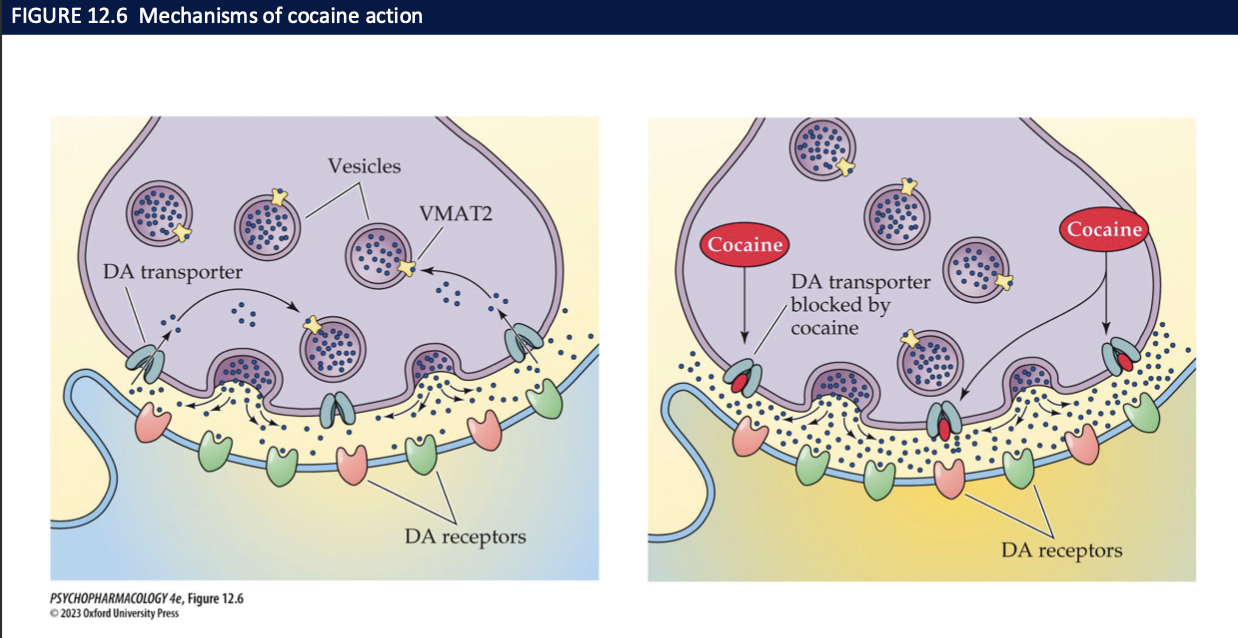
cocaine’s behavioural effects + addictive potential is due to the inhibition of which NT and where
inhibition of DA reuptake → increased dopamine in:
basal ganglia (movement)
PFC (planning, problem solving, decision making)
VTA and NAcc (reward + motivation)
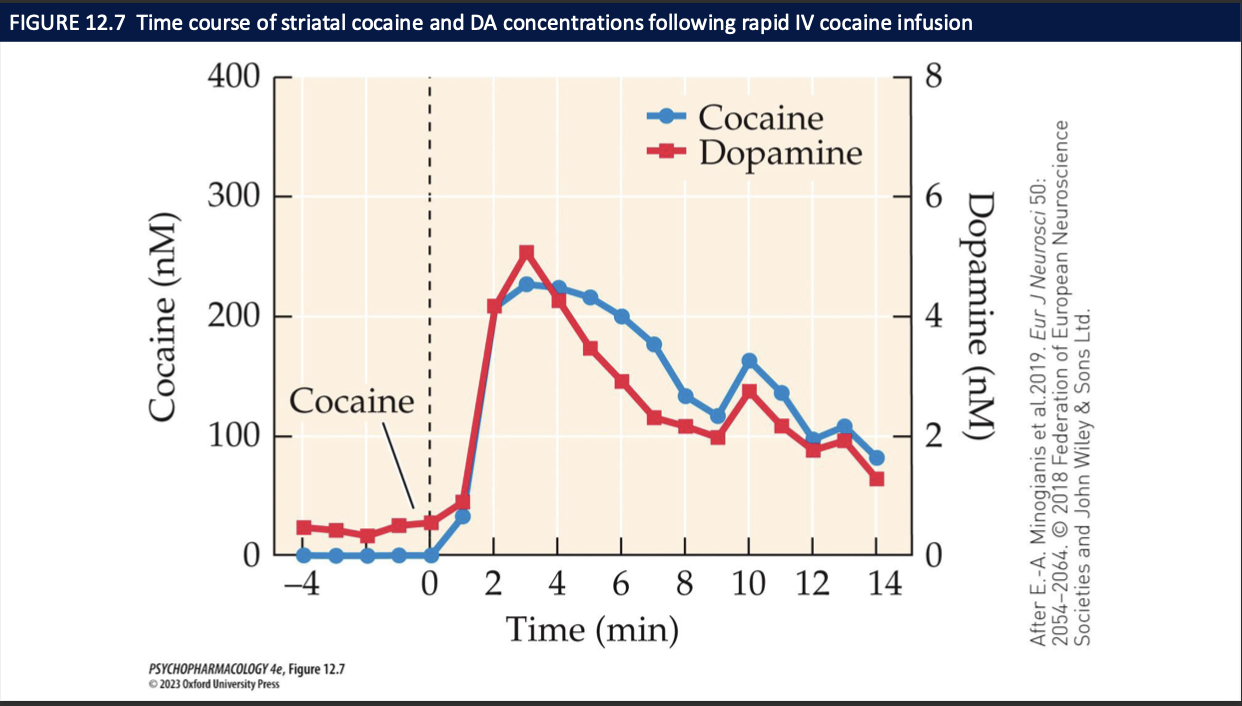
how does cocaine stimulate mood + behaviour (6)
euphoria, elation, arousal, heightened energy + endurance
inflated self esteem + serl-absorption → overconfidence
enhanced physical strength
stereotypies: repetitive + compulsive bhvr
focus more intently + feel mentally sharp but there is a negative effect on cognitive performance
smoked or IV cocaine causes a rush
What are the acute physiological & psychological effects of cocaine (3)
Sympathomimetic: ↑ HR, vasoconstriction, hypertension, hyperthermia
Psych/behavioral: agitation, mania, paranoia, delirium
High-dose risks: seizures, heart failure, stroke, intracranial hemorrhage
Which DA receptors mediate cocaine’s functional effects? (4)
Receptor-selective tools: antagonists & knockout mice
D₁: required for locomotor-stimulating effects;
D₁-KO mice don’t self-administer cocaine → key for reinforcement
D₂/D₃: animals can self-administer; patterns differ from wild-type → contribute, but less critical than D₁
How does experimental cocaine use progress to use disorder? (4)
Most who try don’t progress to misuse
Typical initiation: snorting; some stop due to anxiety on first exposure
Deterrents: cost/availability, legal risk, fear of addiction
Progression: dose escalates and route shifts to crack, freebasing, or IV
What characterizes chronic use patterns and relapse risk? (3)
Cocaine binges: repeated use over hours–days, little/no sleep → withdrawal afterward
Risk factors: psychiatric comorbidity, stress, environmental cues, drug priming
Incubation of craving: craving/relapse increase over time after withdrawal
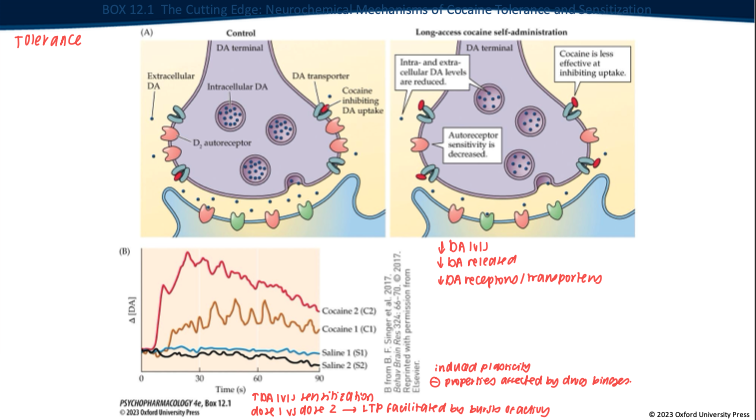
What neurobiological changes occur with chronic cocaine use? (3)
Reduced striatal dopaminergic activity on imaging.
Down-regulation of DA system markers (e.g., DA synthesis capacity, VMAT2, D₂/D₃ receptor availability).
Functional result: tolerance/anhedonia—more drug needed for the same effect, baseline reward tone drops.
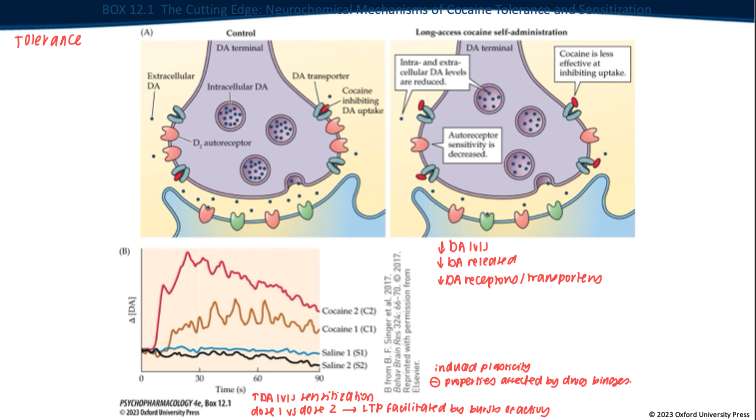
How does chronic cocaine exposure change brain/behavior? What’s the difference between tolerance and sensitization? (4)
Chronic use → neuroadaptations in DA system → behavioral change.
Tolerance: ↓ response with repeated use (need more drug for same effect).
Sensitization: ↑ response to same dose (especially “wanting”/incentive salience).
Expression depends on pattern, context, time since last dose (tolerance → early; sensitization can grow over abstinence).
What are the serious health consequences of repeated/high-dose cocaine use? (4)
Neuro/psych: stroke, seizures, panic attacks, paranoia/psychosis with delusions/hallucinations.
Cardio/resp/renal/GI: arrhythmias, MI, lung injury, kidney injury, GI ischemia.
ENT: septal perforation from chronic snorting.
Pregnancy: fetal growth/neurobehavioral deficits.
What pharmacological strategies are being explored for cocaine use disorder (CUD)? (3)
No FDA-approved meds yet.
Replacement/agonist approaches: full/partial DA agonists or psychostimulants (e.g., amphetamine) as potential maintenance—mixed/limited efficacy.
Vaccines: generate anti-cocaine antibodies to sequester drug in blood or enzyme vaccines to degrade cocaine (ethical/feasibility issues; no approvals).
Which behavioral/psychosocial treatments have evidence for CUD? (4)
Psychosocial programs: individual/group/family counseling.
CBT: cue identification, cognitive restructuring, coping/relapse-prevention skills.
12-step programs: Narcotics/Cocaine Anonymous—peer support/accountability.
Contingency management: voucher/prize-based reinforcement for abstinence—strong evidence, often best when combined with CBT.
Idea behind cocaine chemogenetics to blunt drug seeking?
Engineer ligand-gated ion channels that are opened by cocaine → drug-activated brake.
Where are channels expressed and what happens when cocaine is taken?
Express selectively in reinforcement circuits (e.g., VTA→NAc, lateral habenula).
Cocaine opens channel → hyperpolarizes/suppresses target neurons → ↓ DA surge, ↓ self-admin.
Why doesn’t it kill normal motivation?
Circuit-specific expression → natural rewards (food/sex) largely spared.
What receptor “backbones” are often used to build cocaine-gated channels and what’s the goal of the mutations? (2)
Chimeras based on α7 nicotinic AChR / 5-HT3–like ligand-gated channels with binding-site mutations.
Mutations shift agonist specificity so cocaine (not ACh) opens the channel → selective neuronal silencing during cocaine exposure.
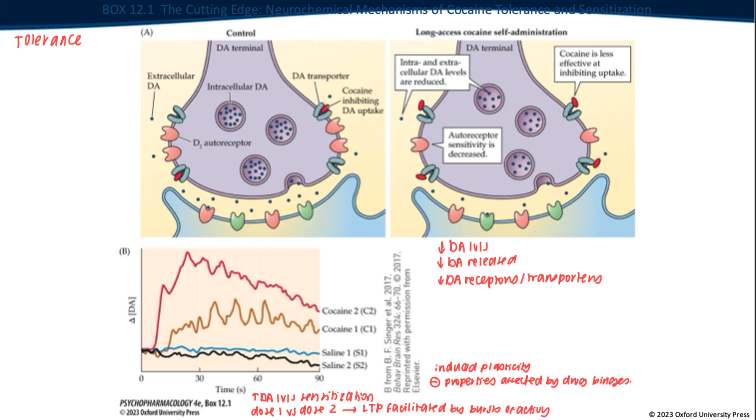
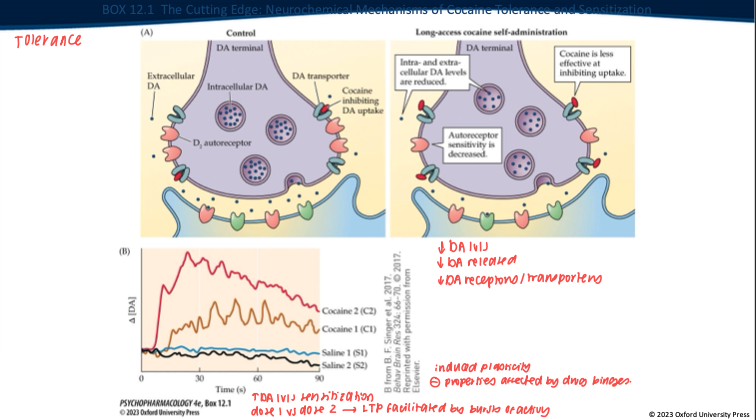
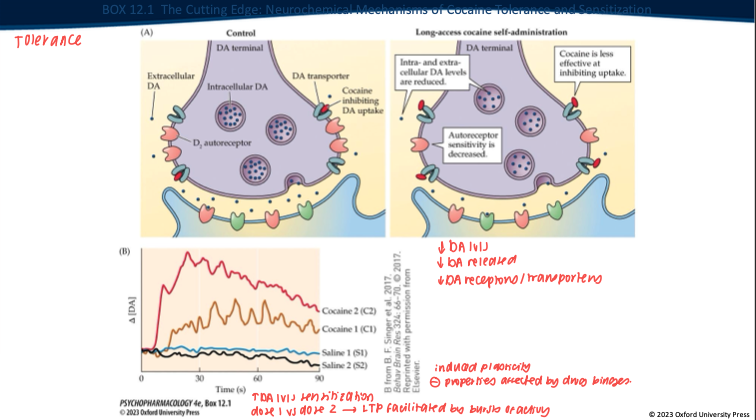
Repeated long-access cocaine produces what DA-synapse changes? (5)
Less DA made/stored → overall DA levels drop.
Nerve terminal releases less DA when it fires.
DAT isn’t blocked as well by cocaine anymore (transporter adapted).
D₂ autoreceptors change (often less responsive).
Result: the same dose gives a smaller DA rise → tolerance.
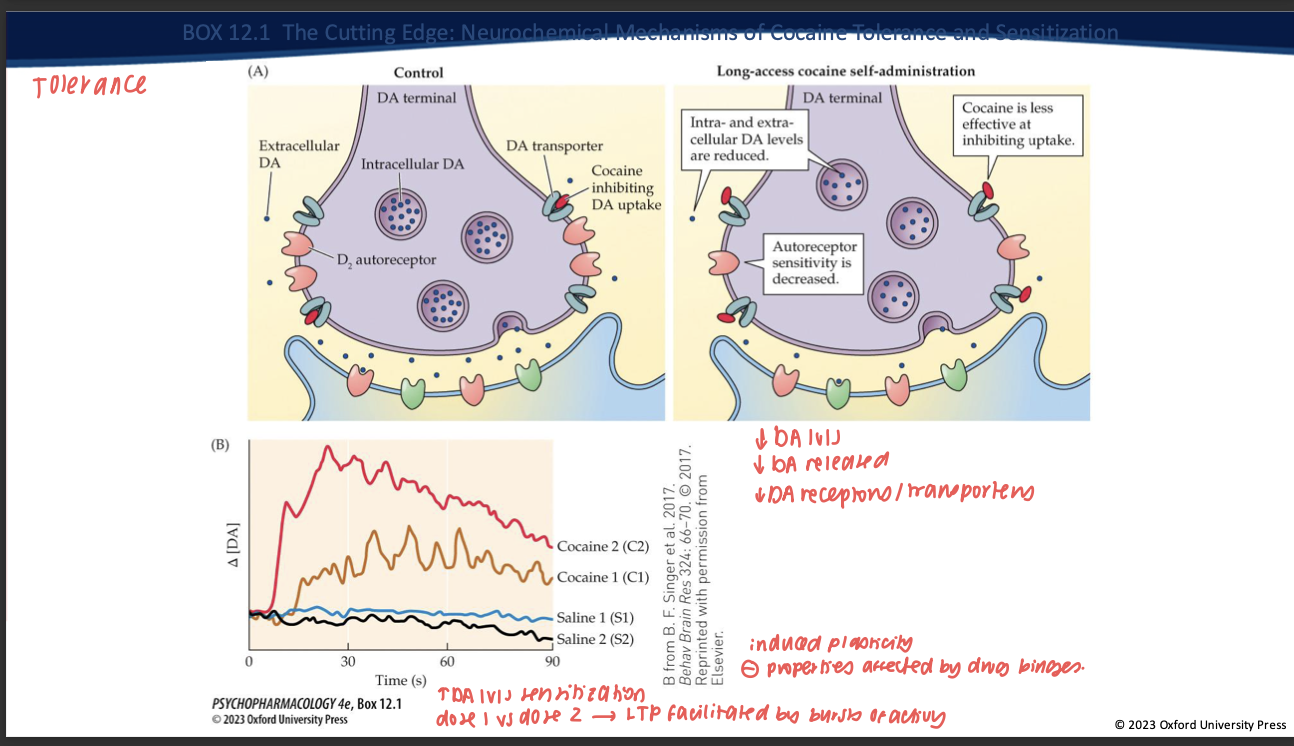
Contrast tolerance vs sensitization with intermittent exposure. (2)
Tolerance: ↓ DA response to a given dose with continuous/high access.
Sensitization: with intermittent/spaced dosing + abstinence → VTA LTP, ↑ burst firing, enhanced cue reactivity → ↑ “wanting” (craving) even if “liking” doesn’t grow.
What is incubation of craving and which process does it align with? (2)
Time-dependent increase in cue-evoked craving during abstinence.
Reflects sensitization-related plasticity (mesolimbic adaptations) rather than tolerance.
amphetamines (4)
globally more popular than cocaine
more potent + sustained effects
administered by several routes
easily + inexpensively synthesized
prevalence of use of amphetamines (4)
27 million ppl worldwide use amphetamine-type stimulants
used to treat ADHD
methamphetamine illegal in Canada
prescription stimulants: Adderall, Vyvanse, Ritalin
forms of amphetamines → L vs R (3)
amphetamine (benzedrine): mixture of left and right-handed molecules (chiral molecules)
left-handed derivatives (levo-amphetamine): raises bp, opens nasal passages, causes headaches, no mood-elevating effects
right-handed derivatives (dextro-amphetamine) → stronger effects in the brain, elevates mood, enhances energy ie. dexedrine
methamphetamines (6)
a methyl group is added to dextro-amphetamine (right-handed)
increases lipid solubulity + potency
better able to penetrate BBB
chemicals to produce meth are readily available → corrosive, highly flammable, toxic
pseudoephedrine/ephedrine in OTC medications are used as the precursor
sales restricted in US
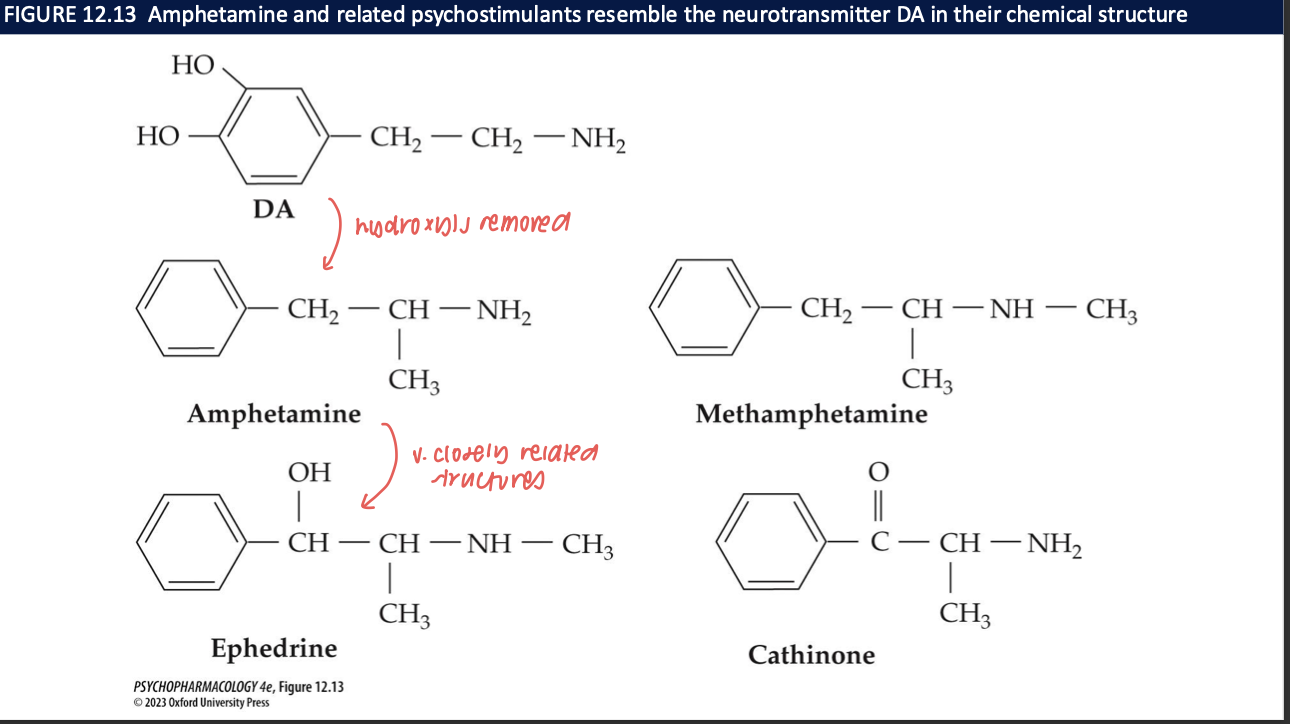
structural differences between DA and amphetamines and ephedrine
DA → remove hydroxyls and add methyl = amphetamines add a methyl = methamphetamines
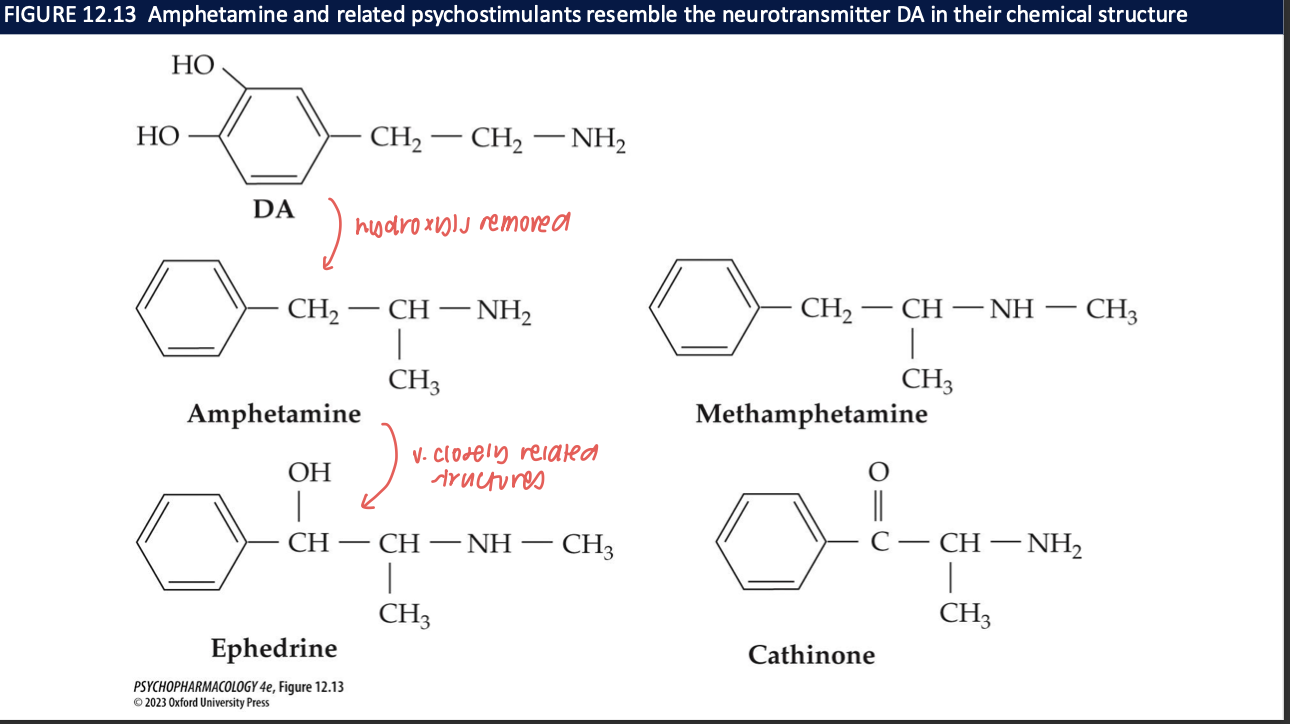
mechanism of action of amphetamines (4)
increases postsynaptic lvls of DA, NE, 5-HT to a greater degree than cocaine by:
blocking reuptake by binding to transporter proteins
increasing release
at high doses, inhibiting MAO → preventing breakdown of catecholamines
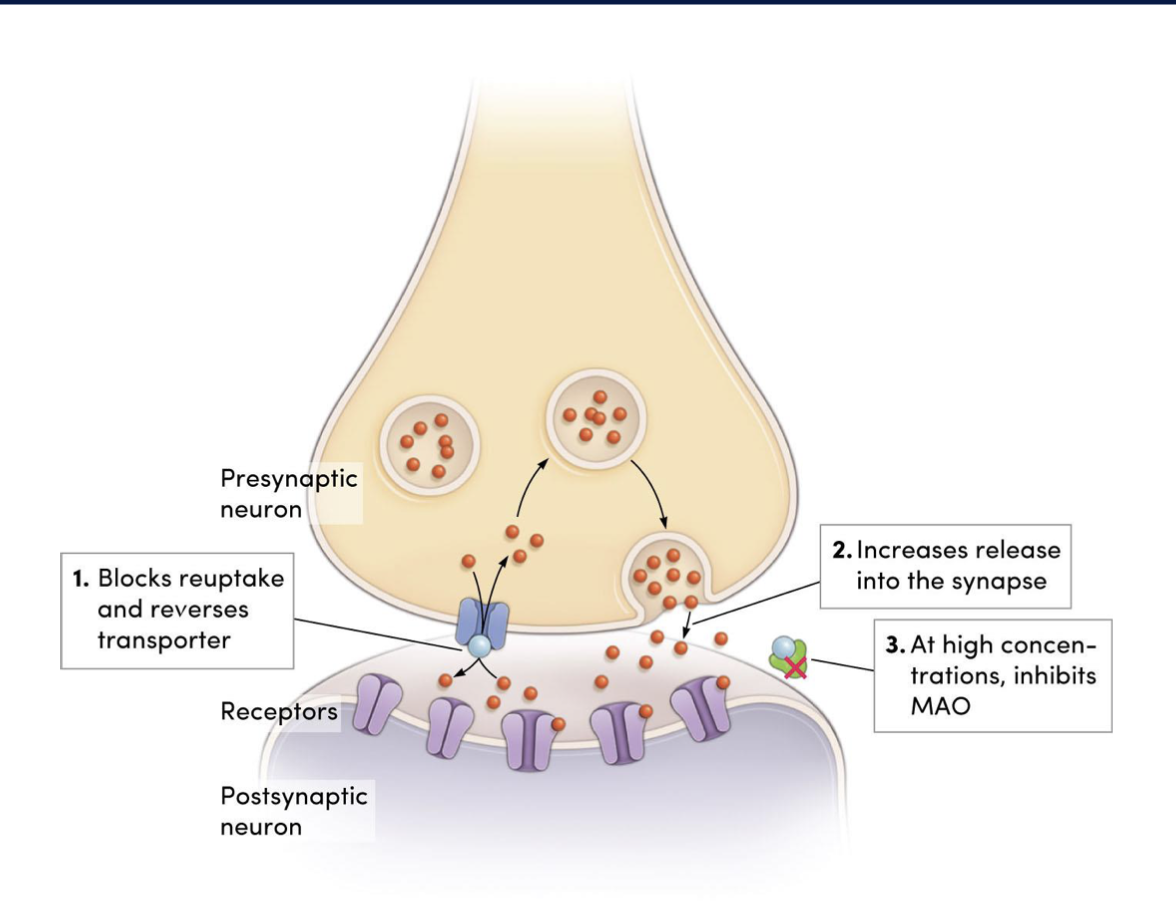
high lvls of amphetamines lead to high lvls of catecholamines resulting in (3)
↑NE: sympathomimetic effects
↑5-HT: delusions + perceptual disturbances
↑DA: locomotor effects, psychotic side effects, reinforcing effects
acute + adverse effects of amphetamines
physiologically + psychologically similar effects to that of cocaine
slower onset of effects + longer duration
side effects + withdrawal seen w amphetamines are qualitatively similar to those of cocaine
medical + therapeutic uses of amphetamines (3)
approved to treat ADHD
narcolepsy: recurring + irresistible attacks of sleepiness during daytime hours, cataplexy, hypnagogic hallucinations, sleep paralysis
short-term weight reduction: not anymore bc of high abuse potential
What are the major consequences of chronic amphetamine use? (9)
Tolerance develops with repeated use.
Skin & dental problems (“meth mouth,” sores).
Cardiovascular: cardiac disease, hypertension; risk of sudden death.
Cerebral hemorrhage (stroke risk).
Renal (kidney) damage.
Seizures.
Amphetamine psychosis (paranoia, hallucinations).
↑ Psychiatric risk: mood/anxiety disorders, addiction.
High doses—esp. methamphetamine: persistent/possibly irreversible neurotoxicity to dopaminergic & serotonergic terminals → long-term cognitive/affective changes.
What are the key administration routes and addiction risks for amphetamines vs methamphetamine? (4)
Amphetamine: taken orally, IV, or subcutaneous (“skin popping”).
Oral absorption is slow; IV gives rapid, intense “high” → higher addiction risk.
Methamphetamine is more potent; can be oral, snorted, IV, or smoked.
Crystal meth (“ice”) = methamphetamine HCl in smokeable crystalline form; highly addictive.
What patterns of use and pharmacokinetics characterize amphetamines? (3)
Binges/runs: repeated injections every ~2 h for 3–6 days, little sleep/food.
Metabolism: slow hepatic metabolism; metabolites excreted in urine.
Long half-lives → a much longer-lasting high from a single dose vs cocaine.
How do amphetamines increase synaptic DA (± NE/5-HT)? (4)
Enter presynaptic terminal (via DAT) and displace DA from vesicles by acting on VMAT2 → ↑ cytosolic DA.
Trigger reverse transport through DAT → non-vesicular DA efflux into synapse.
Kinase signaling (CaMKII/PKC) and Ca²⁺ facilitate DAT phosphorylation → promotes reverse transport.
Net effect: large, rapid DA surge (and at high doses, NE/5-HT too).
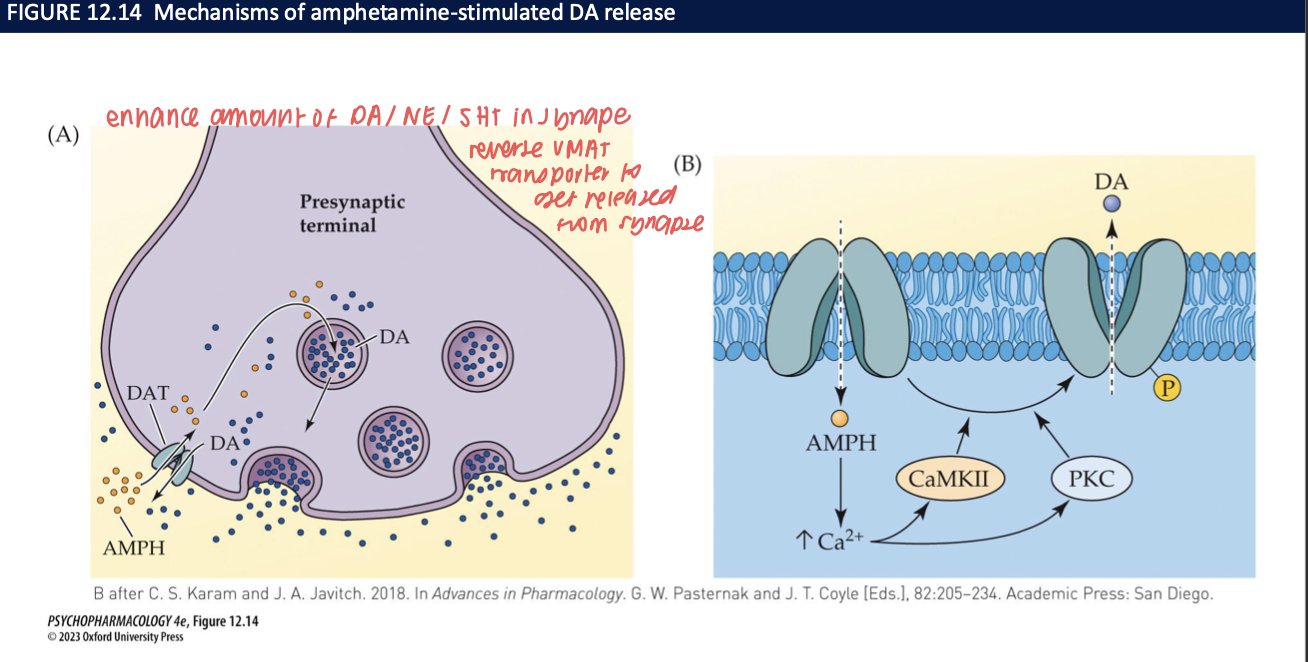
neurobehavioural effects of amphetamines (3)
cause heightened alertness, increased confidence, reduced fatigue, generalized sense of well-being
reduced sleep time, esp. REM, permits sustained physical effort w/o rest or sleep
can enhance athletic performance → banned in competition
how does withdrawal syndrome differ between opioids/alcohol and amphetamines? (3)
withdrawal syndrome less sever than w opioids/alcohol
negative reinforcement has less impact
women more likely to be dependent
where do meth uses show impairments? (5)
multiple cognitive domains
impulse control
verbal learning
working memory
social cognition
What major medical and neurobehavioral risks are linked to high-dose or chronic amphetamine/methamphetamine use? (4)
Psychosis: meth use can precipitate persistent psychotic state (schizophrenia-like) that can last after abstinence.
Neurotoxicity: damage/loss of DA neurons → long-term cognitive/affective deficits; ↑ risk of Parkinson’s disease.
Cardiometabolic & GI: cardiovascular disease, stroke, GI distress; infections due to poor health/immune compromise.
Other systemic: oral disease (“meth mouth”), male sexual dysfunction, premature aging, ↑ mortality.
3,4-methylenedioxymethamphetamine (MDMA) (4)
synthetic amphetamine derivative
entactogen/empathogen → enhances social reward
intermediate profile btw stimulants + hallucinogens
clinical trials: MDMA-assisted psychotherapy for PTSD
what are the primary targets of MDMA and what are the bhvrl effects? (6)
primary target: monoamine transporters
SERT reversed: massive 5-HT release
DAT reversed: moderate DA release
NET reversed: NE release
VMAT2: promotes vesicular monamine realse
leads to mood elevation, empathy, arousal
acute subjective effects of MDMA (3)
euphoria, empathy, emotional openness
enhanced sensory perception → innervation of cortex
increased sociability + energy
acute physiological effects of MDMA(4)
tachycardia, hyperthermia, jaw clenching
risk of dehydration or hyponatremia
duration of effect ~3-6 hours
onset: ~30-60 mins
short-term neurochemical + bhvrl effect of MDMA(2)
serotonin surge → mood elevation + sensory enhancement
oxytocin release → social bonding
long-term neurochemical + bhvrl effect of MDMA (3)
serotonin neurotoxicity → ↑ doses over long-term = cell death
sleep, mood, memory impairments
rebound depression (mid-week crash) → withdrawal effects → Tuesday suicide
ongoing MDMA investigations (3)
MDMA-assisted therapy for PTSD (phase III trials)
potential uses in depression + social anxiety
mechanistic studies on oxytocin + fear extinction
caution with MDMA (2)
neurotoxicity + serotonergic depletion remain concerns
controlled clinical environments essential for safe use
cathinone (khat) + methcathinone (cat)
khat:
leaves of a shrub in East Africa → used for centuries
one of the most popular stimulants worldwide
structurally + functionally similar to amphetamine + DA
cat: synthetic variant → more potent
schedule III drugs
bath salts (4)
The name derives from instances in which the drugs were disguised as bath salts.
synthetic derivatives of cathinone
can produce devastating physical + psychological effects
mephedrone, methylone, MDPV → schedule III drugs