Nucleophilic Properties of Amines
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
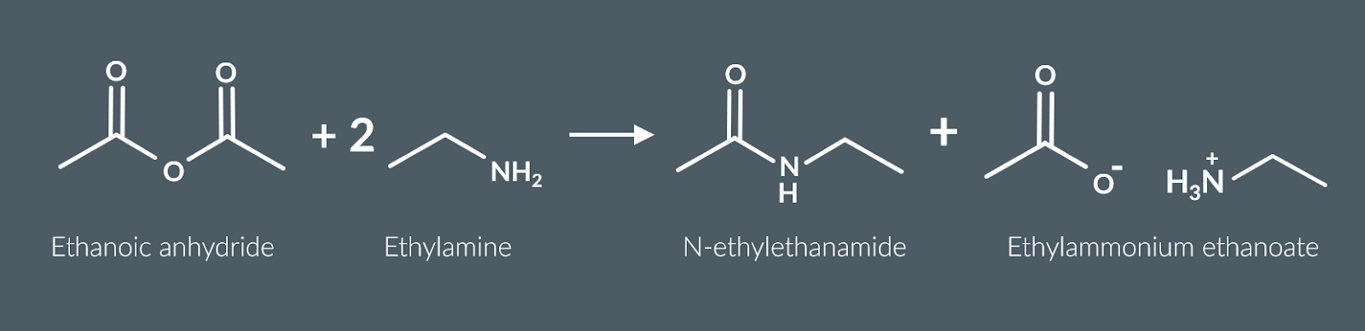
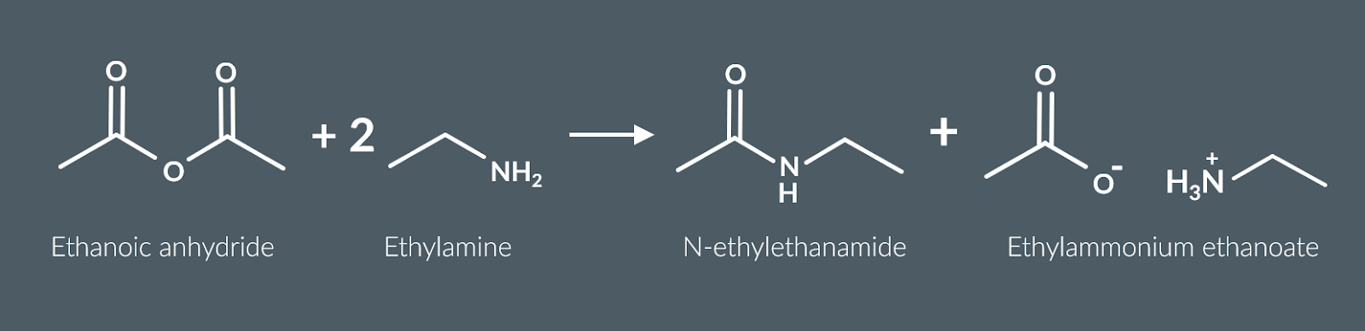
primary amines react with acid anyhydrides to form
a secondary amide and an ammonium salt
Primary amines can act as
nucleophile in nucleophilic addition-elimination reactions.
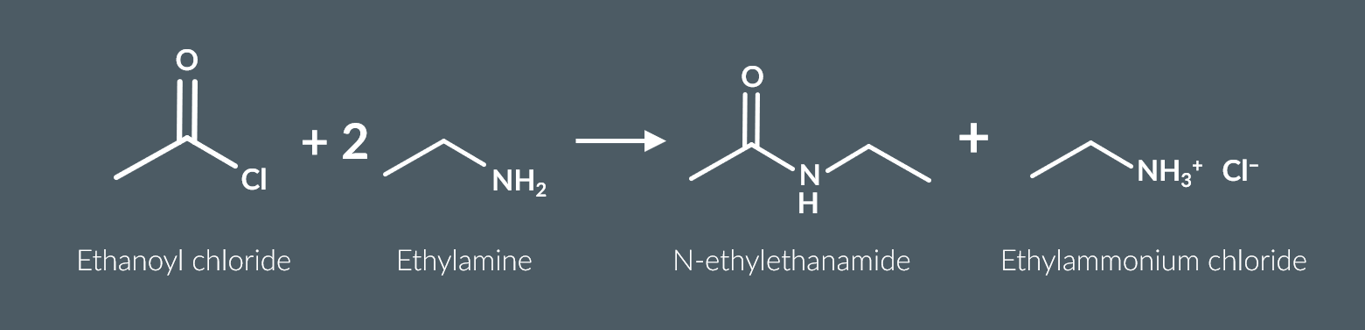
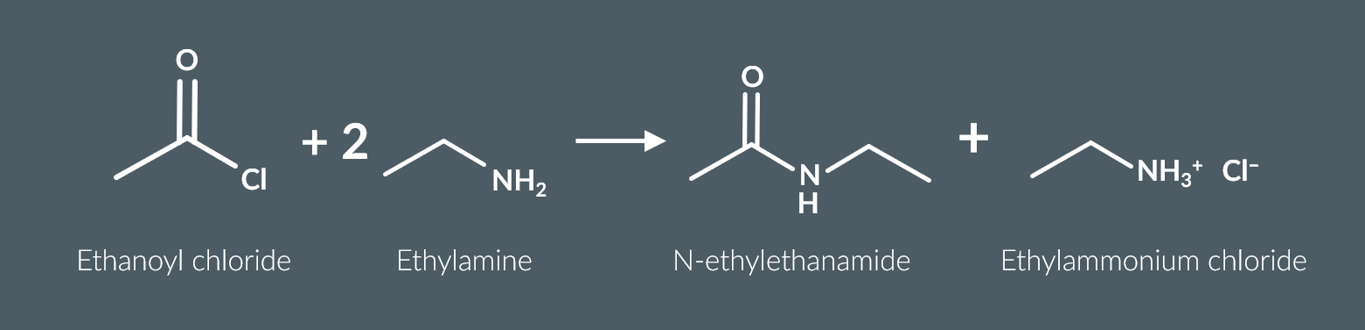
primary amines also react with acyl chlorides to form
a secondary amide and an ammonium salt
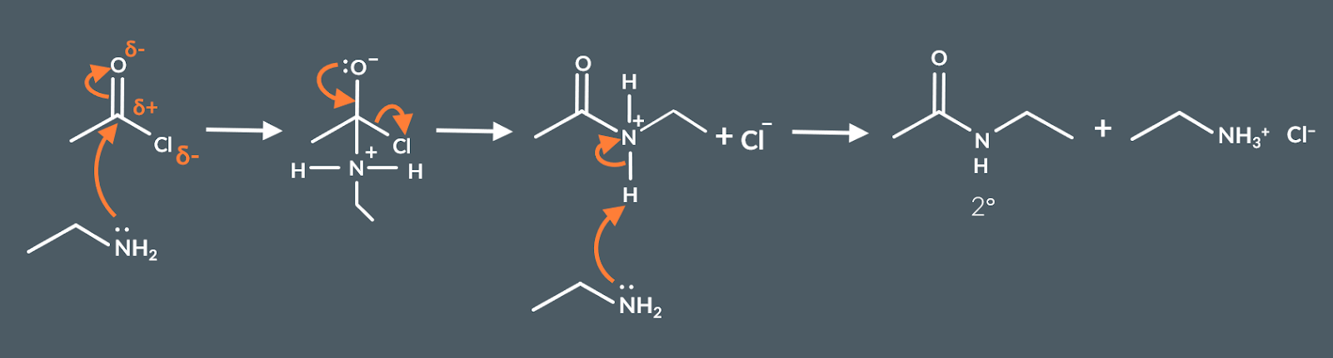
Mechanisms
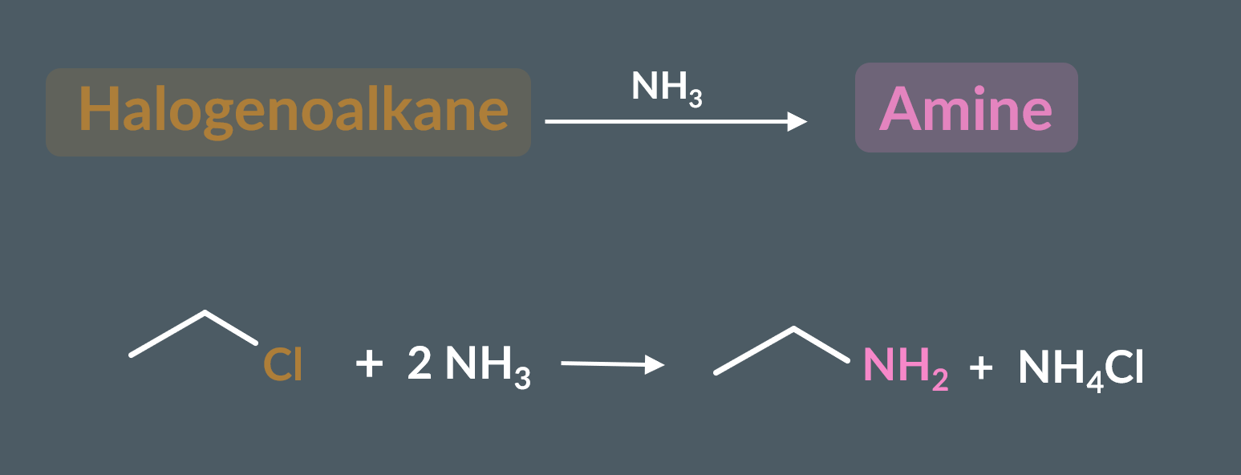
ammonia and halogenoalkanes undergo….. to produce an amine.
what is needed
nucleophilic substitution reaction
However, if there is excess halogenoalkane when ammoni and halgeonoalkanes react what happens
the primary amine can also act as a nucleophile. This means it can react again to produce a secondary amine.
That secondary amine can then act as a nucleophile, and so on
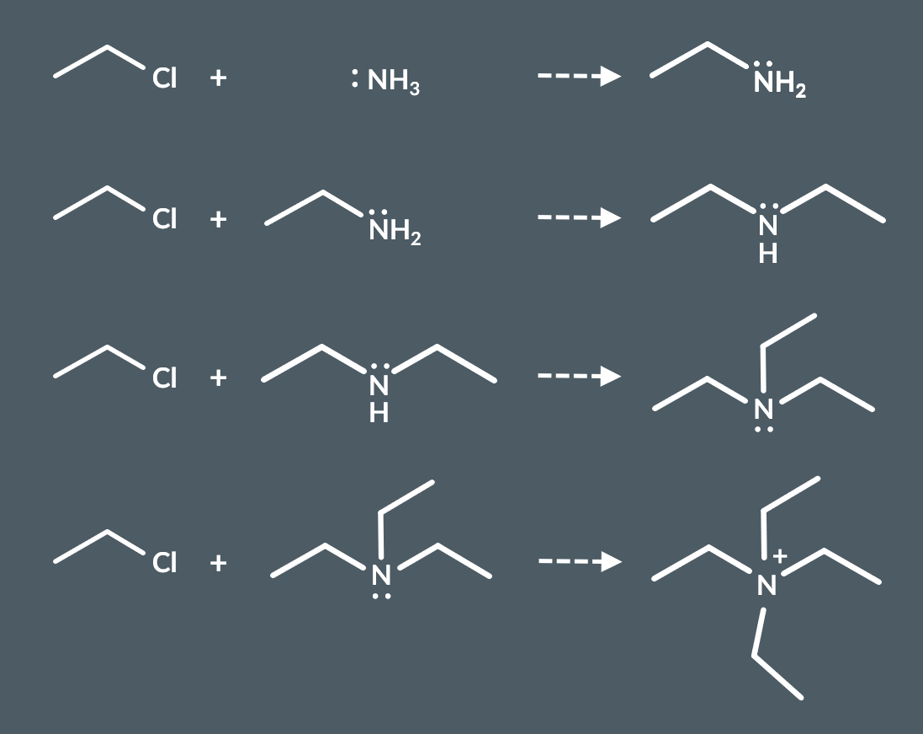
a quaternary ammonium ion.
When nitrogen is bonded to four alkyl groups
quaternary ammonium salt.
When quaternary ammonium ions form a lattice with anions
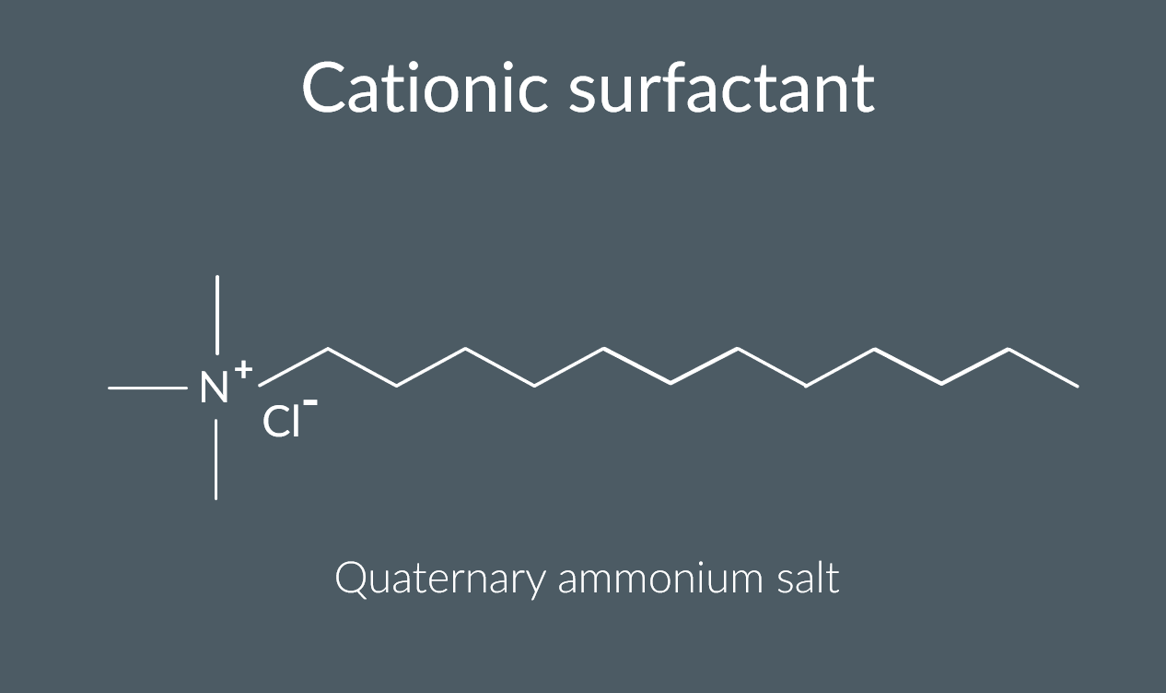
When a quaternary ammonium ion has one long alkyl chain, what are the ends
it has a polar end and a non-polar end.
quaternary ammonium ion useful properties:
it’s often used in cleaning products, as a cationic surfactant.