Chapter 2.1-2.6: The Molecules of Life - Chemistry
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
why is there chemistry in biology class?
biology studies living things - all living things are made up of chemicals
- chemistry helps us understand at the cellular and molecular level
matter
anything and everything that takes up space
elements
substances that cannot be chemically broken down - contain only one kind of atom
atoms
the basic unit of matter
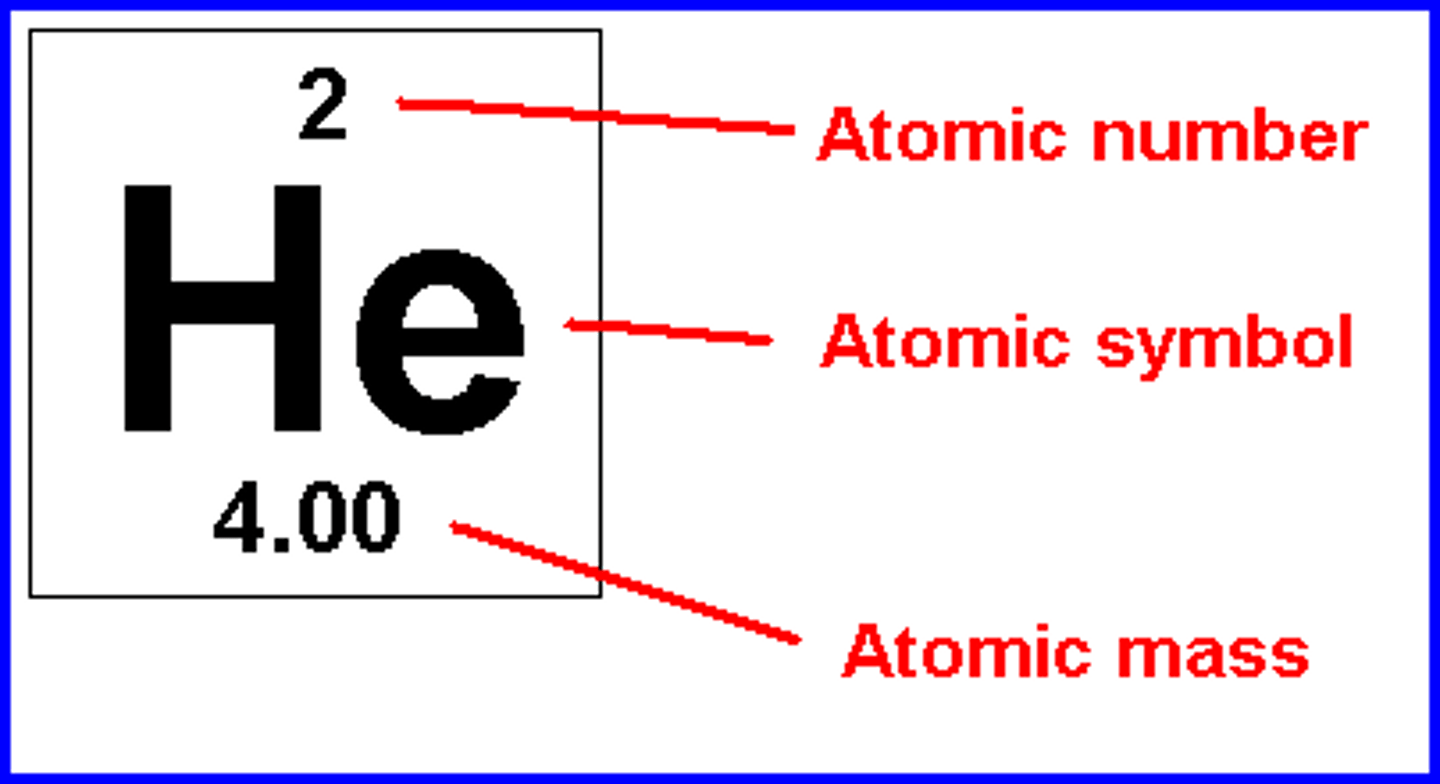
atomic number
# of protons (elements defined by them)
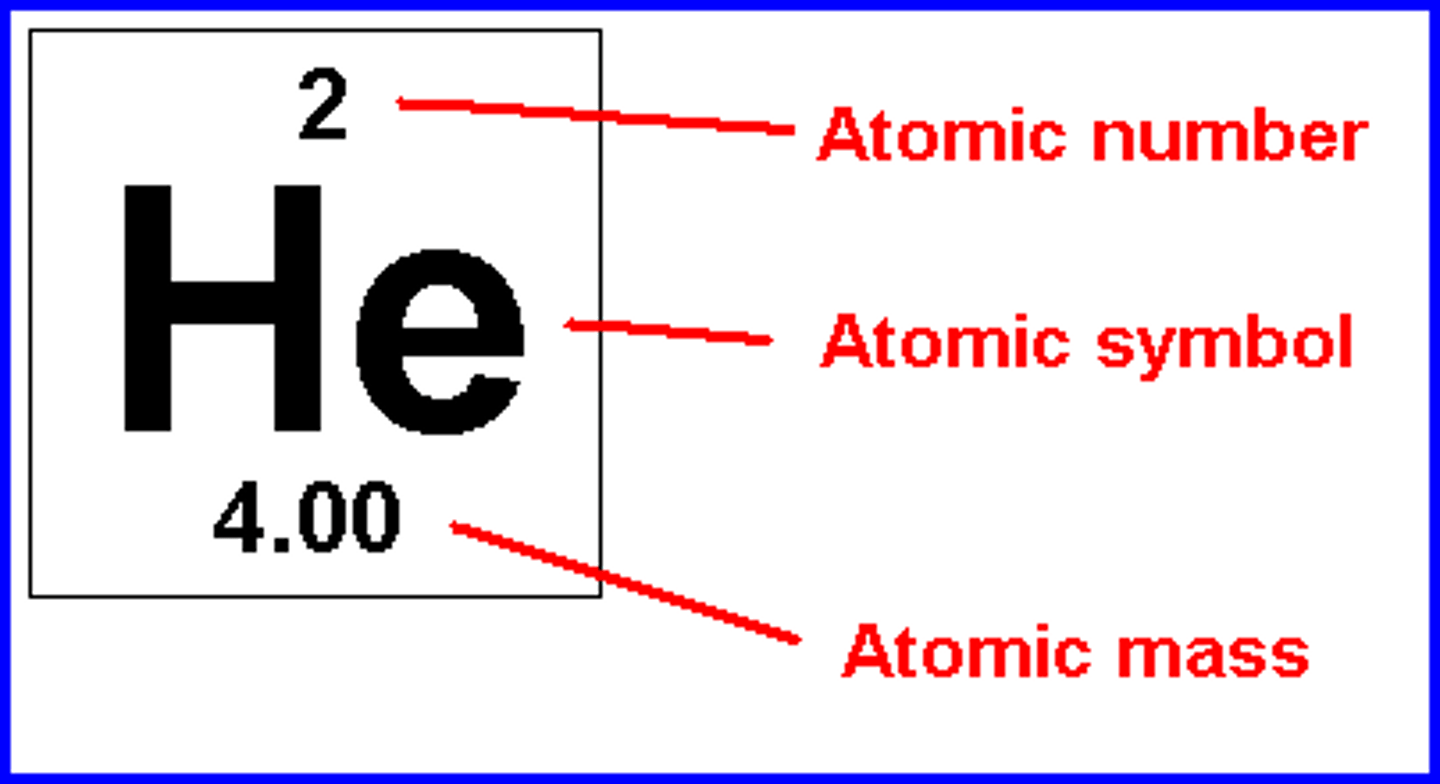
atomic mass
# of protons + neutrons
isotopes
atoms of the same element that have different # of neutrons (proton # stays the same)
ions
atoms with the same # of protons and neutrons are neutral
- some chemical processes result in the loss or gain of electrons
if an atom gains or loses an electron,...
then it can carry a charge
neutral
6 protons
6 neutrons
6 electrons
negatively charged
5 protons
6 neutrons
6 electrons
positively charged
6 protons
6 neutrons
5 electrons
shells
represent energy levels or electron clouds surrounding the atomic nucleus
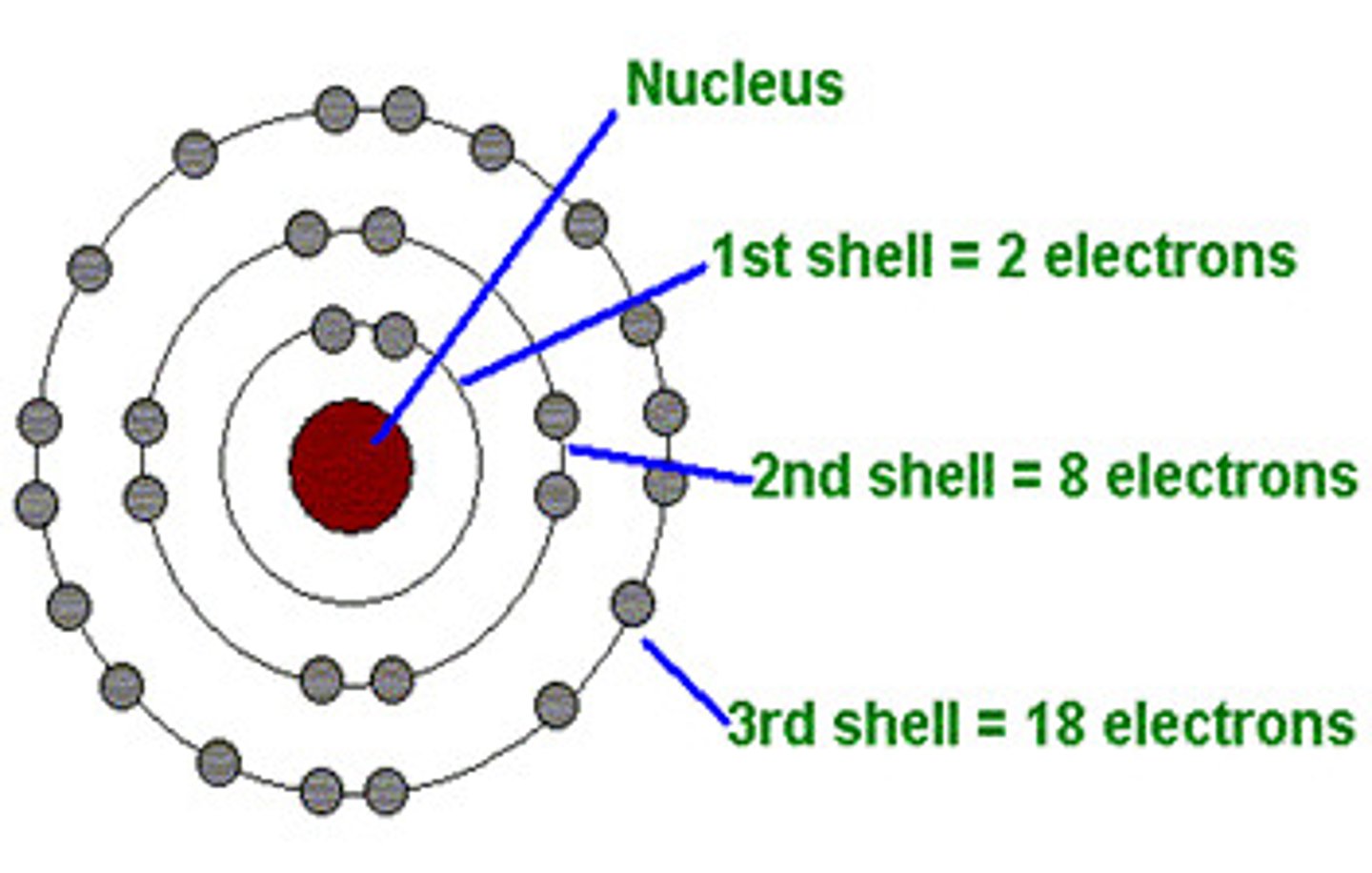
orbitals
describe the most probable regions where electrons can be found within a given shell (max # is 2)
periodic table
elements in the same row have the same # and types of orbitals
- to the right, the atomic # increases
chemical bonds
atoms combine via chemical bonds to form molecules
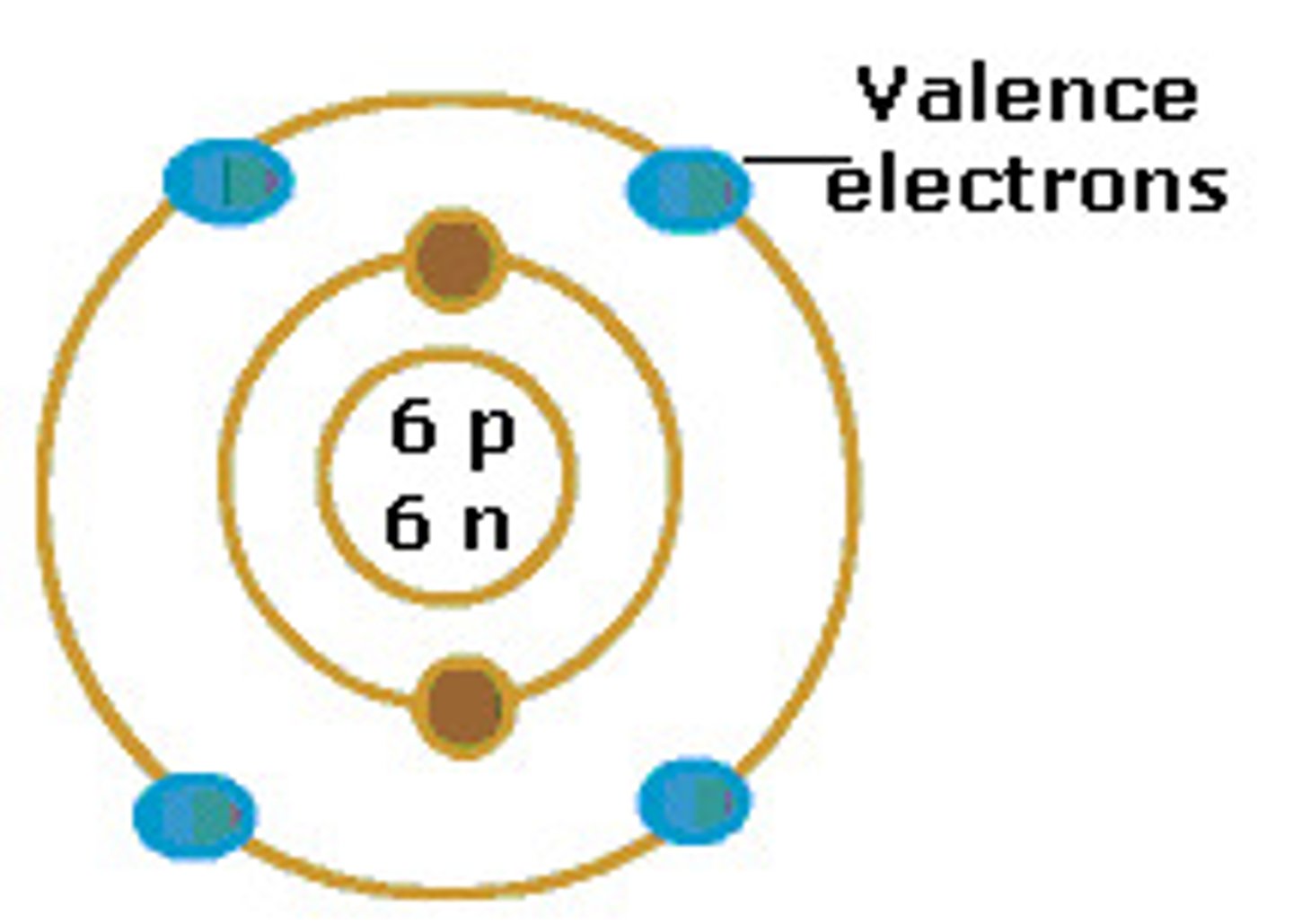
valence electrons
an electron in the outer shell of an atom, which can participate in the formation of a chemical bond
covalent bonds
a chemical bond formed by a shared pair of electrons holding two different atoms together
electronegativity
the property of an atom (in a given chemical element) to attract shred electrons
- as positive protons increase, electrons are held more closely to nucleus
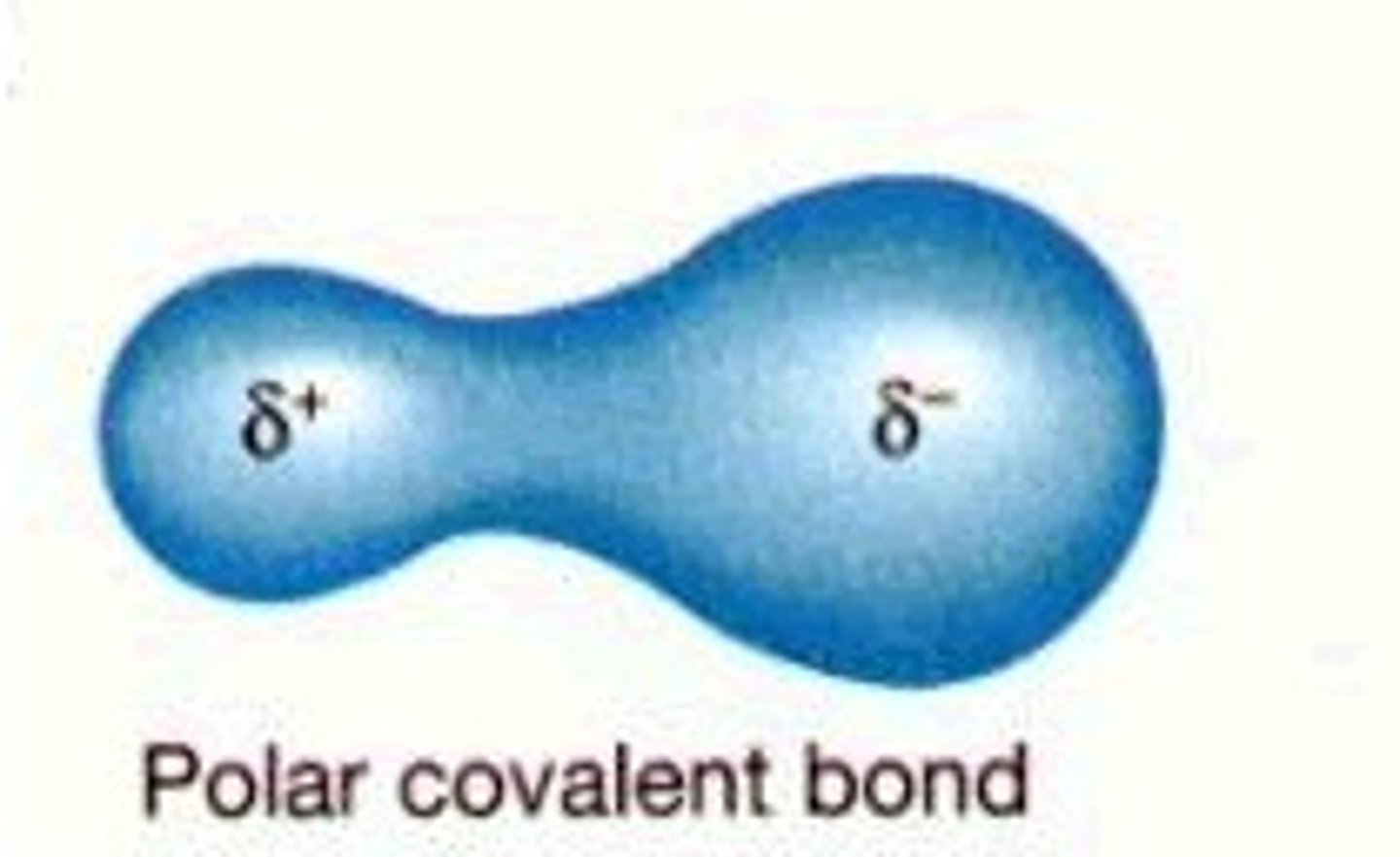
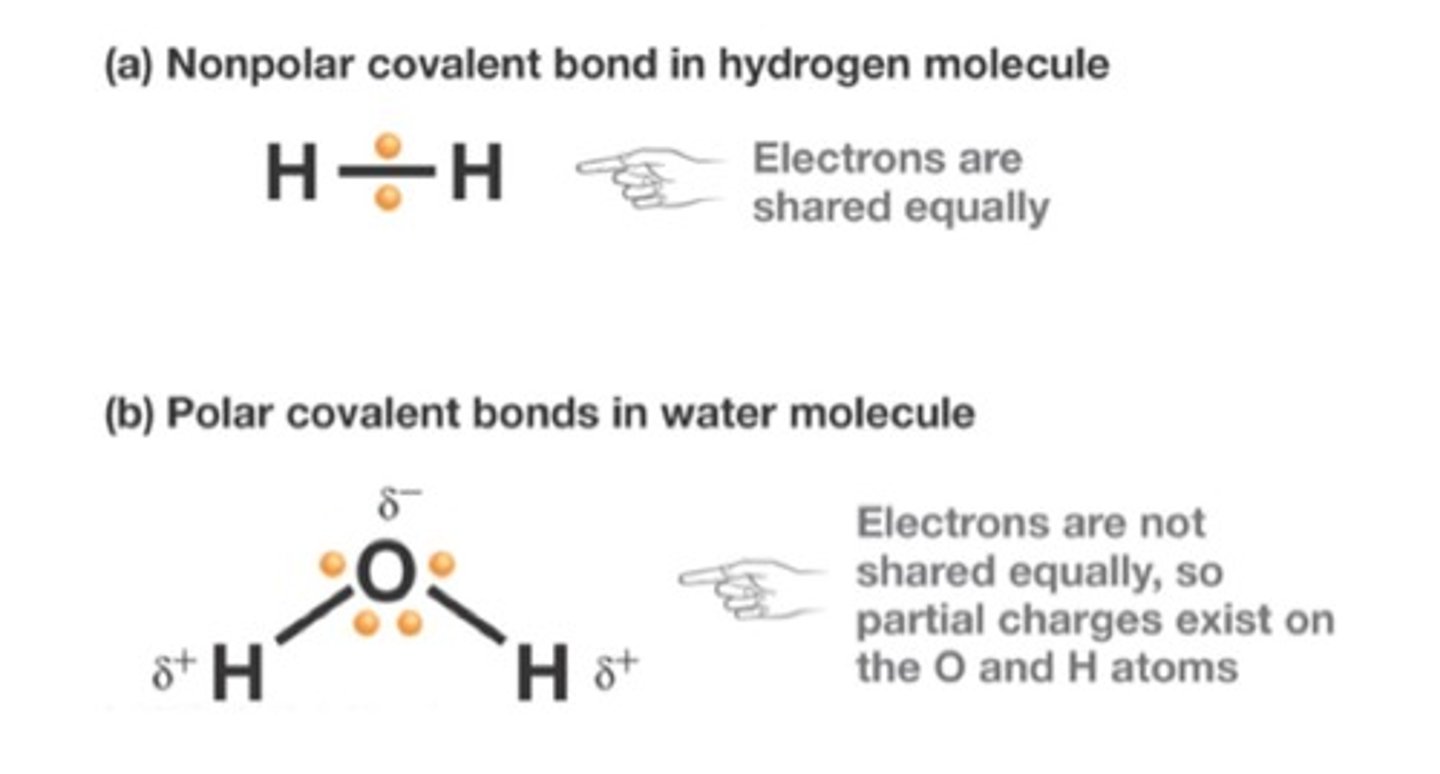
polar covalent bonds
electrons are not equally shared between 2 atoms
- results in partial negative charges on the oxygen and partial positive charges on the hydrogen
nonpolar covalent bonds
form when atoms share electrons equally
- same electronegativity
if an atom gains or loses an electron,...
then it can carry a charge
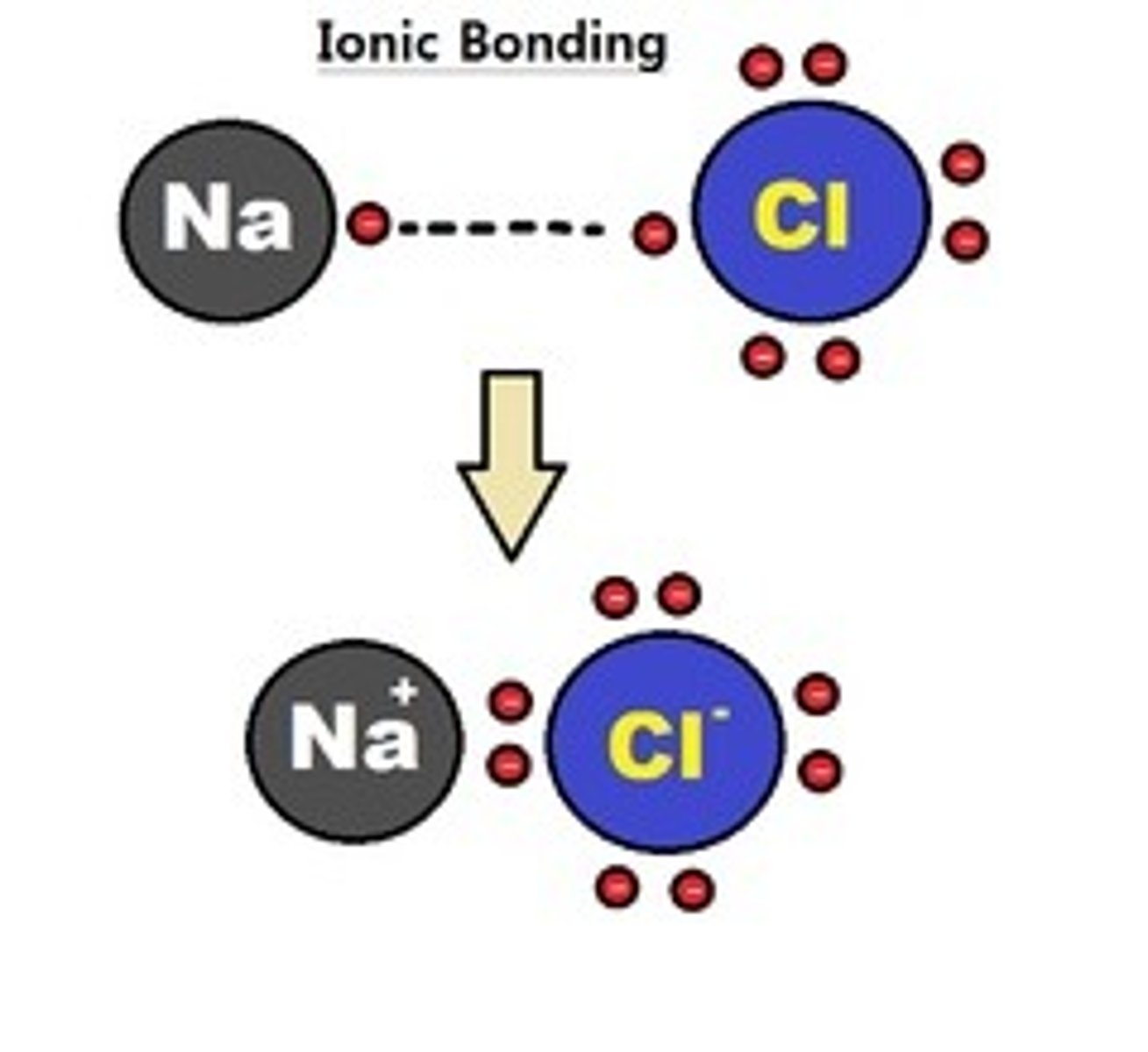
ionic bonds
a chemical bond in which two ions with opposite charges associate with each other due to their difference in electronegativity
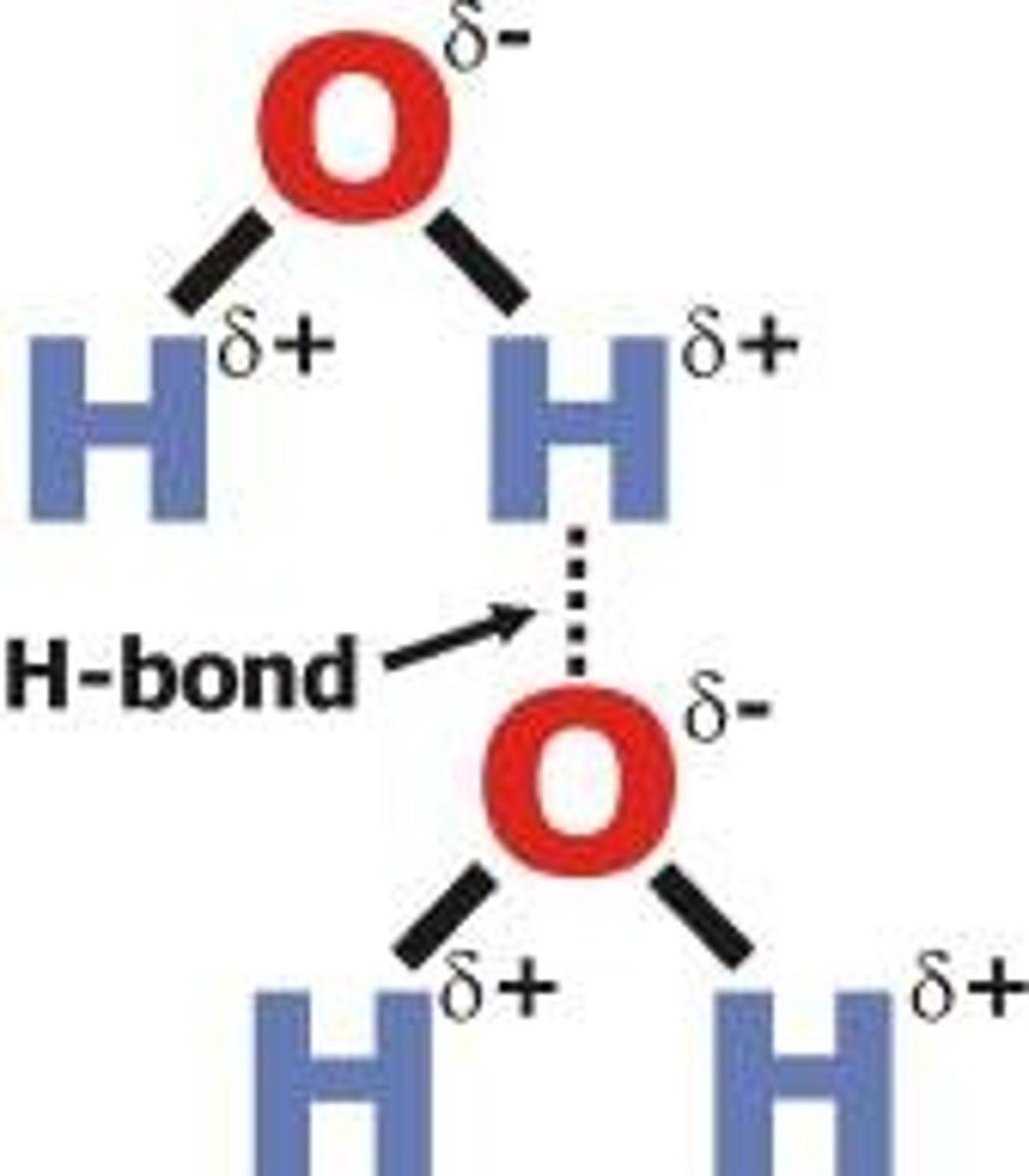
hydrogen bond
forms between two water molecules when the partial positive charge of a hydrogen atom is attracted to the partial negative charge of an oxygen atom
- in liquid water, H-bonds break and reform
cohesion
the attraction between molecules
adhesion
the attraction between molecules and a surface
what is water's unusual property?
water is less dense when solid
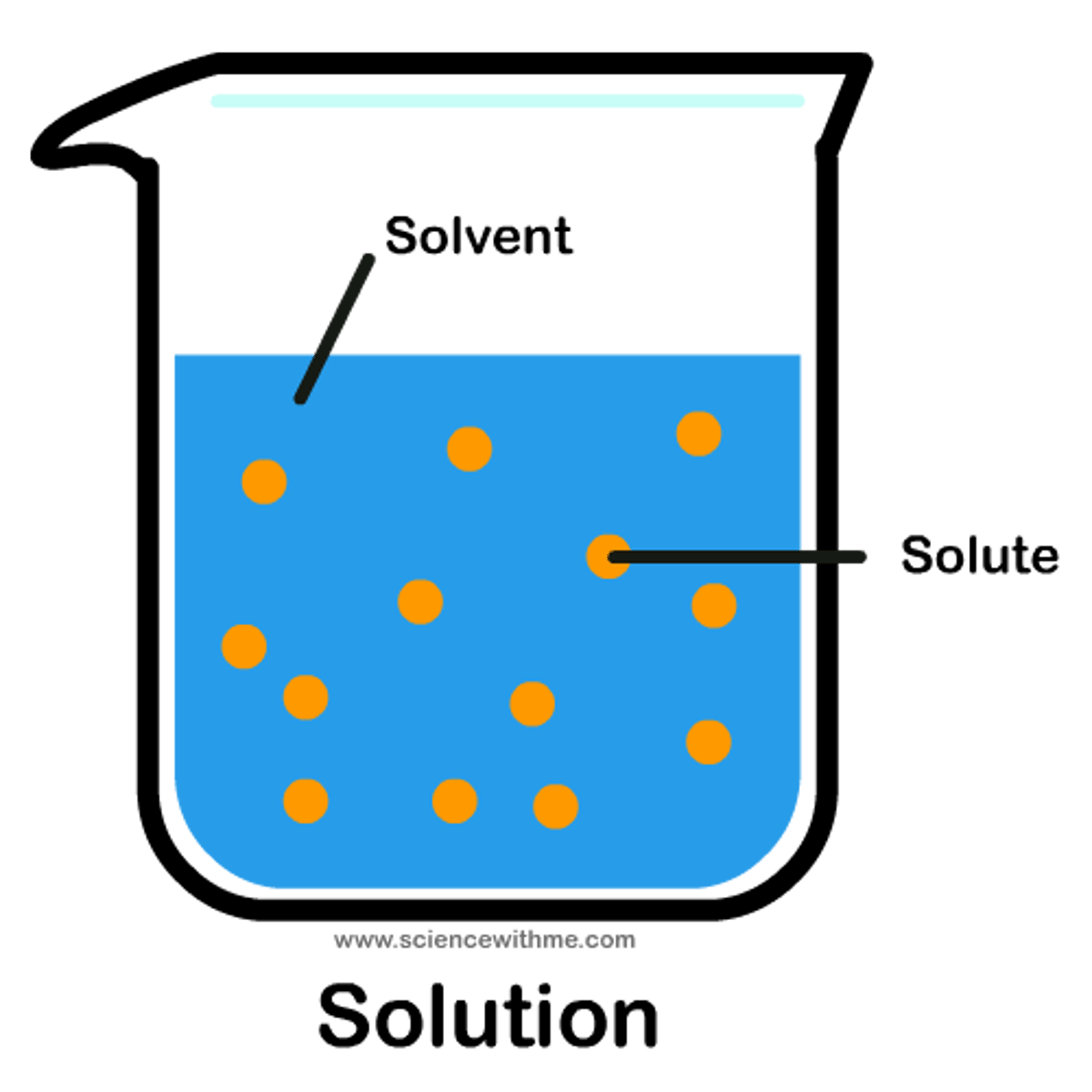
solvent
a liquid capable of dissolving a substance
solute
a dissolved substance
solution
the mixture of solute and solvent
hydrophilic
"water loving"; describes a class of molecules with which water can undergo hydrogen bonding
hydrophobic
"water fearing"; describes a class of molecules poorly able to undergo hydrogen bonding with water
pH
a measure of the concentration of hydrogen ions (H+) in a solution
- ranges from 0 to 14
- a measurement of the concentration of protons in a solution
- pH in most cells is approximately 7 and is tightly regulated because most chemical reactions can be carried out only in a narrow pH range
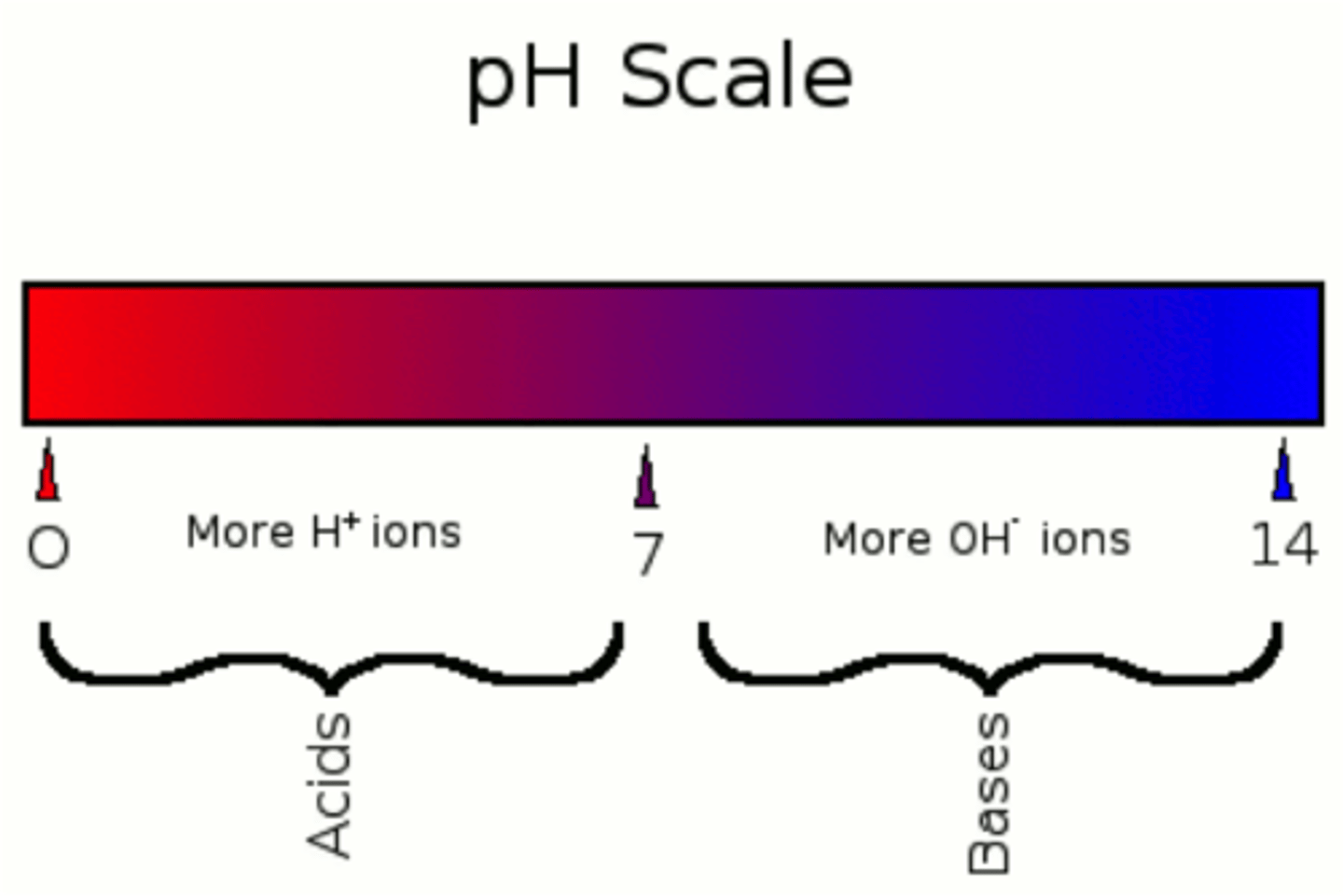
acids
higher concentration of hydrogen ions (H+) and a pH closer to 0 (less than 7)
bases
lower concentration of hydrogen ions (H+) and a pH closer to 14 (greater than 7)
neutral
when the concentrations of protons (H+) and hydroxide ions (OH-) are equal
properties of water
1. polar (regions of partial positive and partial negative charges)
2. cohesive (hydrogen bonding between water molecules)
3. adhesive (hydrogen bonding between water and other molecules)
4. high surface tension (extensive hydrogen bonding on the surface of liquid water)
5. less dense as a solid than liquid (open, crystalline structure of solid water)
6. high specific heat (extensive hydrogen bonding of liquid water)
7. good solvent (hydrogen bonding with other polar molecules)
cohesion via hydrogen bonding
the tendency of water molecules to stick to one another
top four elements in humans
1. carbon
2. oxygen
3. hydrogen
4. nitrogen
(excluding water)
organic molecules
molecules containing carbon
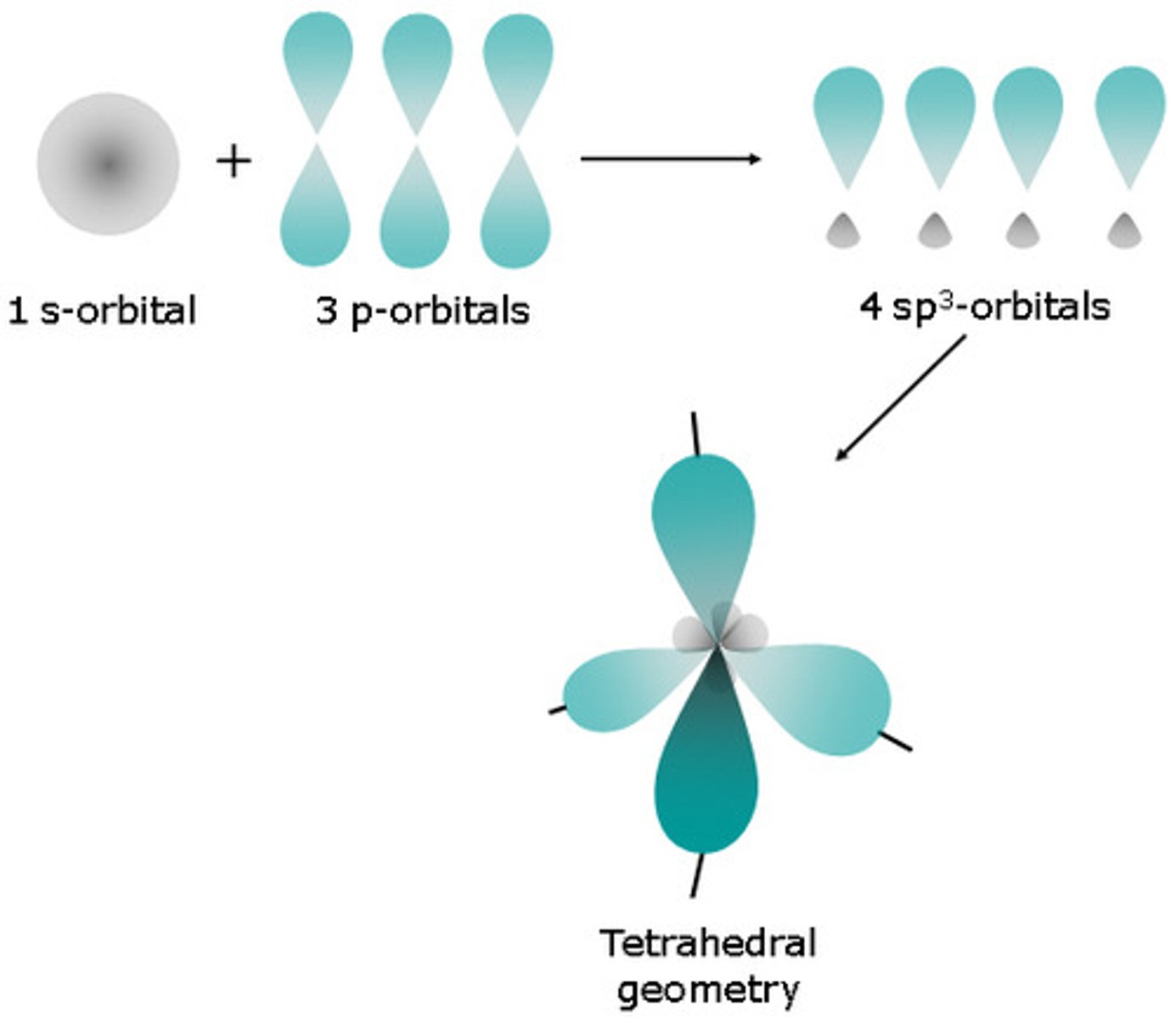
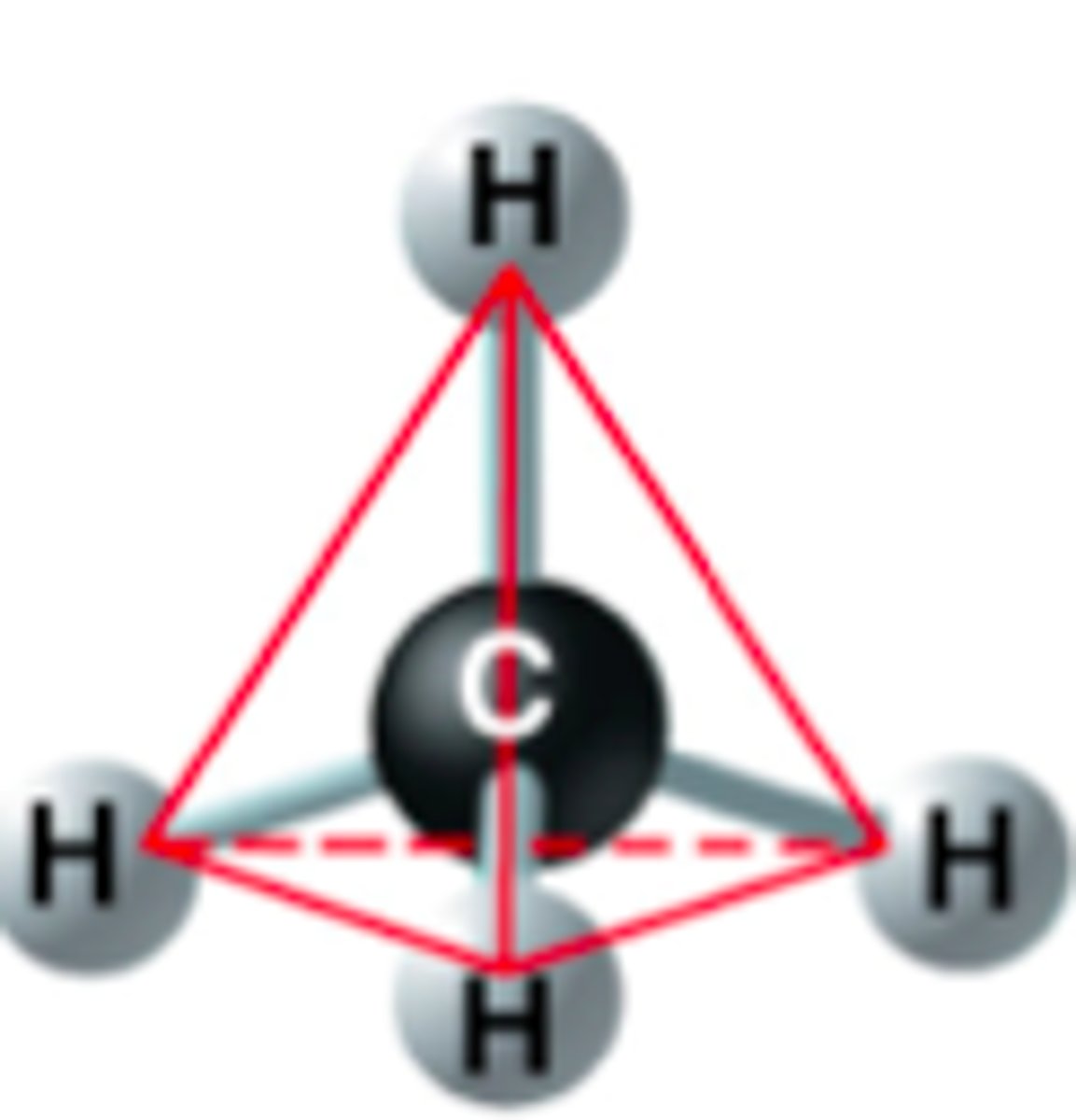
tetrahedron
CH4: because of the shape of the orbitals, the carbon atom lies at the center of a three-dimensional structure, and the four molecular orbitals point toward the four corners of this structure
isomers
same chemical formula, different arrangements of atoms (structure)
4 types of complex organic macromolecules
carbohydrates, proteins, lipids, nucleic acids
proteins
provide structural support and act as catalysts to facilitate chemical reactions
nucleic acids
encode and transmit genetic information
carbohydrates
provide structural support for many organisms and a source of energy
lipids
make up cell membranes, store energy, and are important in cell communication
polymers
chain of repeating units (monomers) that are connected through covalent bonds
amino acids
an organic molecule containing a central carbon atom attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain
- the building blocks (monomers) of proteins (polymers)
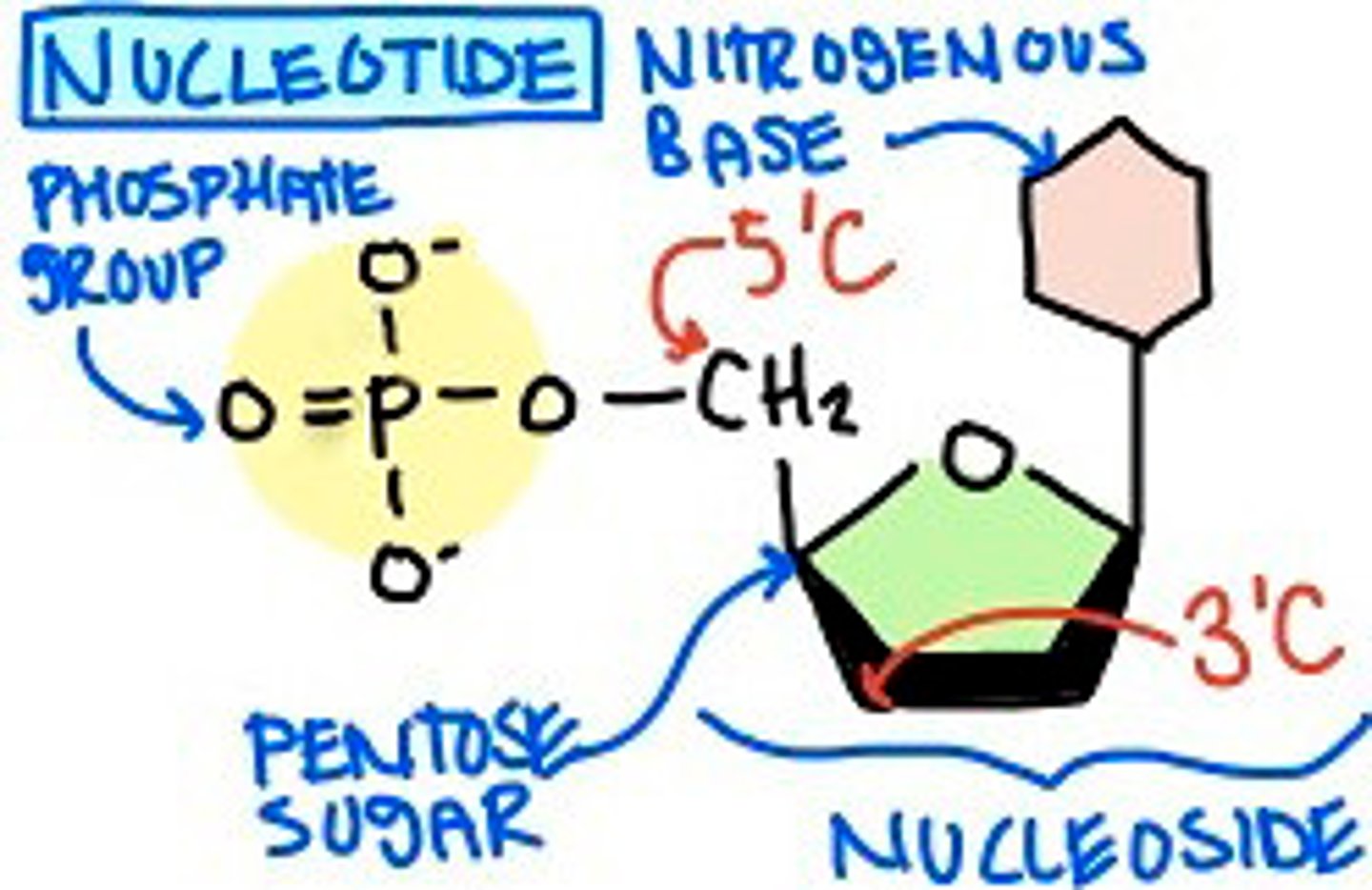
nucleotides
a constituent of nucleic acids, consisting of a 5-carbon sugar, a nitrogen-containing base, and one or more phosphate groups
monosaccharides
a simple sugar (monomer of carbohydrate)
polysaccharides
a polymer of simple sugars
- provide long-term energy storage or structural support
polypeptide
proteins are also called this
- linked amino acids form this
nucleotides are composed of three components:
1. 5-carbon sugar (ribose or deoxyribose)
2. a base containing nitrogen
3. one or more phosphate groups
peptide bond
amino acids are joined through a covalent bond called this
- when they are formed, the carboxyl group releases an oxygen tomorrow, and the nitrogen loses two hydrogen atoms (forms water molecules)
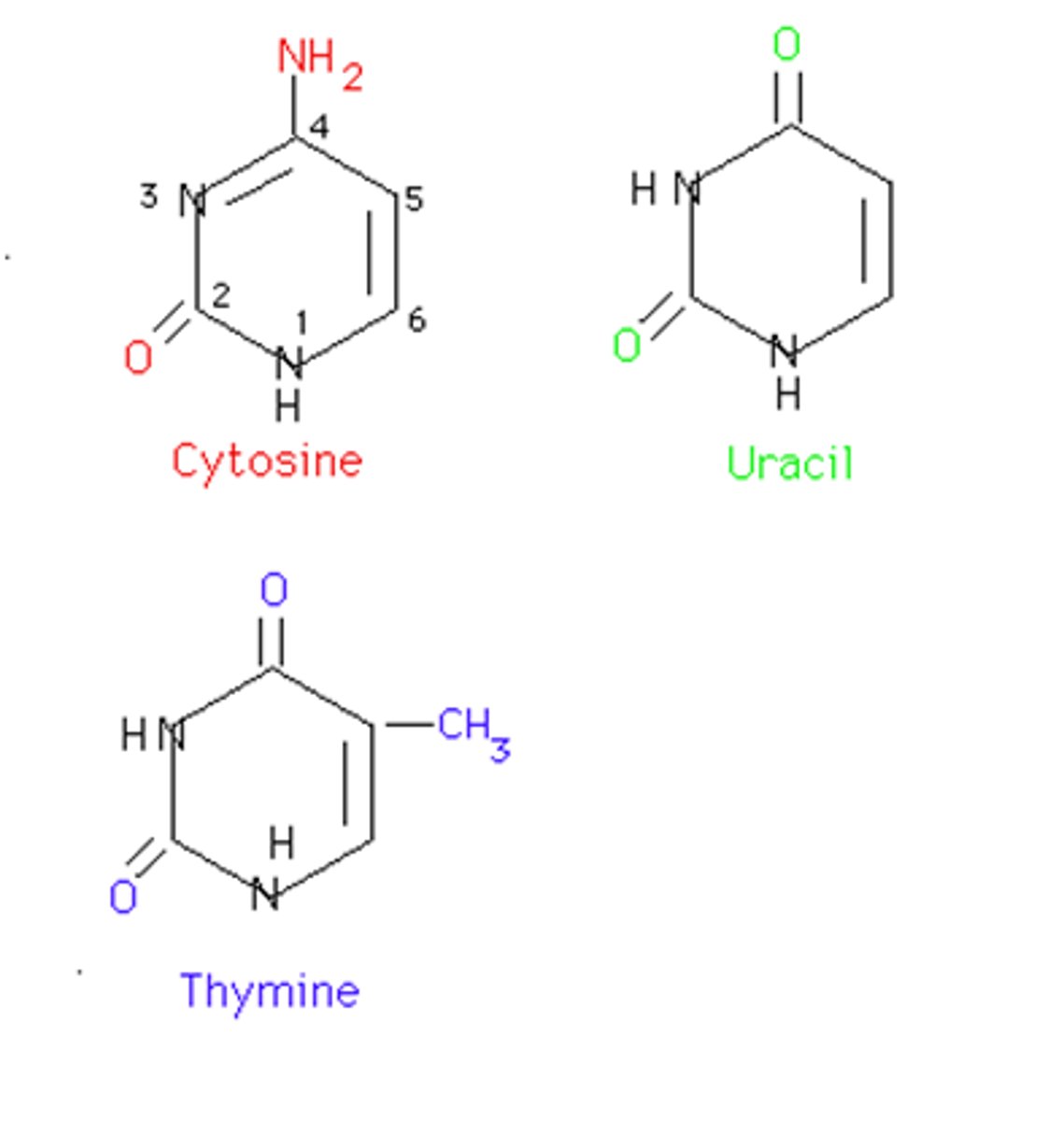
pyrimidine bases
cytosine (C), thymine (T), uracil (U)
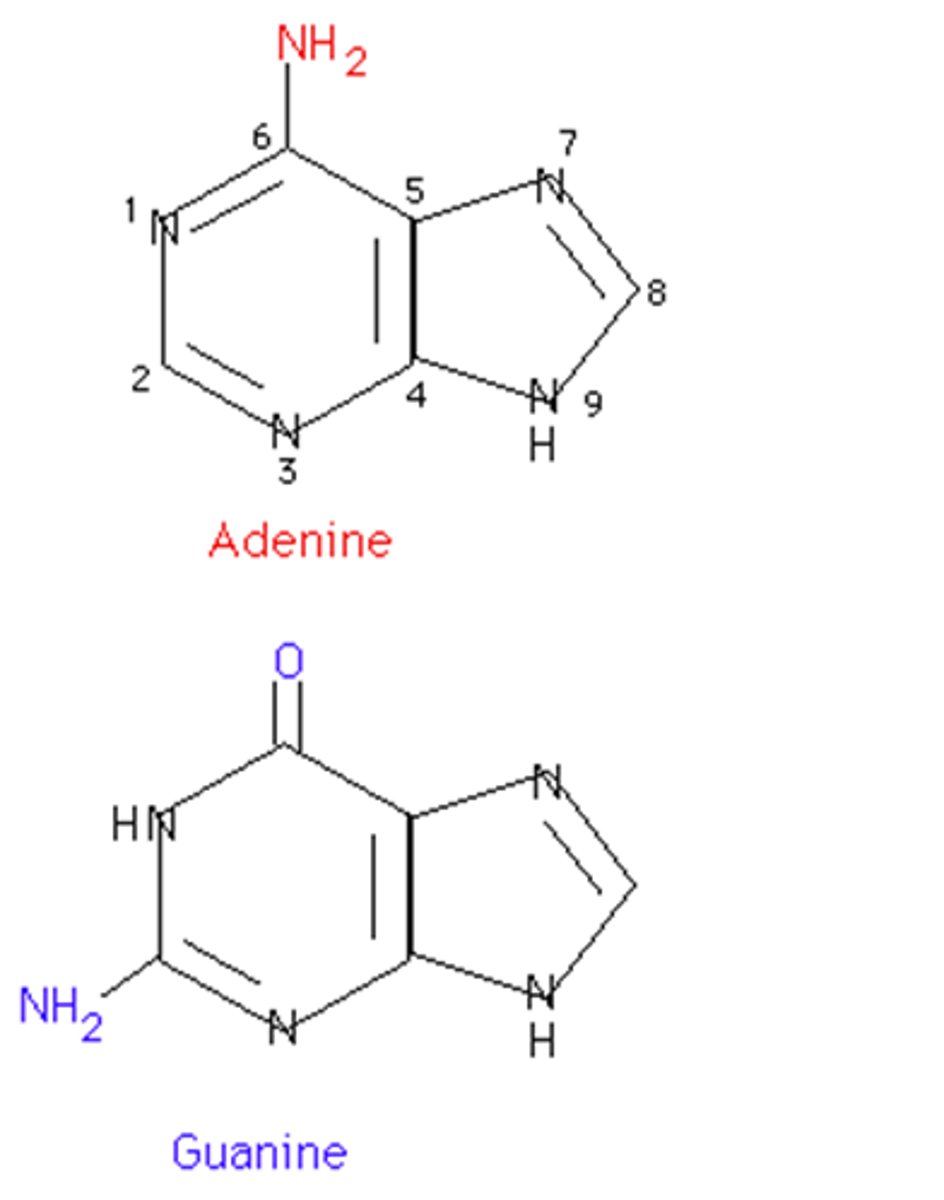
purine bases
guanine (G) and adenine (A)
phosphodiester bond
joins two nucleotides together
- bond is formed between the phosphate group of each nucleotide and the 3'-OH of the last nucleotide
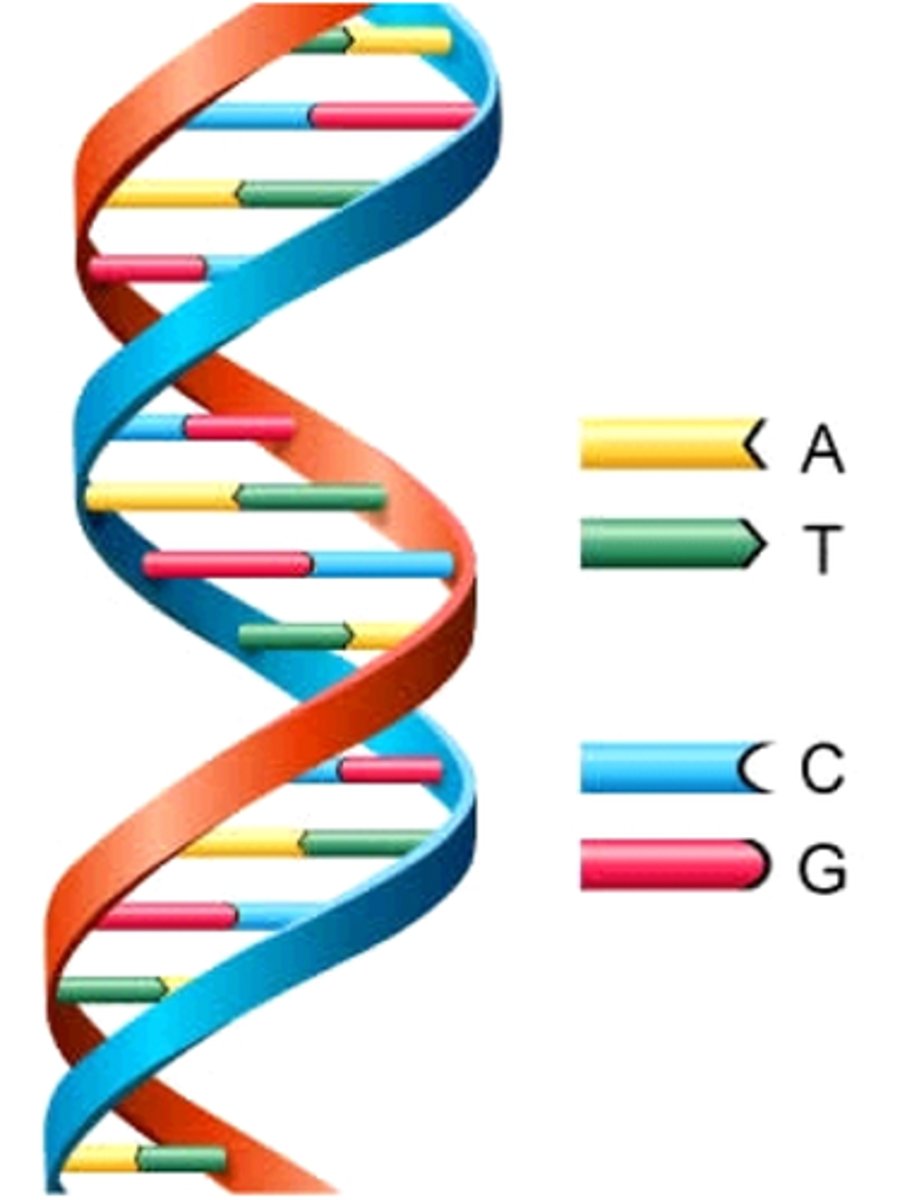
complimentary base pairings
A-T
G-C
types of sugars
1. monosaccharides
2. disaccharides
3. polysaccharides
4. complex carbohydrates
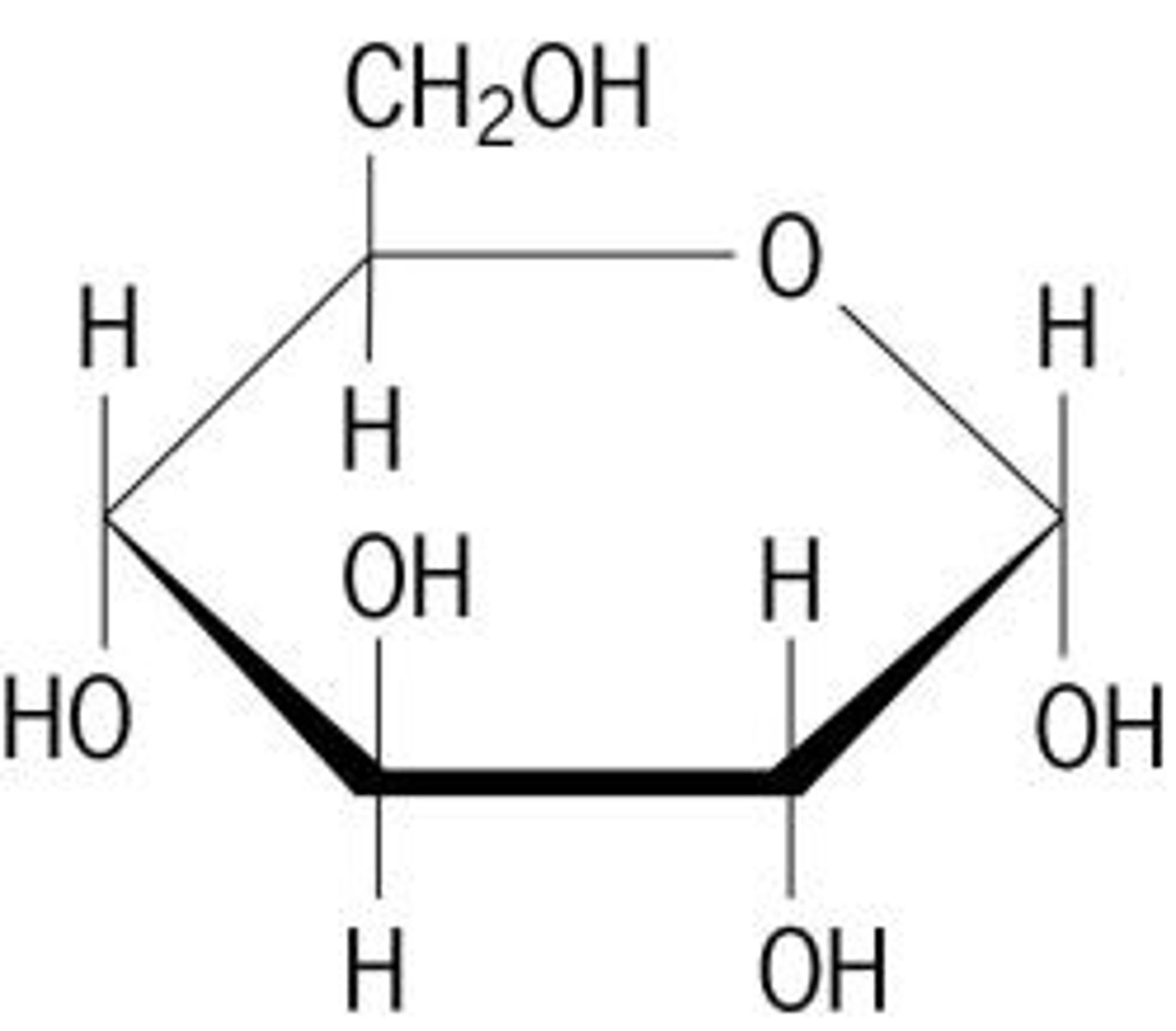
monosaccharide
contains one sugar molecule
- often a subunit, or monomer of more complex sugars
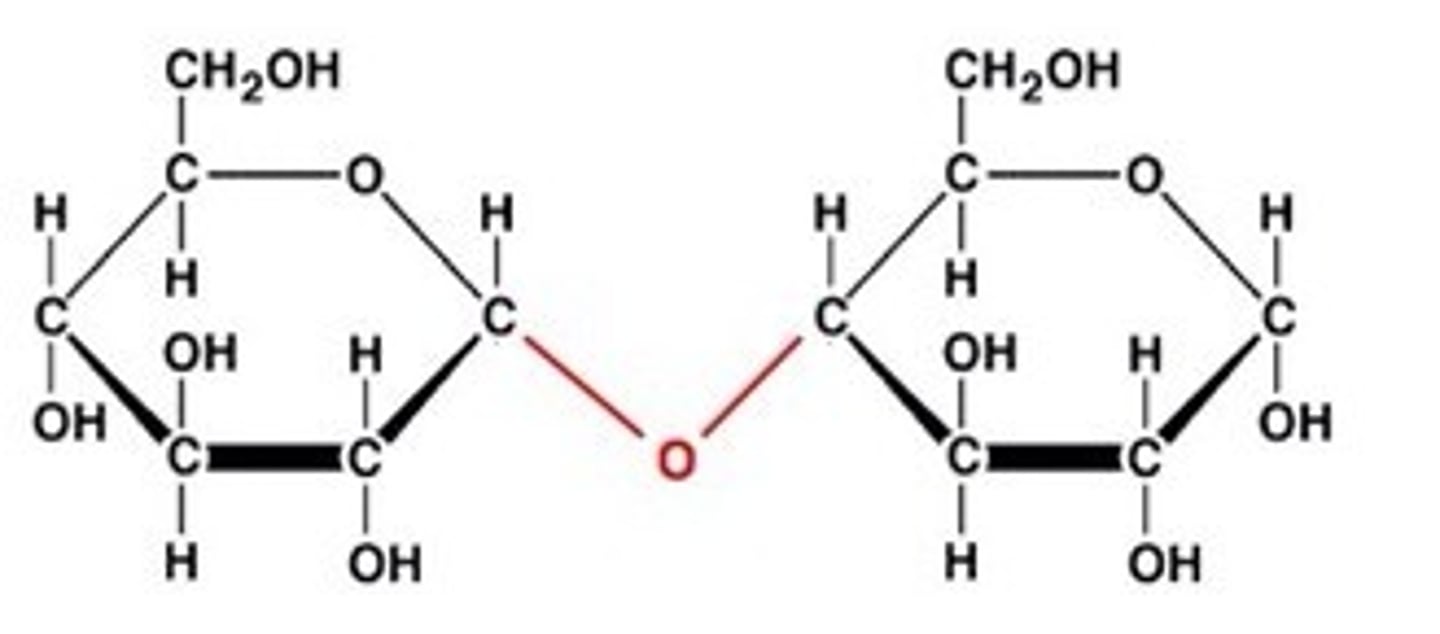
disaccharide
contains two bonded monosaccharides
- usually linked via glycosidic linkages (bonds)
complex carbohydrates
long, braided chains of monosaccharide monomers
lipids: properties & structure
- do not mix well with water (all hydrophobic)
- not made of repeating monomers
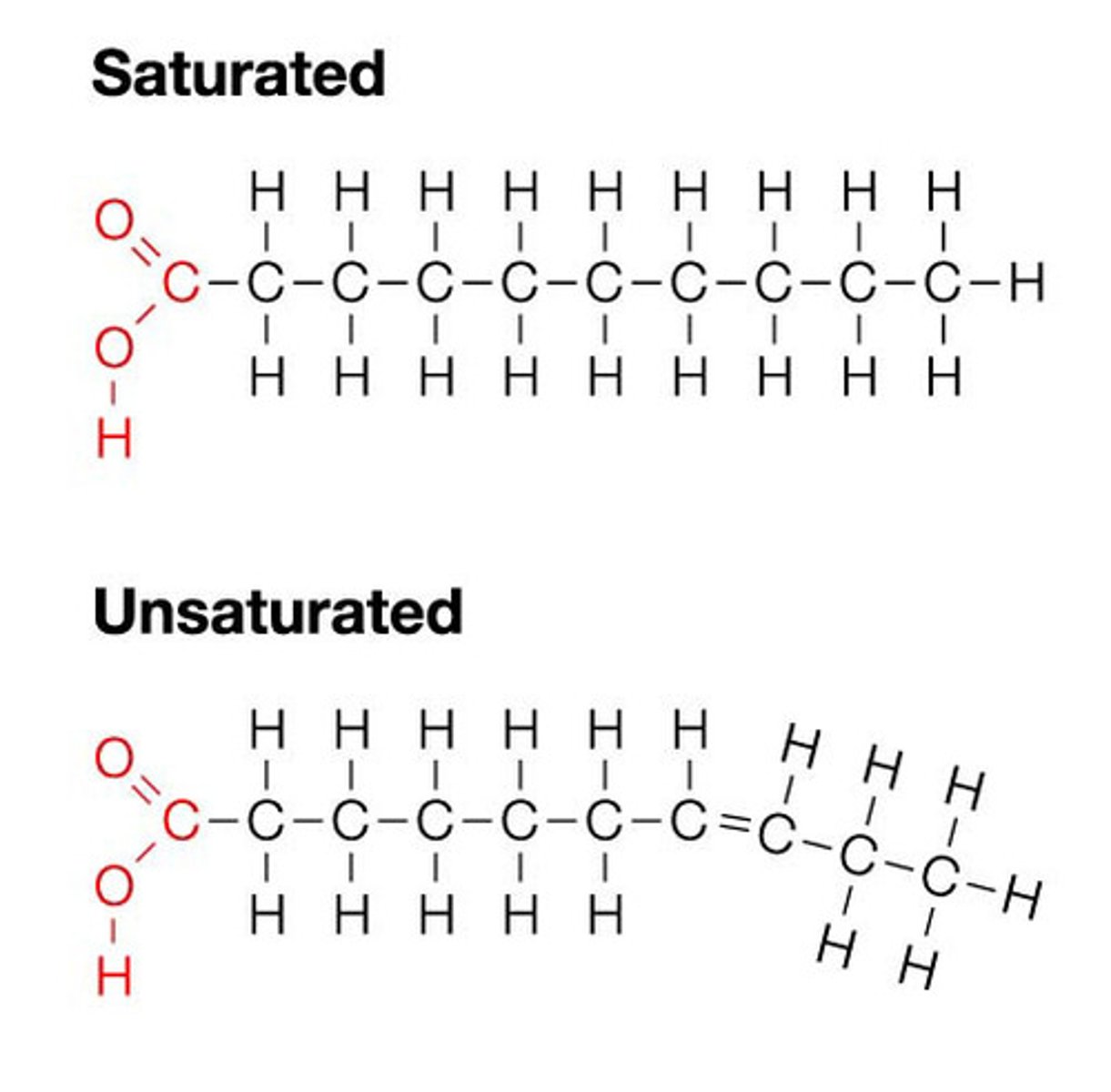
- fatty acids are a type of lipid with long chains of carbon with a carboxyl group at the end
* saturated (single bond) or unsaturated bond (no van der waals forces
--> different structure based on the presence of C-C double bonds
van der waals forces
a slight attraction that develops between the oppositely charged regions of nearby molecules
- sometimes fatty acids line up next to each other (can be very stable in the cell)
- hydrocarbon chains in fatty acids have non-polar covalent bonds
- electrons are moving around the atoms in fatty acids, creating short-lived regions with slight negative charges --> these are attracted to slight positive regions in another atom
* these forces are weaker than hydrogen bonds, but many act together to stabilize molecules --> the length of hydrocarbon chains increases these forces
melting point
the kinks in the fatty acids are caused by unsaturated carbons that have double bonds between them
- this reduces tightness between the molecules, causing a lower melting point
why is animal fat solid at room temperature?
animal fats are saturated (able to stack tightly)
- without double bonds causing kinks in the structure, animal fats can stack closely together and are stabilized by more van der waals interactions than unsaturated fats
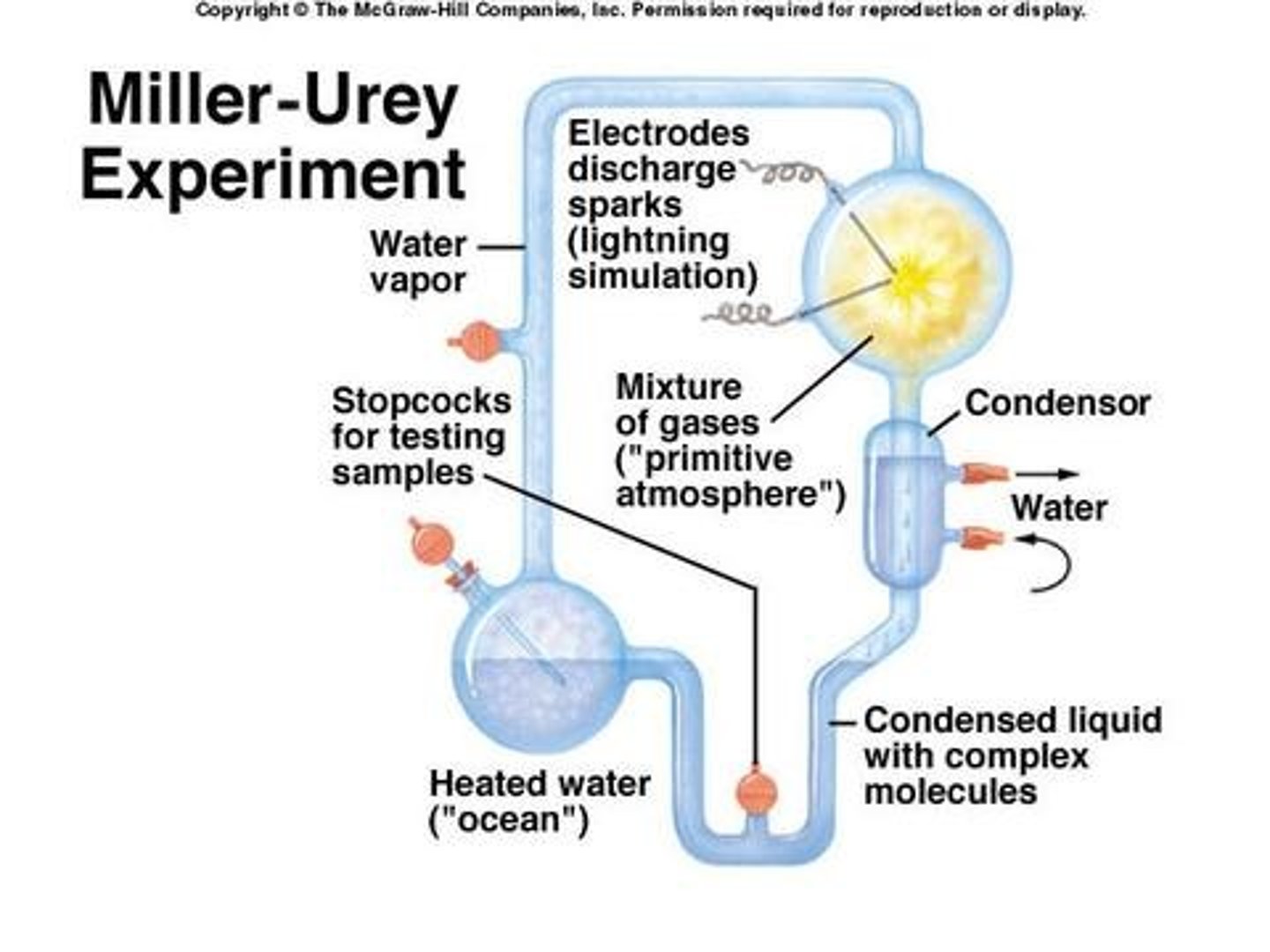
Miller-Urey experiment
subsequent testing has shown that the building blocks of life can form macromolecules