AP CHEM MOLECULAR GEOMETRY (VSEPR)
0.0(0)
Card Sorting
1/12
Earn XP
Description and Tags
BOND ANGLES INCLUDED!!! type shi
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
1
New cards
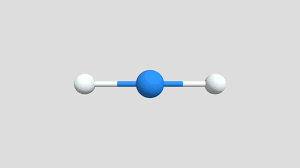
2 bonds, 0 lone pairs (180)
linear
2
New cards
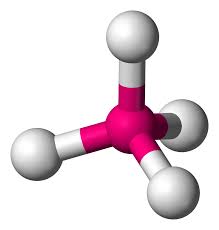
3 bonds, 0 lone pairs (120)
trigonal planar
3
New cards
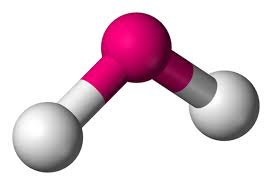
2 bonds, 1 lone pair (<120)
bent
4
New cards
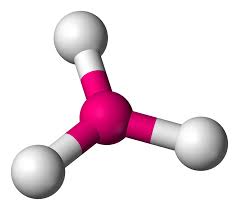
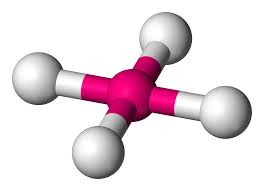
4 bonds, 0 lone pairs (109.5)
tetrahedral
5
New cards
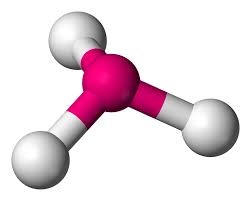
3 bonds, 1 lone pair (<109.5)
trigonal pyramidal
6
New cards
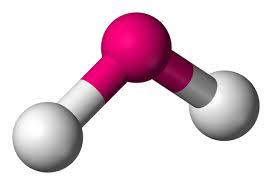
2 bonds, 2 lone pairs (<109.5)
bent
7
New cards
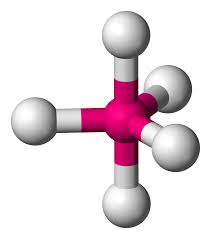
5 bonds, 0 lone pairs (90 & 120)
trigonal bipyramidal
8
New cards
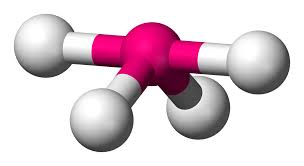
4 bonds, 1 lone pair (90, 120, 180)
seesaw
9
New cards
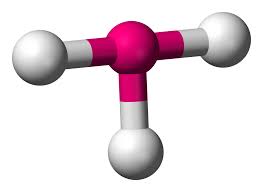
3 bonds, 2 lone pairs (90)
t-shaped
10
New cards
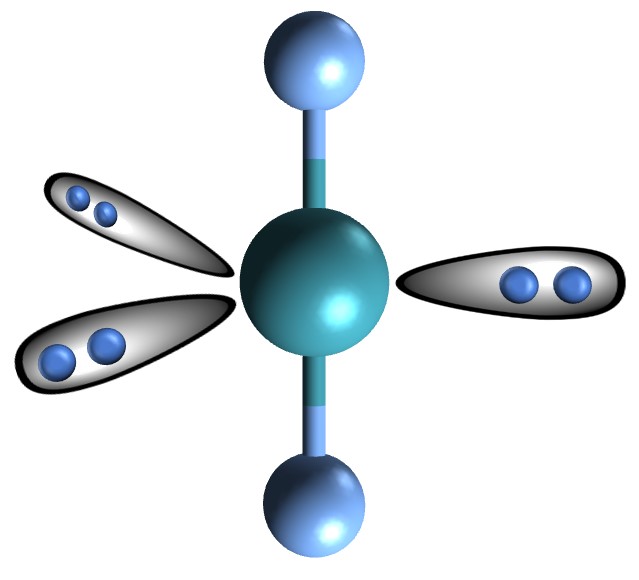
2 bonds, 3 lone pairs (180)
linear
11
New cards
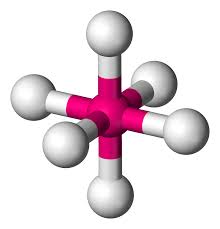
6 bonds, 0 lone pairs (90)
octahedral
12
New cards
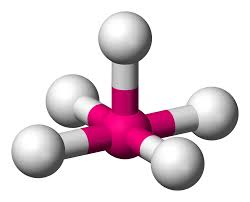
5 bonds, 1 lone pair (90)
square pyramidal
13
New cards
4 bonds, 2 lone pairs (90)
square planar