Alkanes and the Hydrocarbons
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Hydrocarbons
Compounds composed of a carbon and a hydrogen
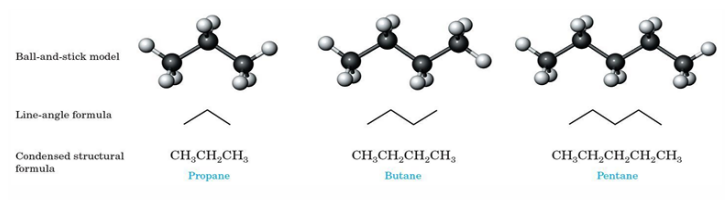
Alkanes
Hydrocarbon that is made out of only carbon-carbon single bonds
Methane and Ethane - first two alkane
Line angle formula
Line - carbon-carbon bond
Vertex - carbon atom
hydrogens are not shown in this formula
Constitution Isomerism
Constitutional Isomers - compounds with the same molecular formula but different structural formula
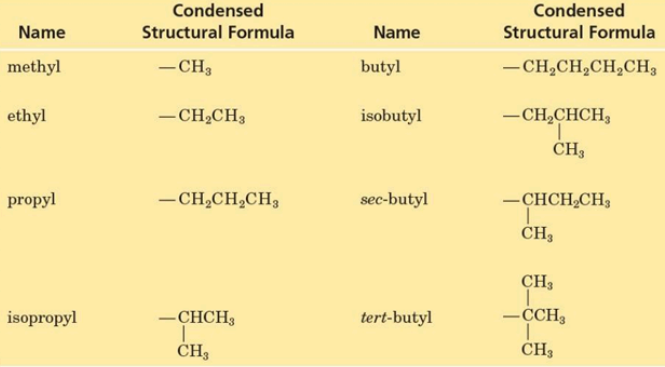
Alkyl Groups
Substituent group derived from an alkane by removal of a hydrogen atom
Commonly represented by the symbol R—
Named by dropping the -ane from the name of the parent alkane and adding the suffix -yl
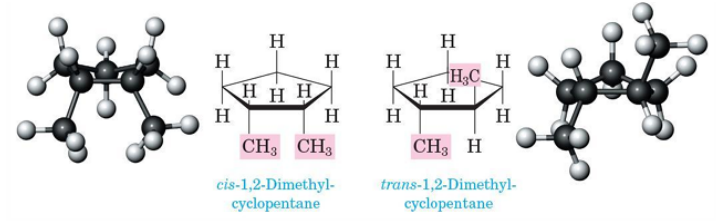
Cycloalkane
Saturated hydrocarbon but with the carbon atoms arranged as rings
cis-trans isomers
cis - same
trans - opposite
Physical Properties (alkanes & cycloalkane)
Alkanes and cycloalkanes almost completely lack of polarity
Electronegativity between carbon and hydrogen is 2.5 - 2.1 = 0.4 NONPOLAR
Alkanes IMF is London dispersion
Alkene and Alkyne
Alkene is carbon-carbon double bond
Alkyne is carbon-carbon triple bond
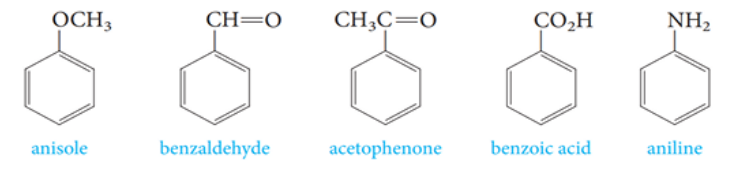
Aromatic Compounds
Compounds that contain benzene rings
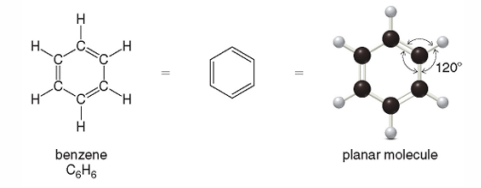
Aromatic Compounds with a common name
widely used before the introduction of systematic method