Reversible Reactions and equillibrium constant 1
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
The rate of reaction is...
the amount of product that is made in a reaction in a specified time period
At what time did this system reach dynamic equilibrium?
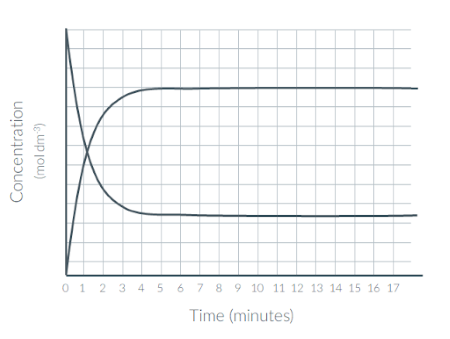
Give your answer to the nearest minute
5 min
Dynamic equilibrium is reached when the…
reversible reactions occur in a closed system
rate of the forward reaction equals the rate of the backward reaction
concentration of the reactants remains constant
concentration of the products remains constant
If the concentration of reactants is greater than the concentration of product, we say that the reaction’s equilibrium position lies to the...
left.
What are the features of a reaction at equilibrium? (3)
The rates of the forward and backward reactions are equal
The reactant and product concentrations are constant
The rates of the forward and backwards reactions are constant
When the concentration of a reactant in equilibrium increases what happensd to the yield of the product?
the yield of the product increases
When the pressure in a system increases, the reaction’s equilibrium position moves...
to the side with fewer gas molecules
When the temperature of a system is increased, the equilibrium position moves…
in the direction of the endothermic reaction
When Le Chatelier decreases the temperature of the system, the reaction’s equilibrium position moves…
in the exothermic direction
Le Chatelier’s principle states that…
when a factor that affects the equilibrium is changed, the equilibrium position will move to counteract that change
Answer le chatliers principle key point:(4)
1)state relevant features of reaction
2)state whether equillibrium position will move to the left or right
3)state why the equillibrium position moved
4) state how this affects the yield of the product
A catalyst is a substance that…
increases the rate of the forward and backward reaction equally and is not used up in a reaction.+doesnt affect the equillibrium position
A catalyst is added to a reaction in equilibrium, what happens to the rates of the forward and backward reactions?
Both increase equally
A reaction takes place in aqueous solution.
2HCl (aq)+MgSO4(aq)⇌MgCl2(aq)+H2SO4(aq)
What happens when water is added to the system?
Adding water to the system will increase the volume of the solution, which decreases the concentration of all the reactants and products. This is because concentration=moles/volume.= The position of equilibrium will shift to counteract this change. The position of equilibrium will shift to the left, because this side has more molecules. This shift increases the amount of moles, and therefore the concentration.
How do Conditions that increase the rate of reaction affect yield...
don’t always increase the yield
When industries carry out reactions, they need to consider…(4)
conditions that increase the rate of reaction
conditions that increase the yield of product
the safety of the equipment
economic costs
How could you decrease the time taken to reach dynamic equilibrium?(4)
Increase the pressure
Increase the temperature
Increase the reactant concentration
Adding a catalyst
All these options increase the rate of reaction, which decreases the time to reach equilibrium
Which formula can we use to predict the amount of product we have at equilibrium, compared to reactant.
concentration of product/concentration of reactants
For a given reaction, the concentration of products at equilibrium divided by the concentration of reactants…
remains constant with increasing initial concentration of reactant
The symbol for the equilibrium constant is...
Kc
If the concentration of products at equilibrium is greater than the concentration of reactants, the value for Kc will be…
more than 1.
If the concentration of products at equilibrium is less than the concentration of reactants, the value for Kc will be…
less than 1.