biochem exam 3
0.0(0)
Card Sorting
1/248
There's no tags or description
Looks like no tags are added yet.
Last updated 1:15 PM on 4/11/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
249 Terms
1
New cards
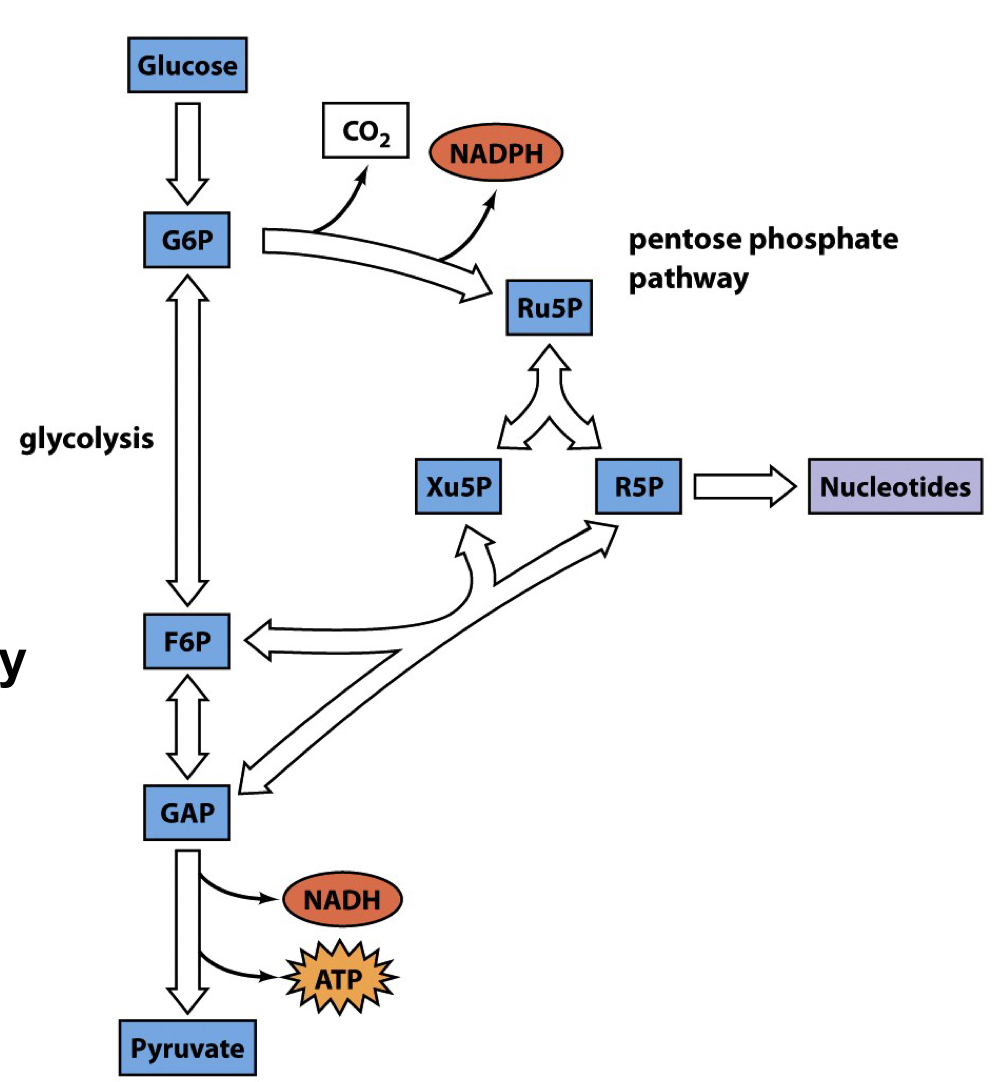
relationship between the PPP and glycolysis
glucose can be covered to GFP, which can be converted to F6P (glycolysis) or Ru5P (PPP, releasing CO2 and NADPH). Ru5P can be made into R5P (for nucleotide synthesis) or Xu5P. Xu5P can be interconverted with F6P, and R5P can be interconverted with GAP. F6P can also be converted to GAP. GAP then goes on to form pyruvate, releasing NADH and ATP in the process
2
New cards
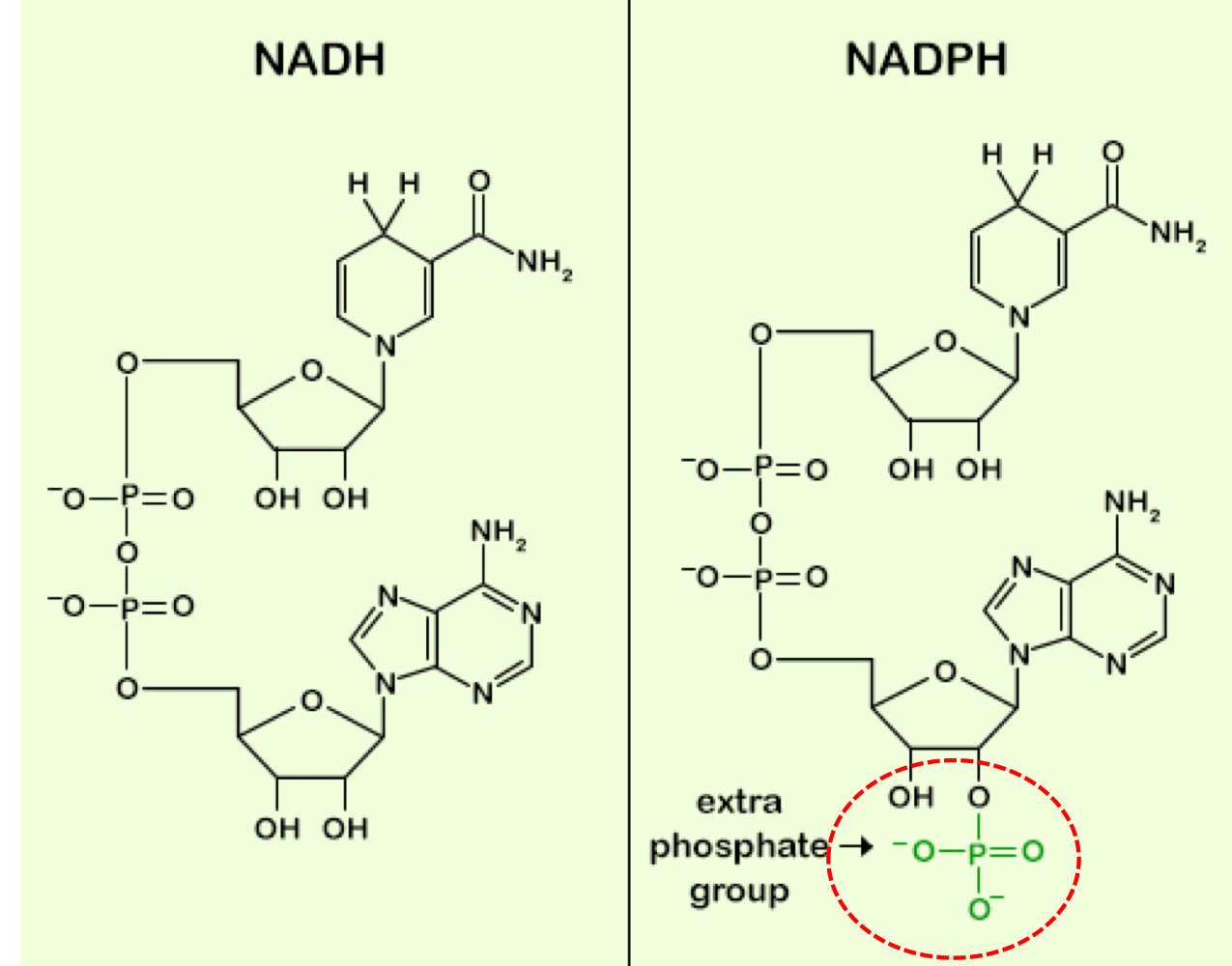
NADH vs NADPH
NADH is used for ATP synthesis. when the NAD+/NADH ratio is titled to NAD+ (1000/1 ratio), fuel substrate oxidation is maximized. NADPH is used for reductive biosynthesis. when the NADP+/NADPH ratio is tilted to NADPH (1/1000 ratio), reduction during biosynthesis reactions is maximized. NADH is used for oxidation and NADPH is used for reduction
3
New cards
when does glucose enter the PPP?
after conversion to glucose-6-phosphate
4
New cards
the primary functions of the PPP
1. synthesis of NADPH for reductive biosynthesis
2. synthesis of ribose 5-phosphate (R5P) for the biosynthesis of ribonucleotides (RNA, DNA)
5
New cards
tissues in which the PPP is active
\- tissues that synthesize **fatty acids or steroids** (liver, mammary and adrenal glands, adipose tissue) due to requirements for **NADPH**
\- tissues undergoing **rapid cell division** (bone marrow) due to a need for **R5P**
\- red blood cells to produce **NADPH** to maintain **reduced Fe2+ in hemoglobin**
\- tissues undergoing **rapid cell division** (bone marrow) due to a need for **R5P**
\- red blood cells to produce **NADPH** to maintain **reduced Fe2+ in hemoglobin**
6
New cards
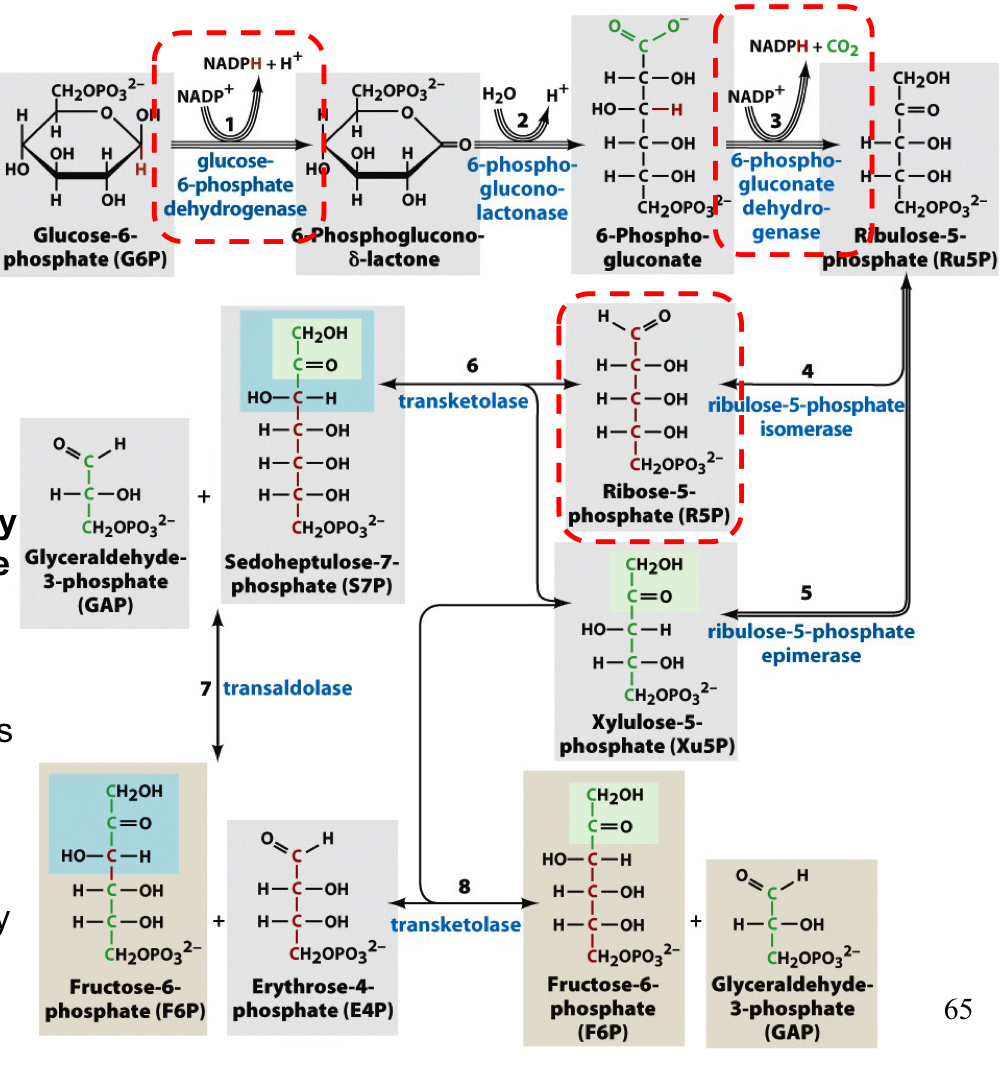
PPP overview
1. oxidative reactions (1-3): glucose-6-phosphate is turned into 6-phosphoglucono-δ-lactone by glucose-6-phosphate dehydrogenase, which is turned into 6-phosphogluconate by 6-phosphoglucono-lactonase, which is turned into ribulose-5-phosphate (Ru5) by 6-phosphogluconate dehydrogenase
2. isomerization and epimerization reactions (4-5): Ru5P is turned into Xu5P by an epimerase or into R5P by an isomerase
1. carbon shuffling (6-8): R5P and Xu5P undergo carbon shuffling by a transketolase or transaldolase
7
New cards
the main control point for the PPP
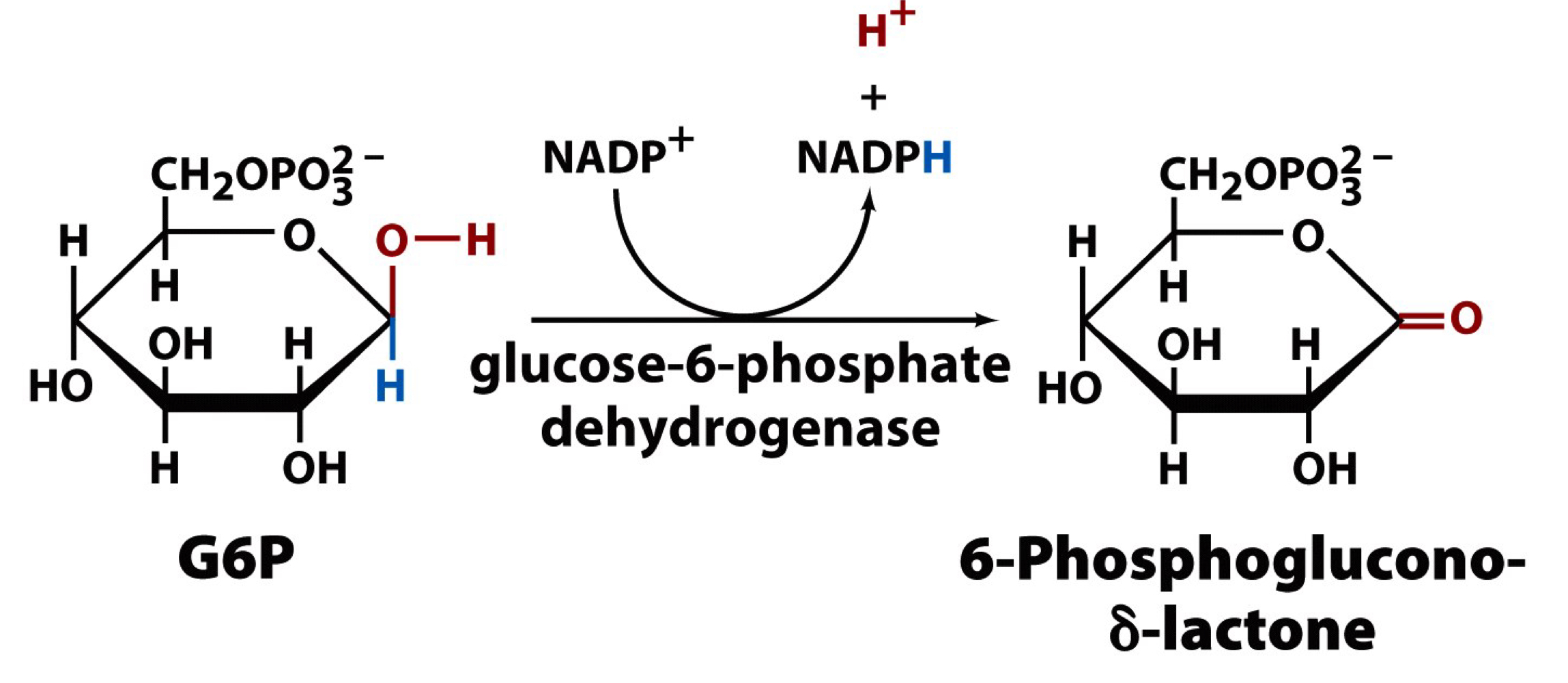
the first reaction, the reaction of glucose-6-phosphate to 6-phosphoglucono-δ-lactone by glucose-6-phosphate dehydrogenase, releasing an NADPH and an H+
8
New cards
the first reaction of the PPP
reaction of glucose-6-phosphate to 6-phosphoglucono-δ-lactone by glucose-6-phosphate dehydrogenase, releasing an NADPH and an H+
the hydride ion transfer from C1 of G6P to NADP+ to generate NADPH
highly regulated by NADP+ activity (build up of NADP+ stimulates activity)
G6P is oxidized to a lactone
the hydride ion transfer from C1 of G6P to NADP+ to generate NADPH
highly regulated by NADP+ activity (build up of NADP+ stimulates activity)
G6P is oxidized to a lactone
9
New cards
the second reaction of the PPP
6-phosphoglucono-δ-lactone hydrolyzes to 6-phosphogluconate by 6-phosphoglucono-lactonase or spontaneously, using an H2O and releasing an H+
10
New cards
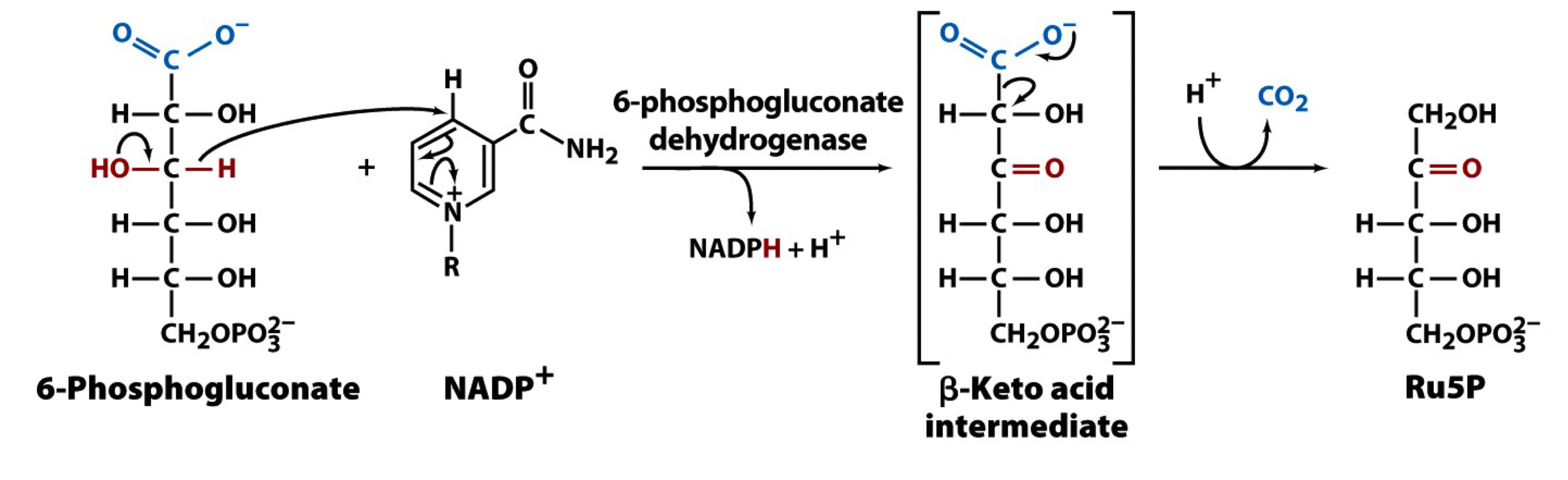
the third reaction of the PPP
6-phosphonogluconate is oxidized by 6-phosphonogluconate dehydrogenase to generate the second molecule of NADPH, releasing CO2 and forming ribulose-5-phosphate (Ru5P)
11
New cards
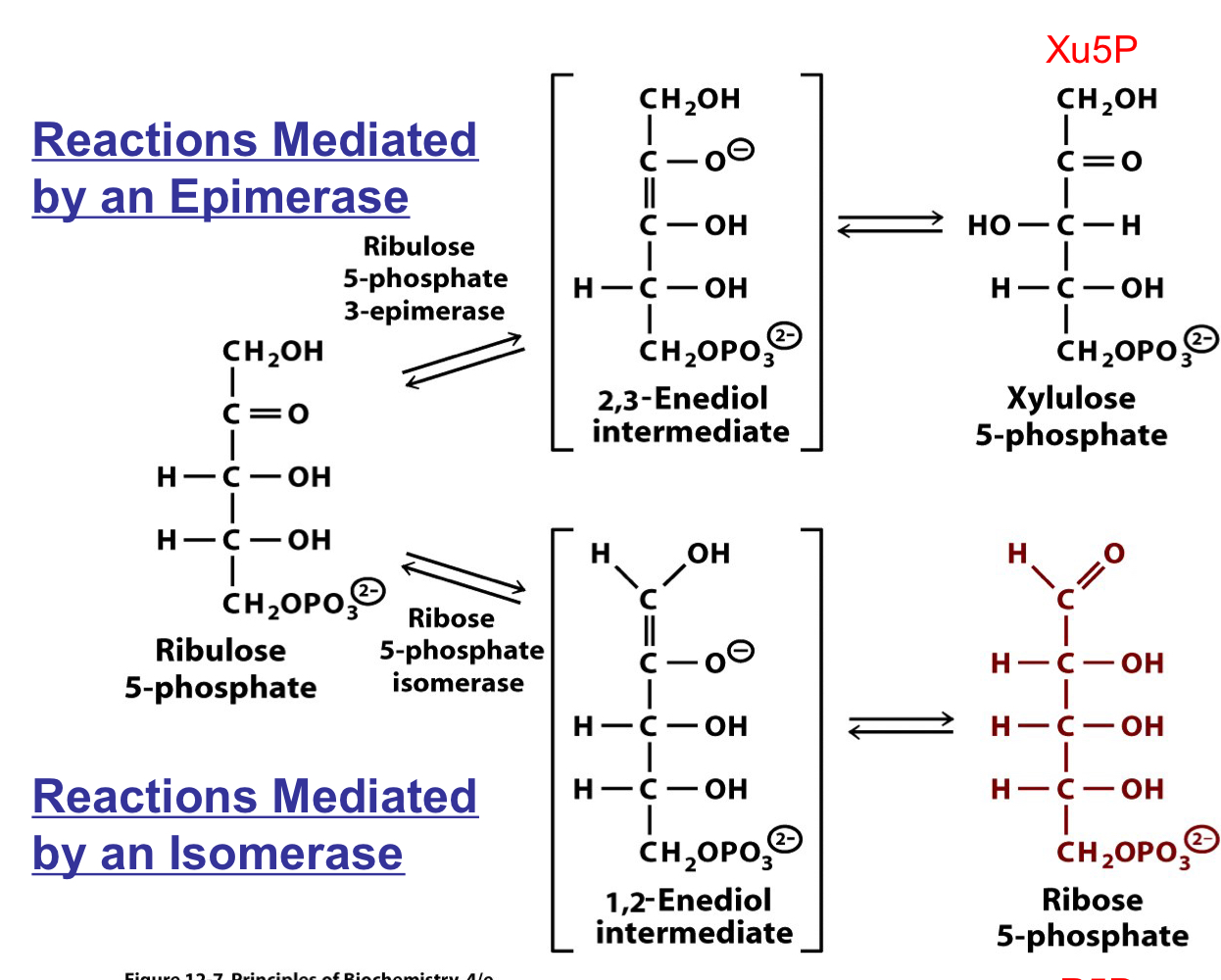
fourth and fifth reactions of the PPP
4. ribulose-5-phosphate (Ru5P) is converted to ribose 5-phosphate (R5P) by an isomerase
5. ribulose-5-phosphate (Ru5P) is converted to xylulose 5-phosphate by an epimerase
if only NADPH is required, the ratio is 2:1 Xu5P:R5P, setting the stage for recycling of the carbon compounds to re-enter glycolysis
R5P can be siphoned off as a precursor for nucleotides in rapidly dividing cells
12
New cards
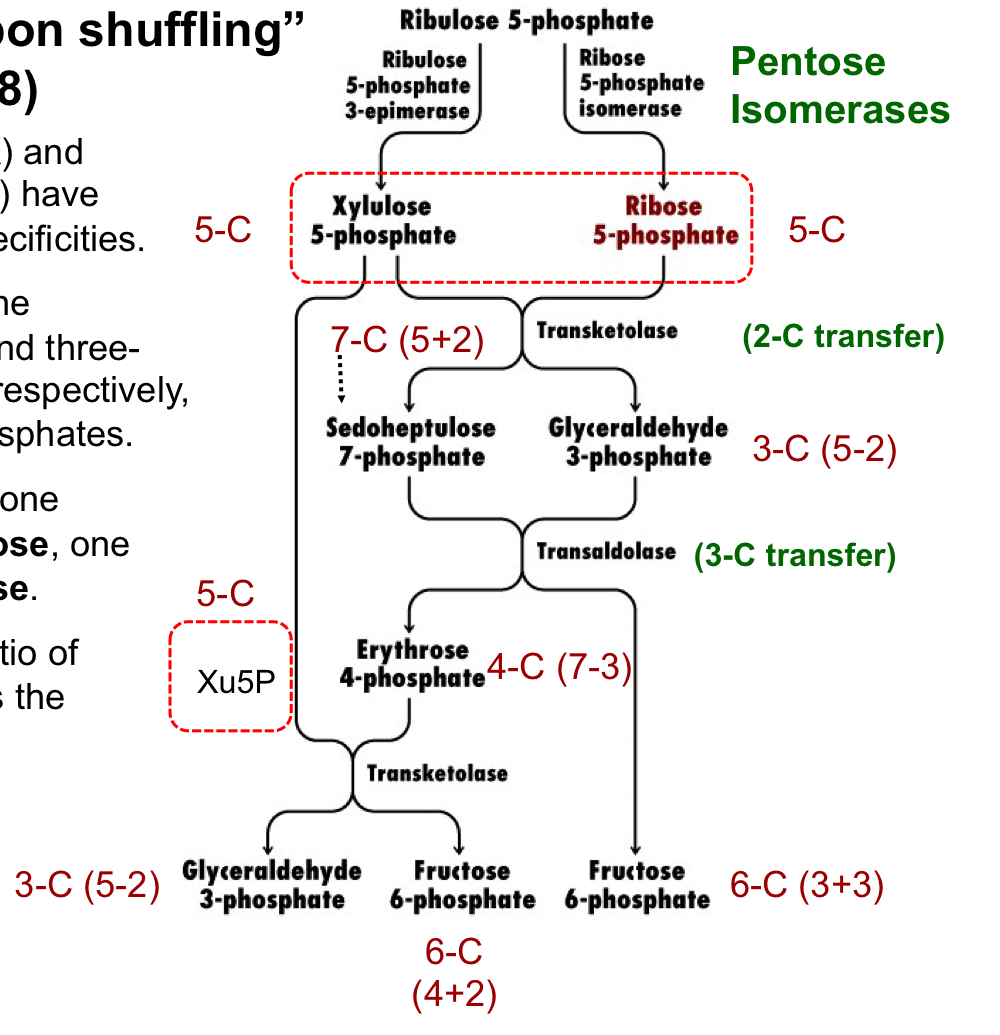
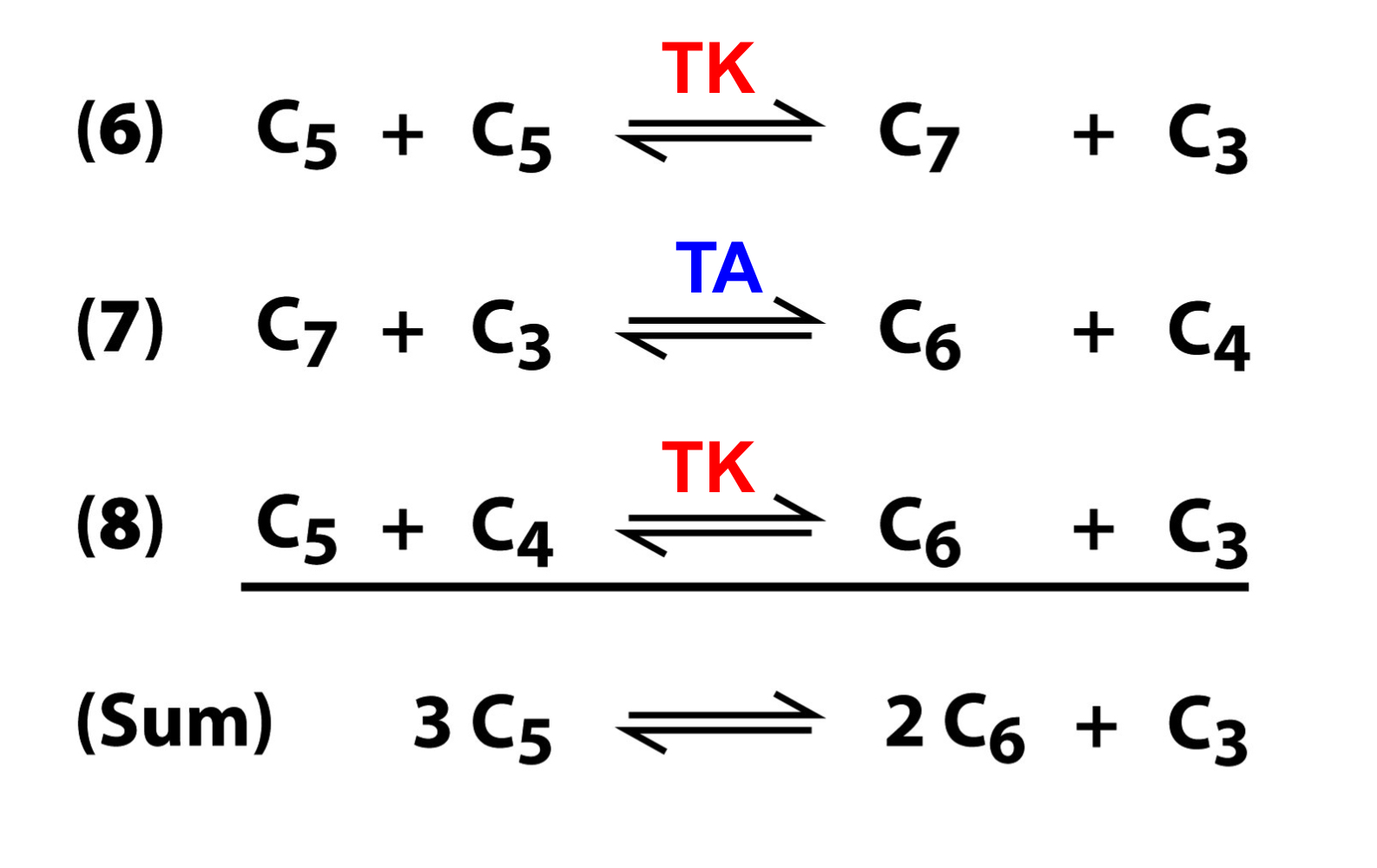
carbon shuffling of the PPP
transketolase moves 2 carbons from Xu5P (5C) to R5P (5C), generated a 7C cmpd and a 3C cmpd
transaldolase moves 3 carbons from the 7C cmpd to the 3C cmpd, forming a 4C cmpd and a 6C cmpd
transketolase transfers 2 carbons from Xu5P to the 4C cmpd to form a 3C cmpd and a 6C cmpd
the net result is a 3C cmpd and two 6C cmpds
transaldolase moves 3 carbons from the 7C cmpd to the 3C cmpd, forming a 4C cmpd and a 6C cmpd
transketolase transfers 2 carbons from Xu5P to the 4C cmpd to form a 3C cmpd and a 6C cmpd
the net result is a 3C cmpd and two 6C cmpds
13
New cards
transketolase and transaldolase
have broad substrate specficities and catalyze the exchange of 2 (TK) and 3 (TA) carbon fragments
one substrate is an aldose and one is a ketone
one substrate is an aldose and one is a ketone
14
New cards
the main goal of carbon shuffling in the PPP
to convert pentoses back into intermediates of glycolysis or gluconeogenesis
15
New cards
carbon shuffling PPP equations summary
16
New cards
the reaction catalyzed by the enzyme aldolase has a ΔG = +23 kJ/mol. in muscle cells, the reaction proceeds in the forward direction. how can this occur?
the concentration of reactant(s) must be significantly greater than product(s) in the cells
17
New cards
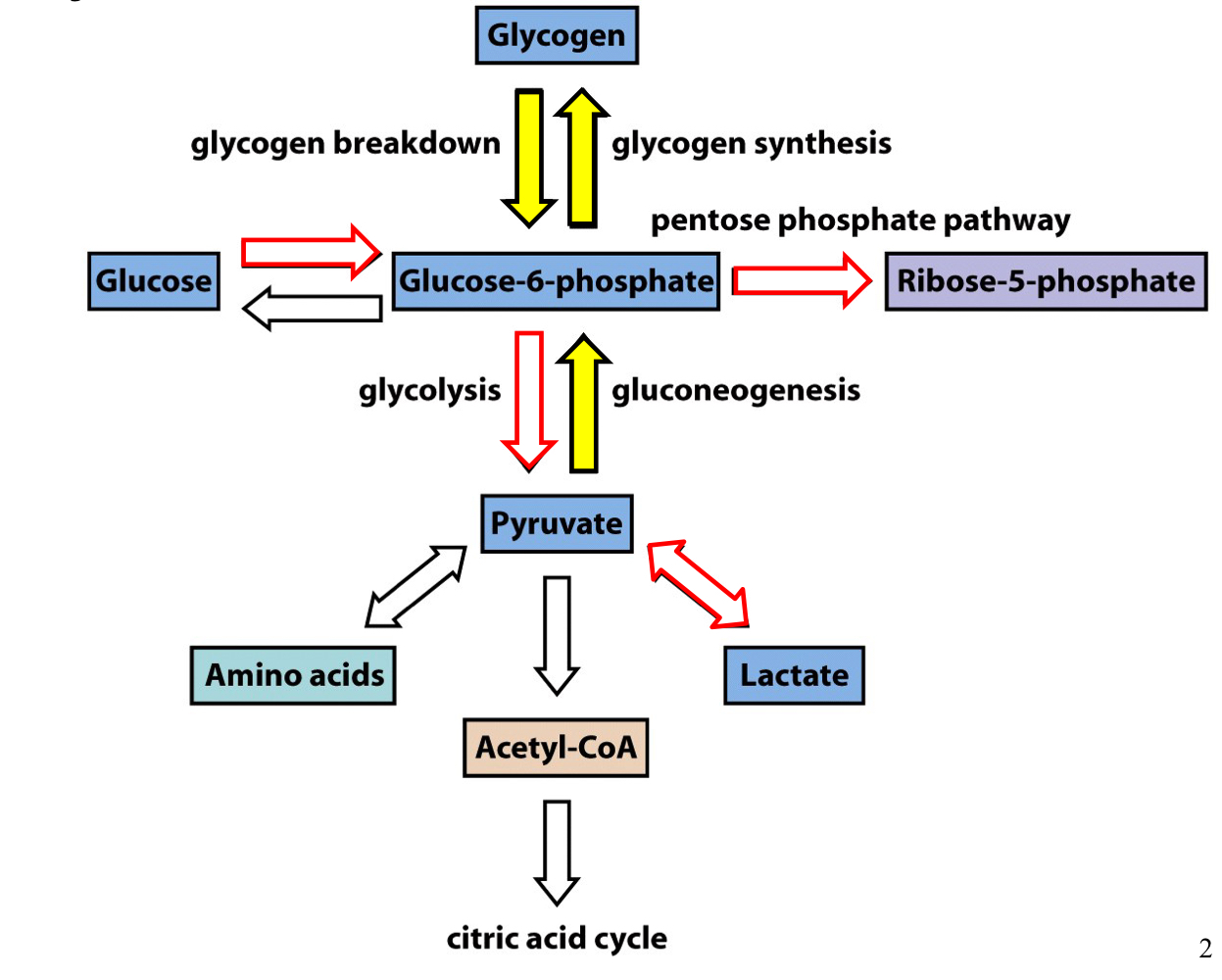
the fates of G6P
1. G6P can be converted back into glycogen through glycogen synthesis
2. glycogen can be converted into G6P through glycogen breakdown
1. pyruvate can be converted into G6P through gluconeogenesis
18
New cards
the importance of the liver
the liver participates in the interconversion of all types of metabolic fuels (carbohydrates, amino acids, fatty acids)
products of digestion pass immediately via the portal vein into the liver for metabolism or redistribution
the liver regulates distribution of dietary fuels and supplies fuel from its reserves
products of digestion pass immediately via the portal vein into the liver for metabolism or redistribution
the liver regulates distribution of dietary fuels and supplies fuel from its reserves
19
New cards
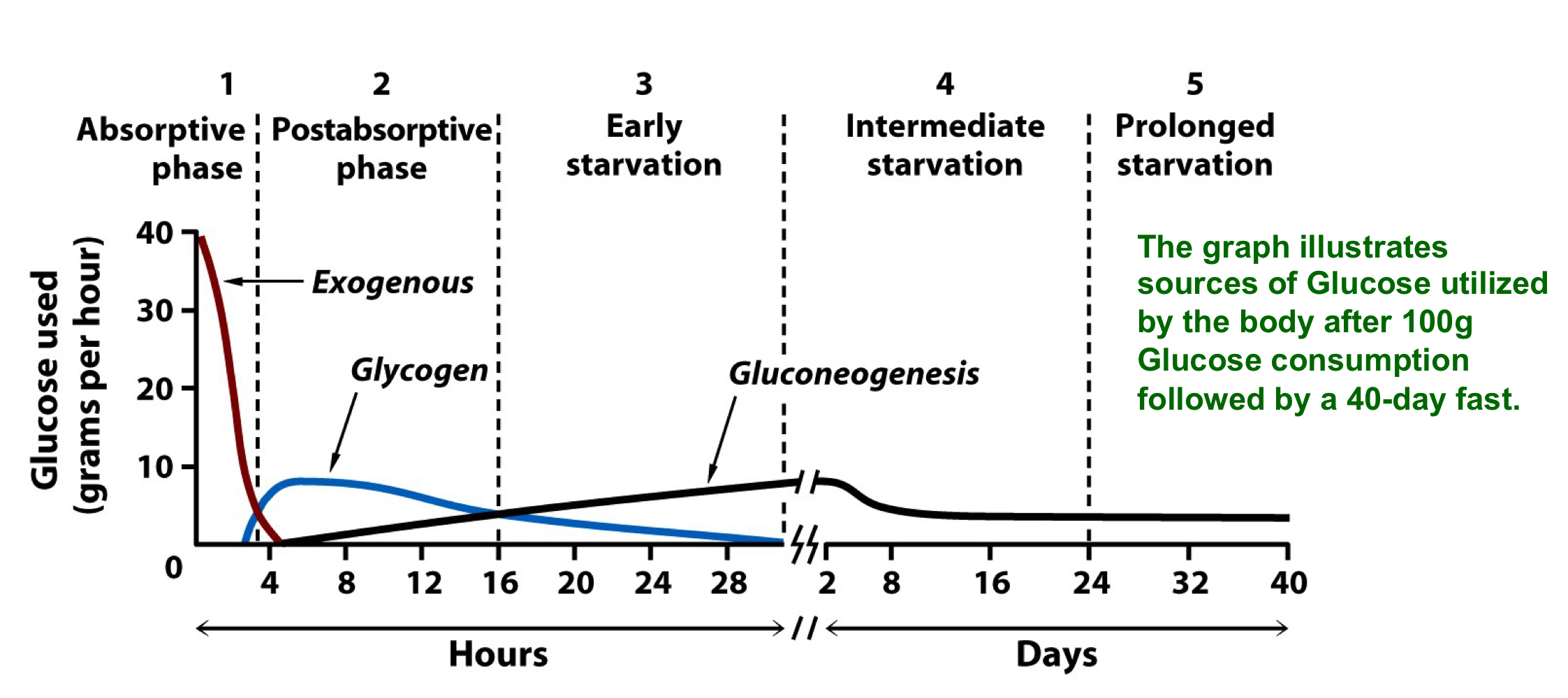
glucose homeostasis during fasting
during fasting and starvation, the body maintains blood glucose by first breaking down stored glycogen to release glucose
later the liver begins to synthesis new glucose from non carbohydrate sources via gluconeogenesis
the liver and kidney can synthesize glucose form non-carbohydrate precursors such as lactate and alanine
under fasting conditions, liver gluconeogenesis supplies almost all of the body’s glucose
later the liver begins to synthesis new glucose from non carbohydrate sources via gluconeogenesis
the liver and kidney can synthesize glucose form non-carbohydrate precursors such as lactate and alanine
under fasting conditions, liver gluconeogenesis supplies almost all of the body’s glucose
20
New cards
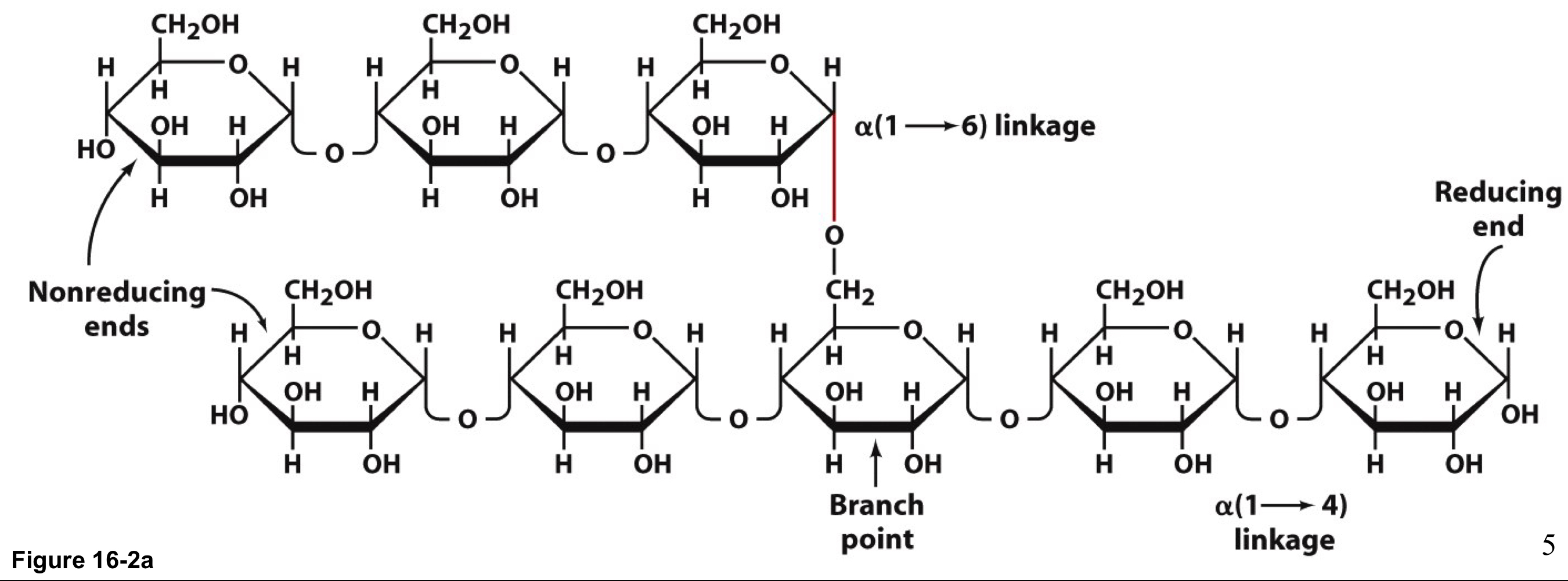
glycogen molecule structure
α-1-4 linked linear glucose monosaccharides with branch points with α-1-6 linkages every 8-14 residues
can contain up to 120,000 glucose molecules
forms granules that also contain the enzymes that catalyze synthesis/degradation of glycogen
can contain up to 120,000 glucose molecules
forms granules that also contain the enzymes that catalyze synthesis/degradation of glycogen
21
New cards
enzymes that catalyze glycogen degradation
1. glycogen phosphorylase
2. glycogen debranching enzyme
3. phosphoglucomutase
22
New cards
glycogen phosphorylase in glycogen degradation
removes glucose resides one at a time from the nonreducing ends of glycogen
only acts on α-1-4 linkages of glycogen
glycogen breakdown yields glucose 1-phosphate, which can be converted to glucose 6-phosphate for metabolism via glycolysis and the citric acid cycle
only acts on α-1-4 linkages of glycogen
glycogen breakdown yields glucose 1-phosphate, which can be converted to glucose 6-phosphate for metabolism via glycolysis and the citric acid cycle
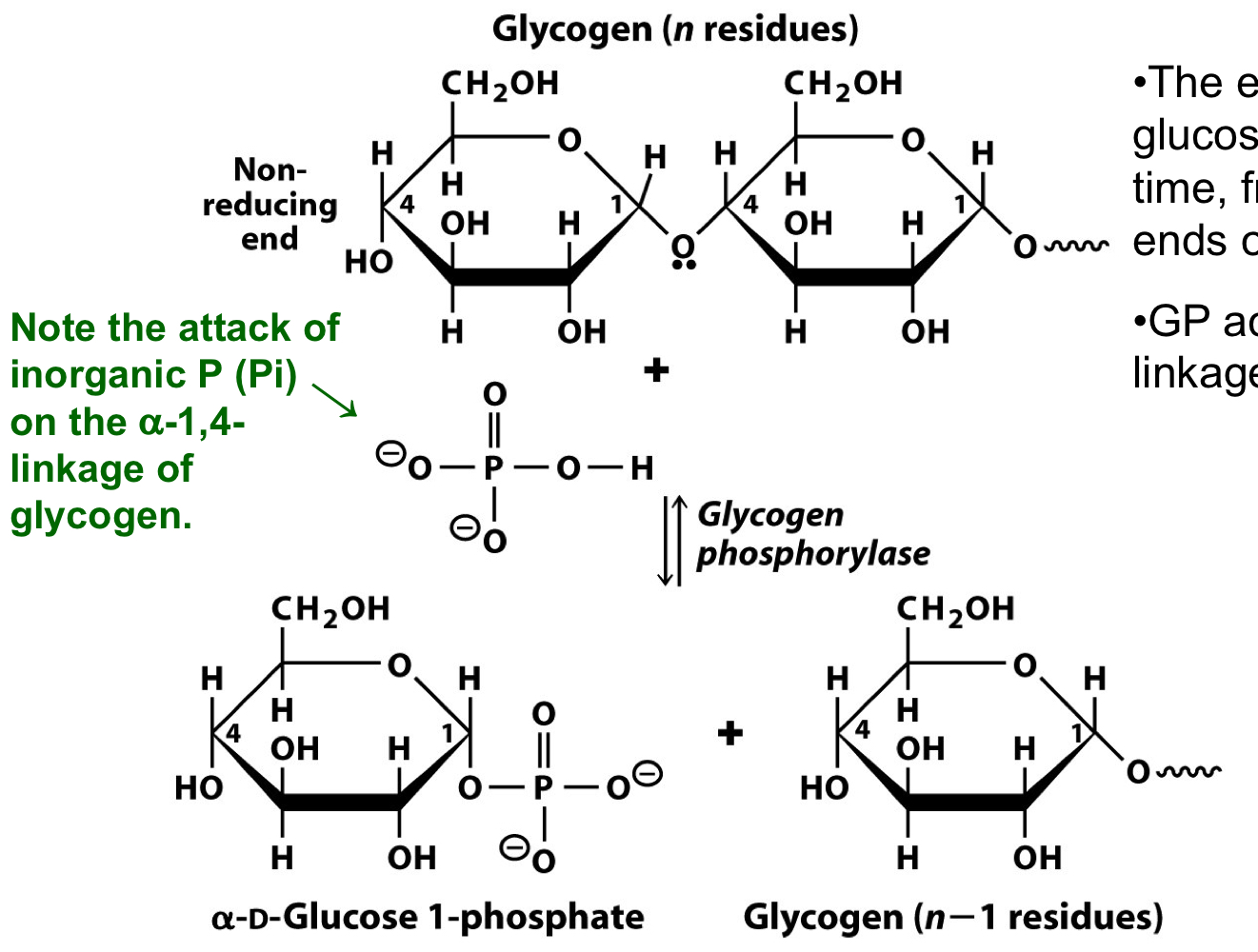
23
New cards
glycogen phosphorylase steps
1. formation of an enzyme-Pi-glycogen ternary complex
the enzyme binds via a Schiff base to a PLP cofactor (the phosphate group of PLP acts as a general acid-base catalyst
2. during C1-O1 bond cleavage, the terminal glycosyl residue is converted into an oxonium ion (in a half chair conformation) intermediate by proton transfer through the Pi from the PLP phosphate (acid catalysis)
3. the oxonium ion reacts with the Pi forming the alpha conformation of glucose-1-phosphate
the glycogen (n-1) cycles back for another round of degradation
24
New cards
glycogen debranching enzyme
resolves limit branch structures in the glycogen polymer so GP can continue to degrade the linear regions
a SLOW process
a SLOW process
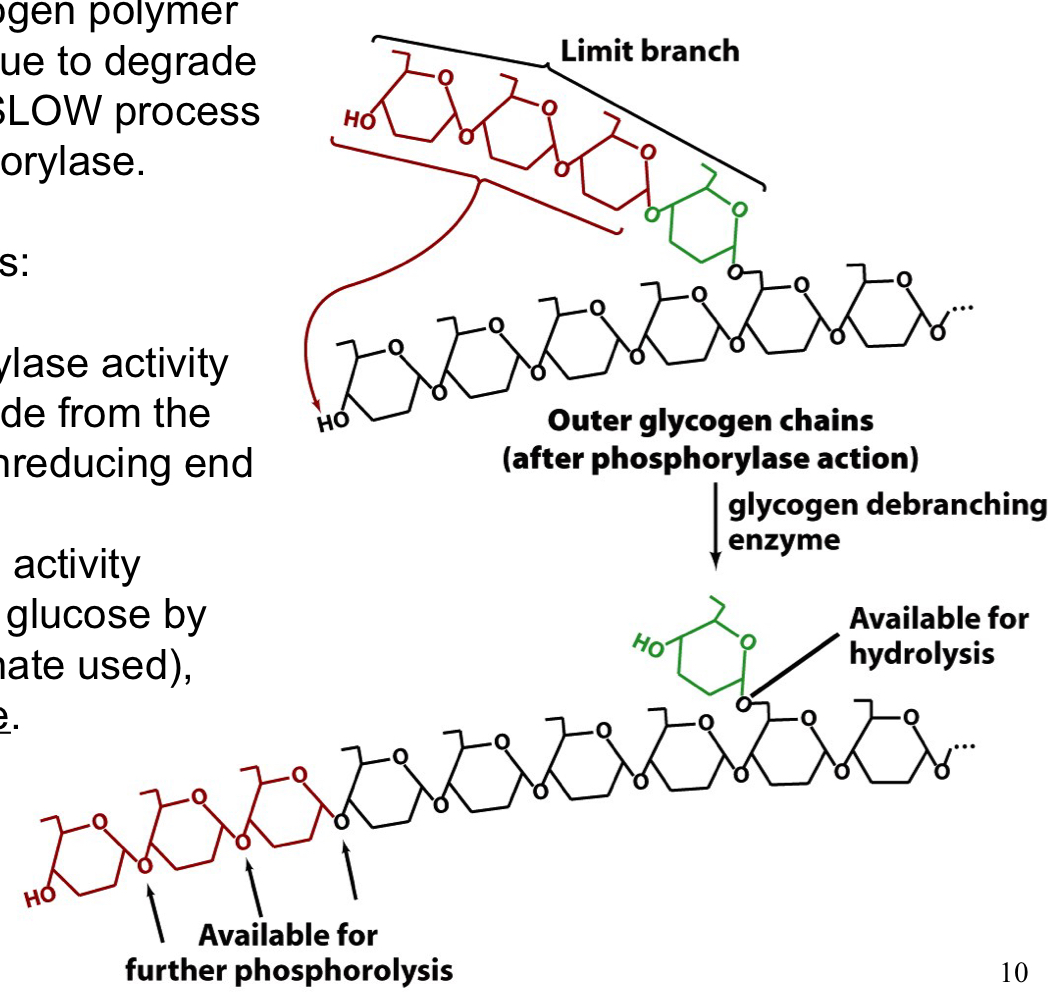
25
New cards
glycogen debranching enzyme activities
1. α-1-4 transglycosylase activity: transfers a trisaccharide from the limit branch to the nonreducing end of an adjacent strand
1. α-1-6 glucosidase activity: cleaves off remaining glucose by hydrolysis (no phosphate used), releasing a free glucose
26
New cards
phosphoglucomutase
converted glucose-1-phosphate (G1P) to glucose-6-phosphate (G6P) for use in other metabolism (like glycolysis)
a double phosphorylation using a Ser-bound phosphate to phosphorylate the C6 position but then the Ser-bound phosphate is regenerated by removal of the C1 phosphate
a double phosphorylation using a Ser-bound phosphate to phosphorylate the C6 position but then the Ser-bound phosphate is regenerated by removal of the C1 phosphate
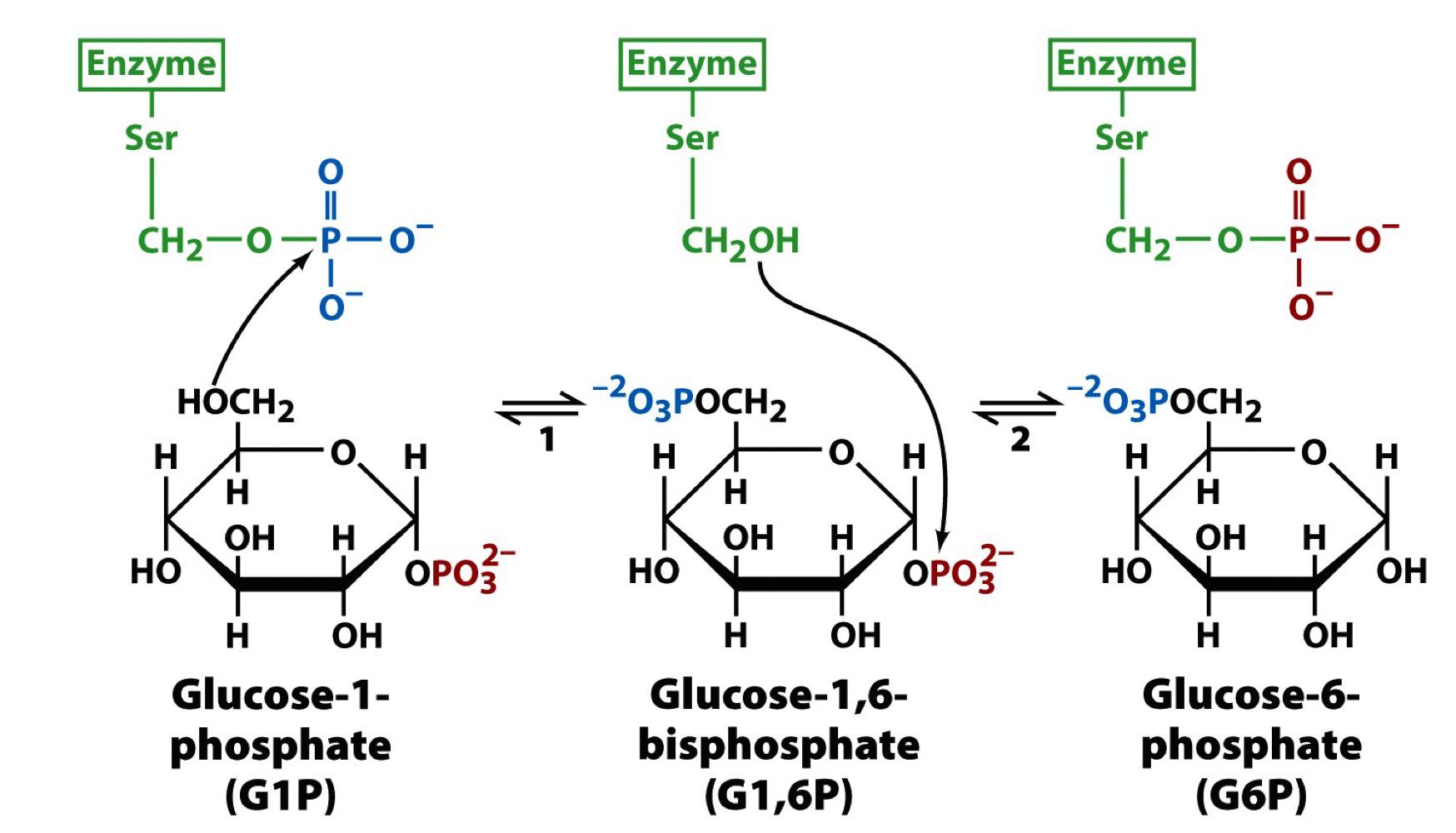
27
New cards
under anaerobic conditions in the muscle, what is the net yield of ATP from glycolysis when glycogen is used as a starting material (assume from α-1-4 linkages)?
3 ATP
bypassing the committed step = less energy
bypassing the committed step = less energy
28
New cards
glycogen breakdown vs glycogen synthesis
breakdown is thermodynamically favorable while synthesis is not, so the processes occur by separate pathways
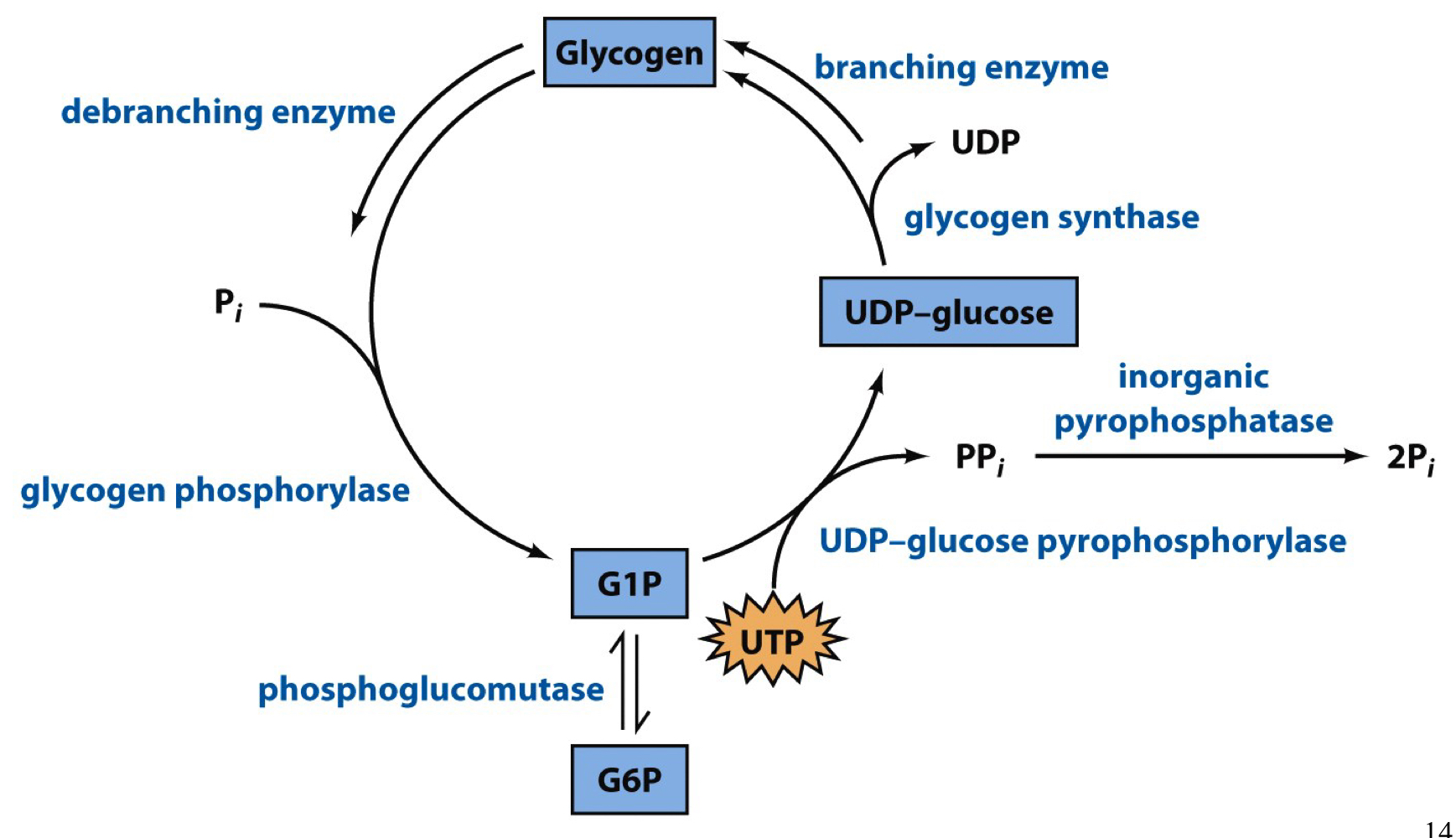
29
New cards
the enzymes used in glycogen synthesis
1. UDP-glucose pyrophosphorylase
2. glycogen synthase
3. branching enzyme
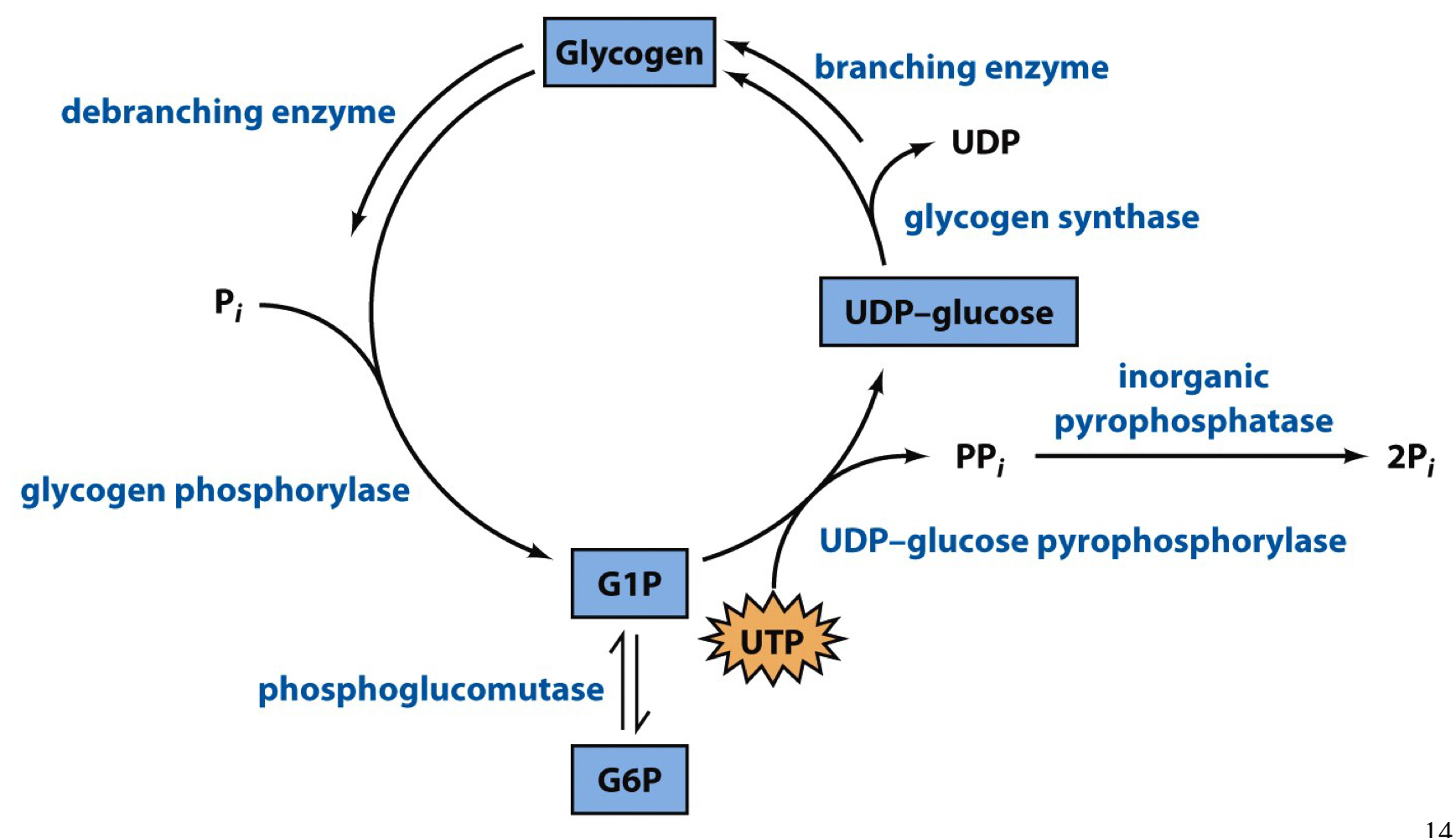
30
New cards
UDP-glucose pyrophosphorylase
phosphorylation oxygen of glucose-1-phosphate attacks the α-phosphorus atom of UTP to generate UDP-glucose
the γ and β phosphorus groups are released as pyrophosphate, which is then further hydrolyses by pyrophosphatase. this coupled step provides the energetic “pull” towards UDP-glucose formation
UDP-glucose is now activated to donate glucose to a growing glycogen chain
the γ and β phosphorus groups are released as pyrophosphate, which is then further hydrolyses by pyrophosphatase. this coupled step provides the energetic “pull” towards UDP-glucose formation
UDP-glucose is now activated to donate glucose to a growing glycogen chain
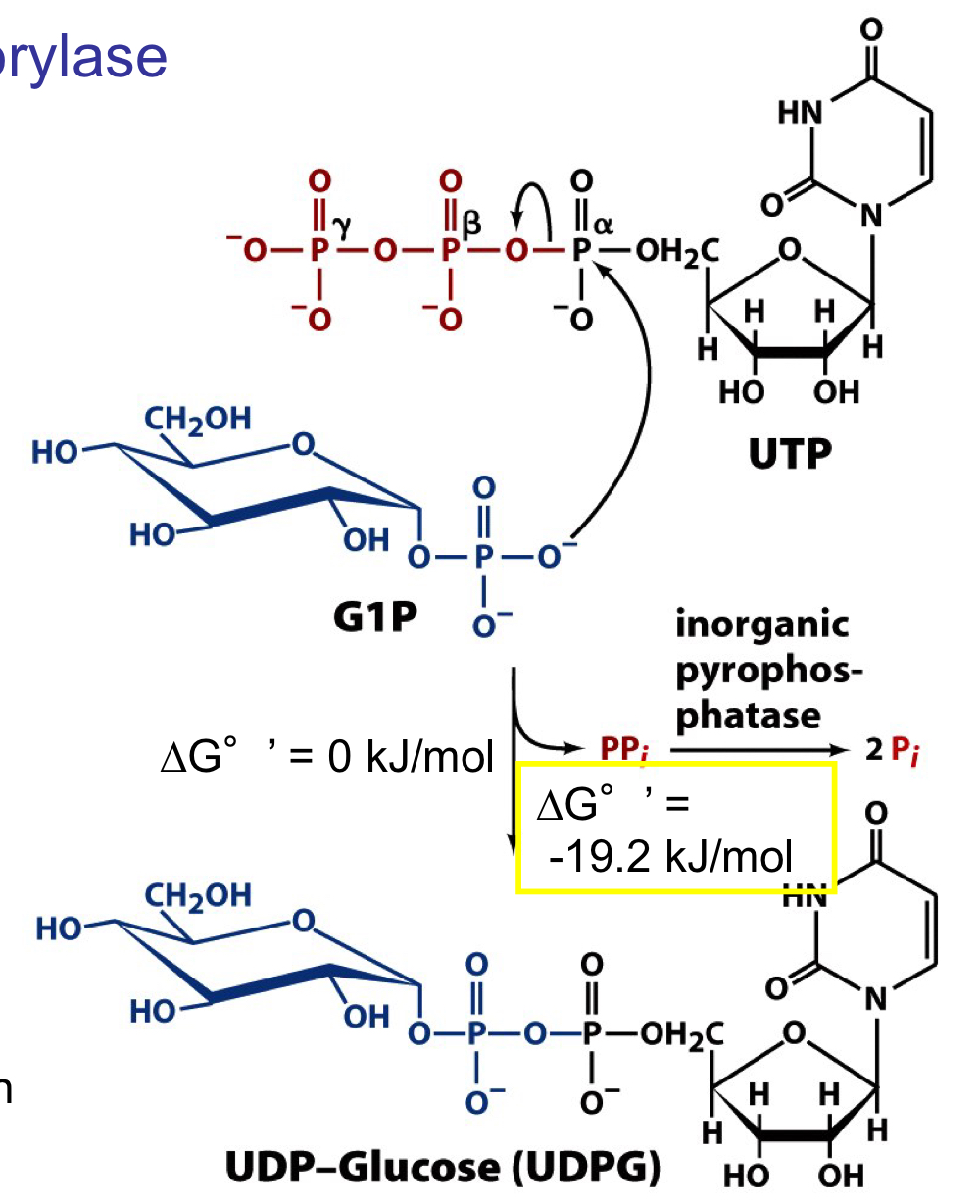
31
New cards
glycogen synthase
the UDP of UDP-glucose is a good leaving group, so it departs and generates an electrophilic oxonium ion at C1 that can be attached by the C4 hydroxyl group of the non-reducing end of the glycogen chain
only introduces α-1-4 linkages
only introduces α-1-4 linkages
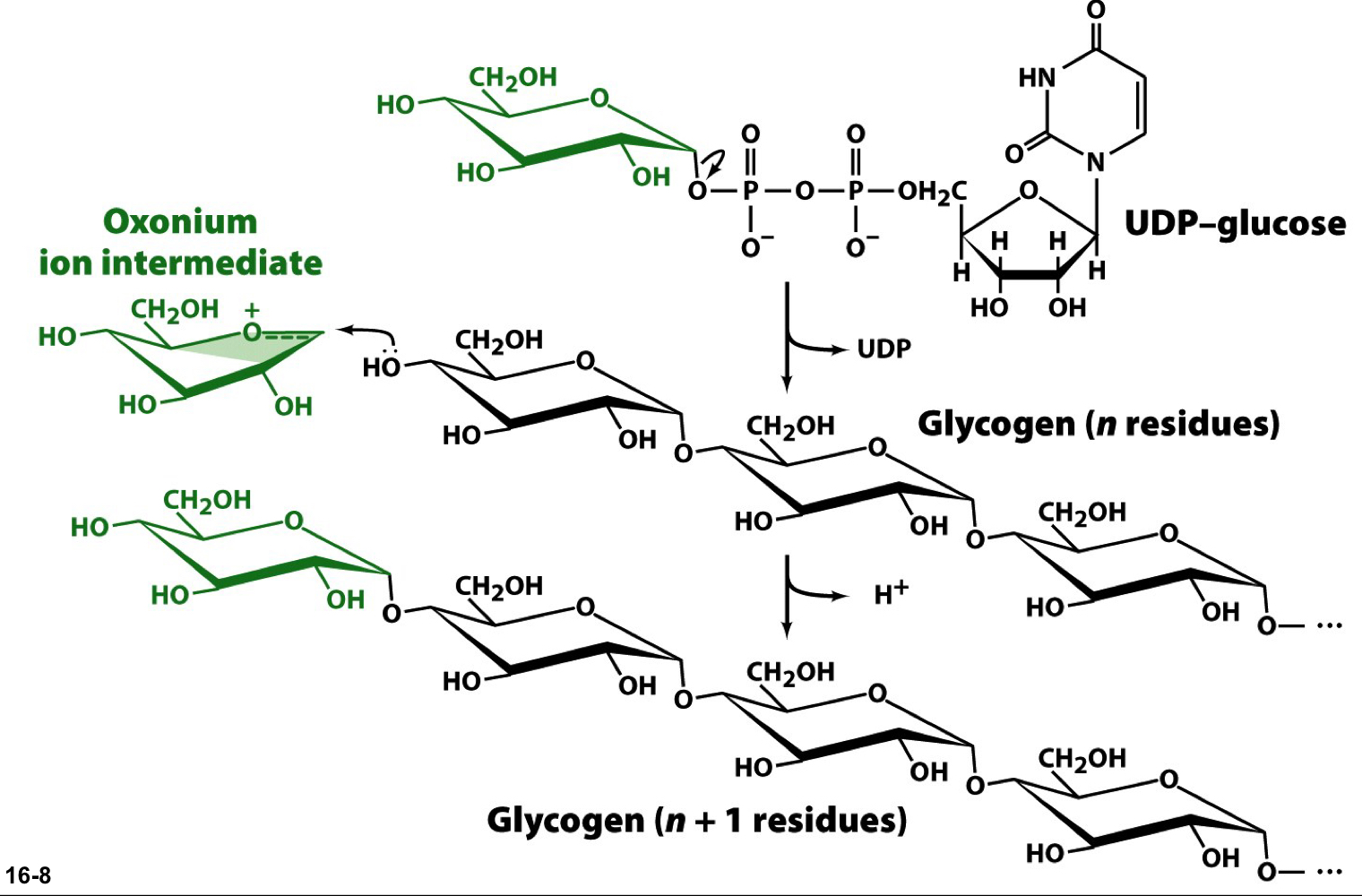
32
New cards
branching enzyme
creates α-1-6 branch points
a 7 residue section is transferred from a linear chain at least 11 residues long to another point on that chain or to an adjacent chain
branch points are separated by at least 4 residues
a 7 residue section is transferred from a linear chain at least 11 residues long to another point on that chain or to an adjacent chain
branch points are separated by at least 4 residues
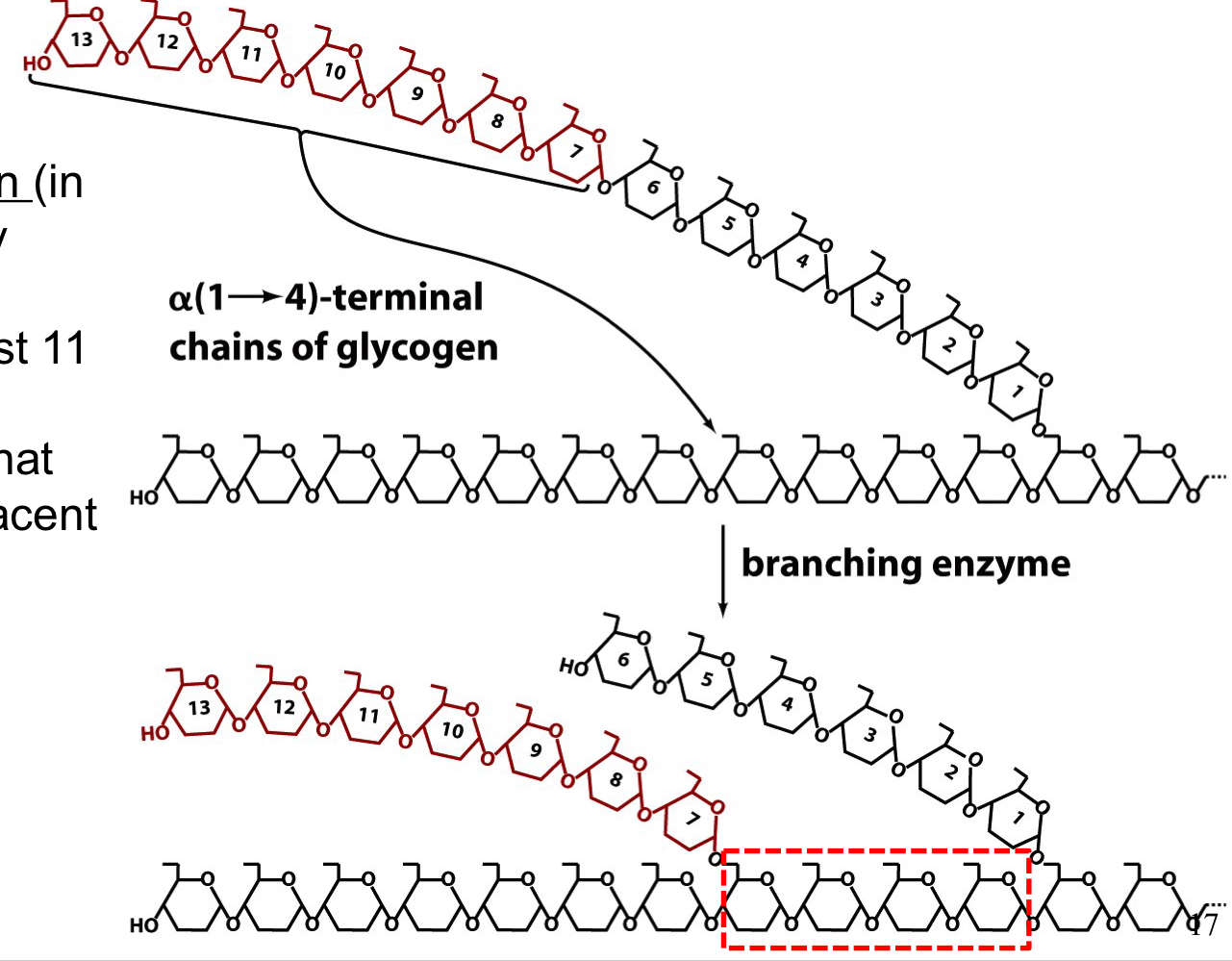
33
New cards
the magic number for glycogen branching
13 residues
highly branched structures are dense with glucose and have many ends for glucose release by phosphorylase (fast) but require significant debranching (slow)
low branched structures don’t require as much deb ranching but also can’t pack as much glucose into a small shape and don’t have as many non-reducing ends for glucose release
highly branched structures are dense with glucose and have many ends for glucose release by phosphorylase (fast) but require significant debranching (slow)
low branched structures don’t require as much deb ranching but also can’t pack as much glucose into a small shape and don’t have as many non-reducing ends for glucose release
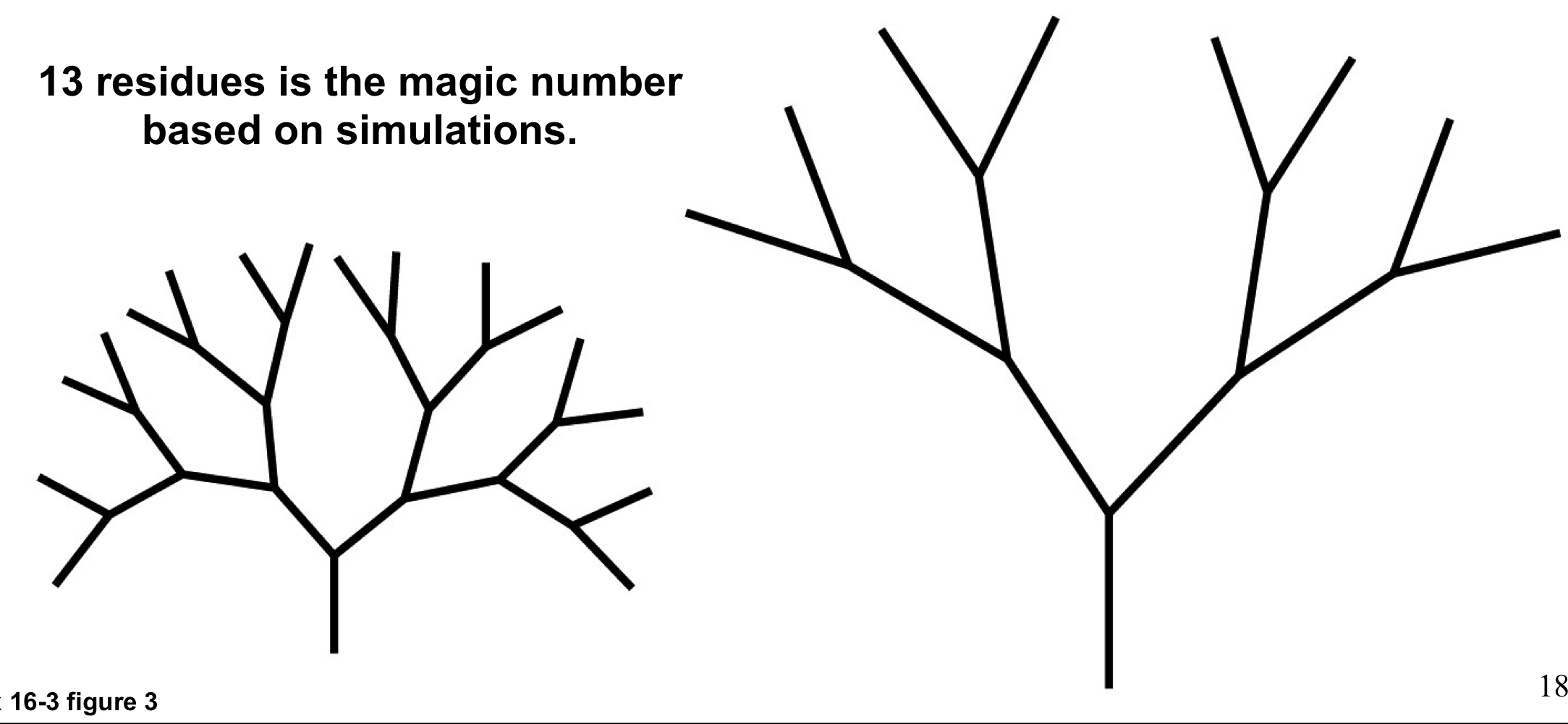
34
New cards
name of glycogen branching enzyme
amyloid-1,6-glucosidase
35
New cards
name of glycogen debranching enzyme
amyloid-(1,4→1,6)-transglycosylase
36
New cards
glycogen storage disease general
accumulation of carbohydrate intermediates
mutations in the enzymes involved in glycogen metabolism can lead to an enlarged liver, hypoglycemia, muscle cramps/weakness, and a variety of renal and cardiac symptoms
mutations in the enzymes involved in glycogen metabolism can lead to an enlarged liver, hypoglycemia, muscle cramps/weakness, and a variety of renal and cardiac symptoms
37
New cards
type I glycogen storage disease
deficient in the enzyme glucose-6-phosphatase, affects the liver, common name von Gierke’s disease, glycogen structure is normal
prevents the release of glucose into the bloodstream, causing an accumulation of glycogen in the liver and kidneys
prevents the release of glucose into the bloodstream, causing an accumulation of glycogen in the liver and kidneys
38
New cards
type II glycogen storage disease
deficient in the enzyme α-1,4-glucosidase, affects all lysosomes, common name Pompe’s disease, normal glycogen structure
39
New cards
type 0 glycogen storage disease
deficient in the enzyme glycogen synthase, affects the liver, normal glycogen structure but deficient in quantity
opposite of type I
issues with glycogen synthesis, causing a deficiency of glycogen in the body, causing issues with hyperglycemia after meals and hypoglycemia
opposite of type I
issues with glycogen synthesis, causing a deficiency of glycogen in the body, causing issues with hyperglycemia after meals and hypoglycemia
40
New cards
reciprocal regulation
the same activity or modification can have different effects on different pathways
when one is active the other is inactive
when one is active the other is inactive
41
New cards
reciprocal regulation of glycogen phosphorylase and glycogen synthase
reciprocally regulated both covalently and allosterically
covalent regulation occurs by phosphorylation and dephosphorylation
phosphorylating glycogen phosphorylase b (inactive) changes it to glycogen phosphorylase a (active)
phosphorylating glycogen synthase a (active) changes it to glycogen synthase b (inactive)
covalent regulation occurs by phosphorylation and dephosphorylation
phosphorylating glycogen phosphorylase b (inactive) changes it to glycogen phosphorylase a (active)
phosphorylating glycogen synthase a (active) changes it to glycogen synthase b (inactive)
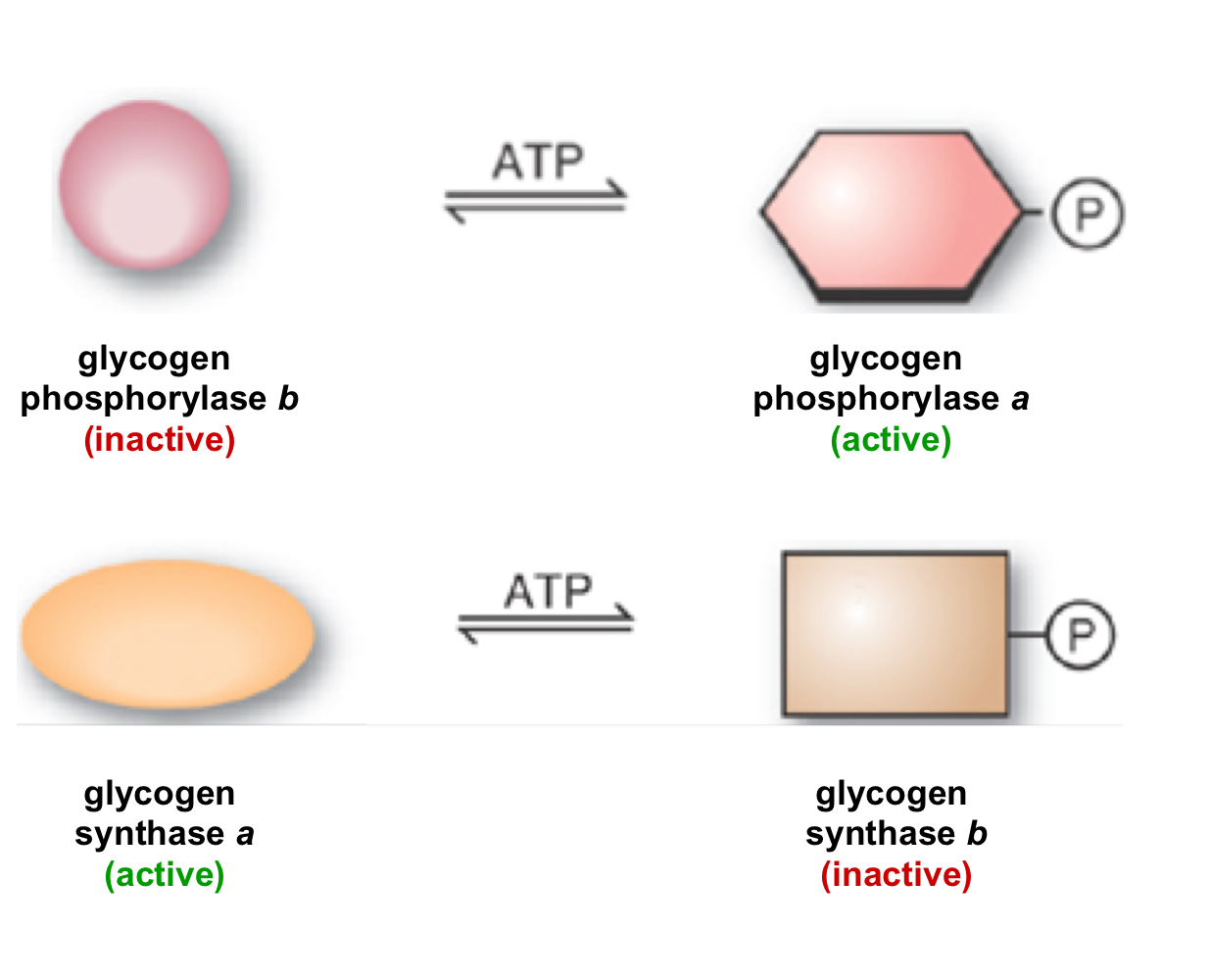
42
New cards
the site of phosphorylation on glycogen phosphorylase
Ser 14
glycogen phosphorylase forms a homodimer
glycogen phosphorylase forms a homodimer
43
New cards
b vs a form of glycogen phosphorylase
the b form is unphosphorylated and the a form is phosphorylated
44
New cards
regulation of glycogen phosphorylase
the T state has an open, inactive active site
the R state is active
the phosphorylated a form of the enzyme has the equilibrium shifted towards the R (active) state
the R state is active
the phosphorylated a form of the enzyme has the equilibrium shifted towards the R (active) state
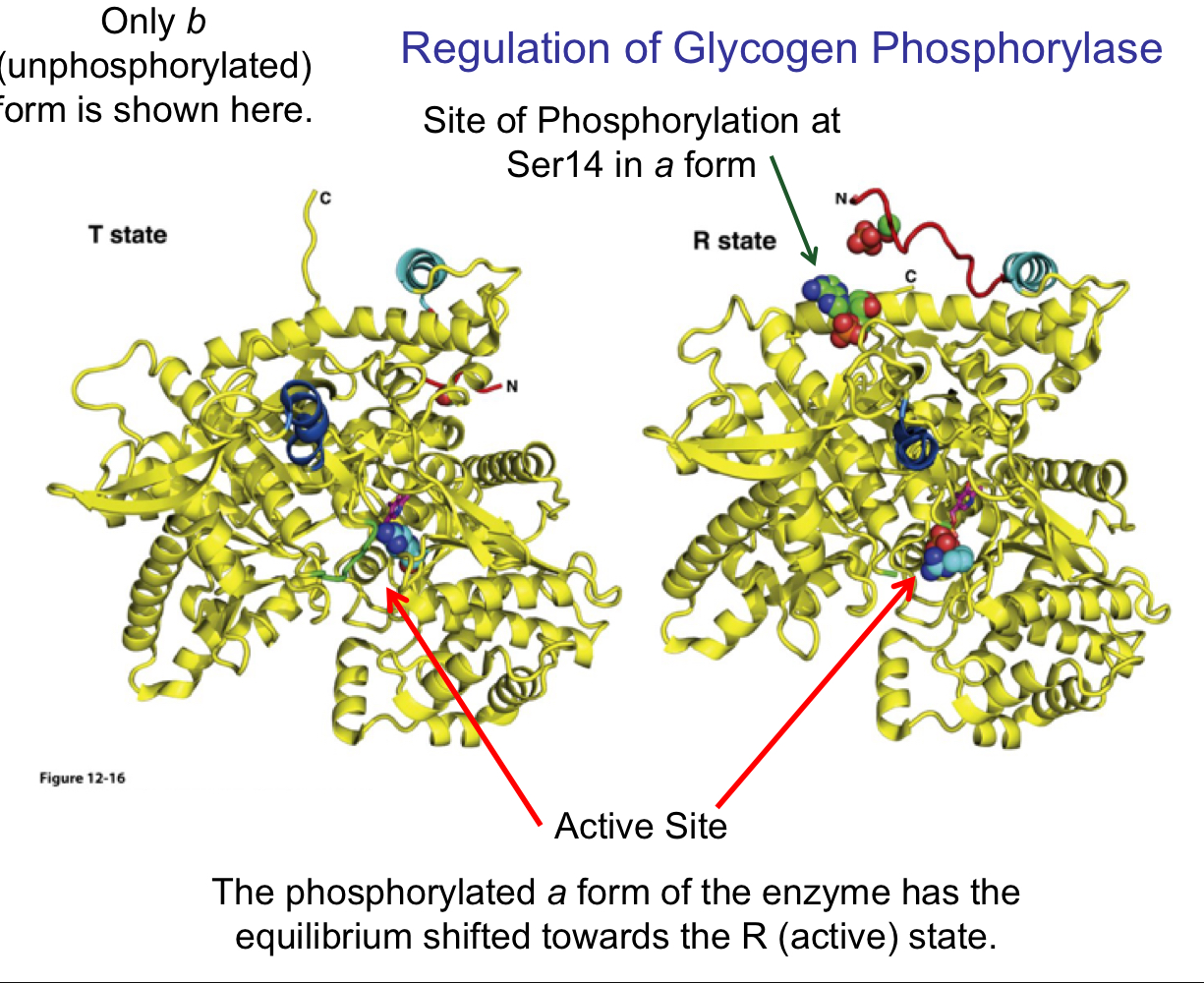
45
New cards
phosphorylation effect on glycogen phosphorylase regulation
in addition to promoting shift to active R form, phosphorylation also changes which other allosteric regulations (ATP, G6P, glucose) can affect GP
in the unphosphorylated (b) active (R) form of GP, ATP and/or G6P inactivate it, changing to the T form
in the unphosphorylated (b) inactive (T) form of GP, AMP activates it, changing to the R form
in the phosphorylated (a) active (R) form of GP, glucose inactivates it, changing to the T form
phosphorylase kinase uses 2 ATP to phosphorylate the T form of GP (still inactive)
phosphoprotein phosphatase uses 2 H2O to dephosphorylate the T form of GP (still inactive)
in the unphosphorylated (b) active (R) form of GP, ATP and/or G6P inactivate it, changing to the T form
in the unphosphorylated (b) inactive (T) form of GP, AMP activates it, changing to the R form
in the phosphorylated (a) active (R) form of GP, glucose inactivates it, changing to the T form
phosphorylase kinase uses 2 ATP to phosphorylate the T form of GP (still inactive)
phosphoprotein phosphatase uses 2 H2O to dephosphorylate the T form of GP (still inactive)
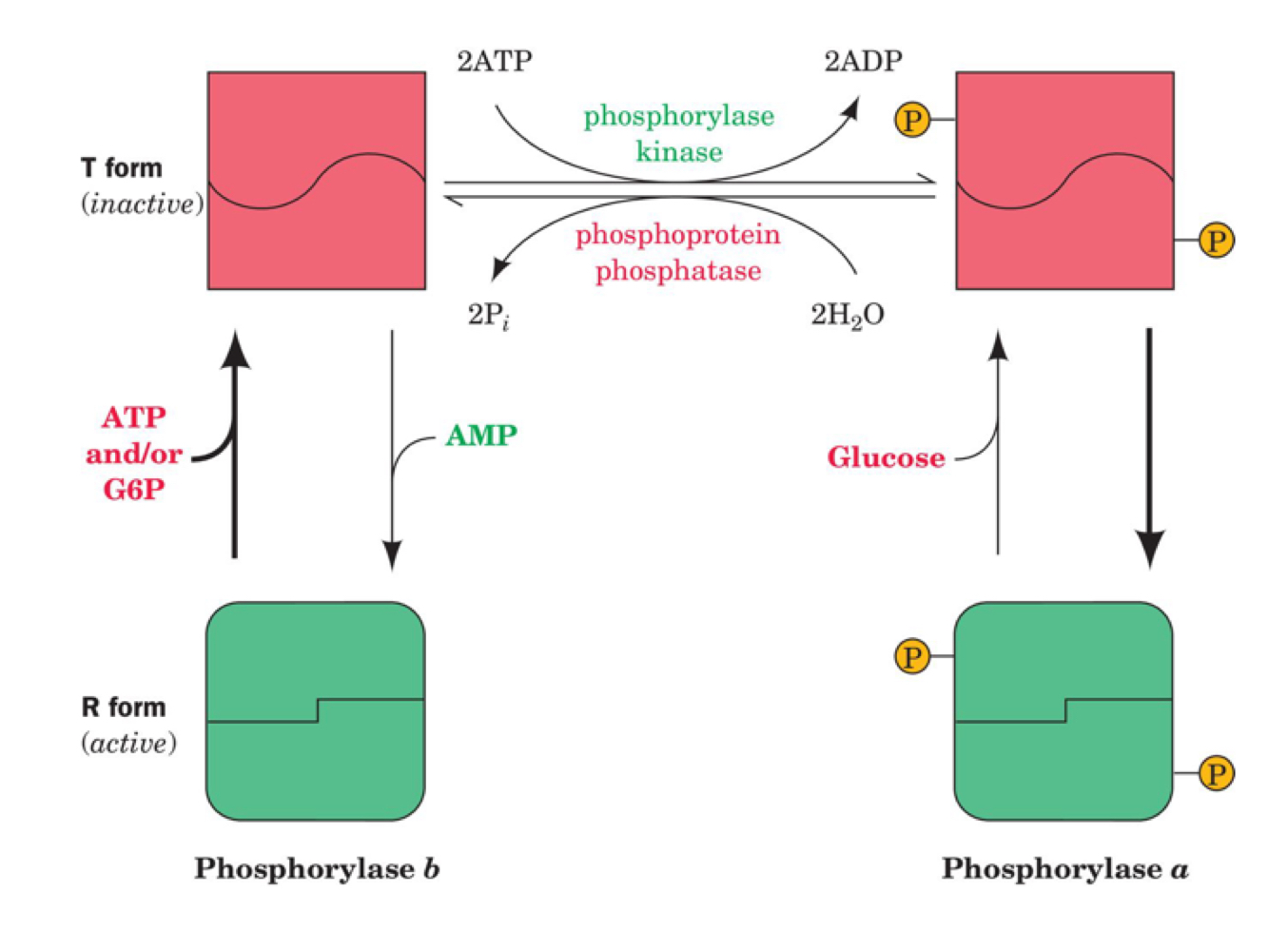
46
New cards
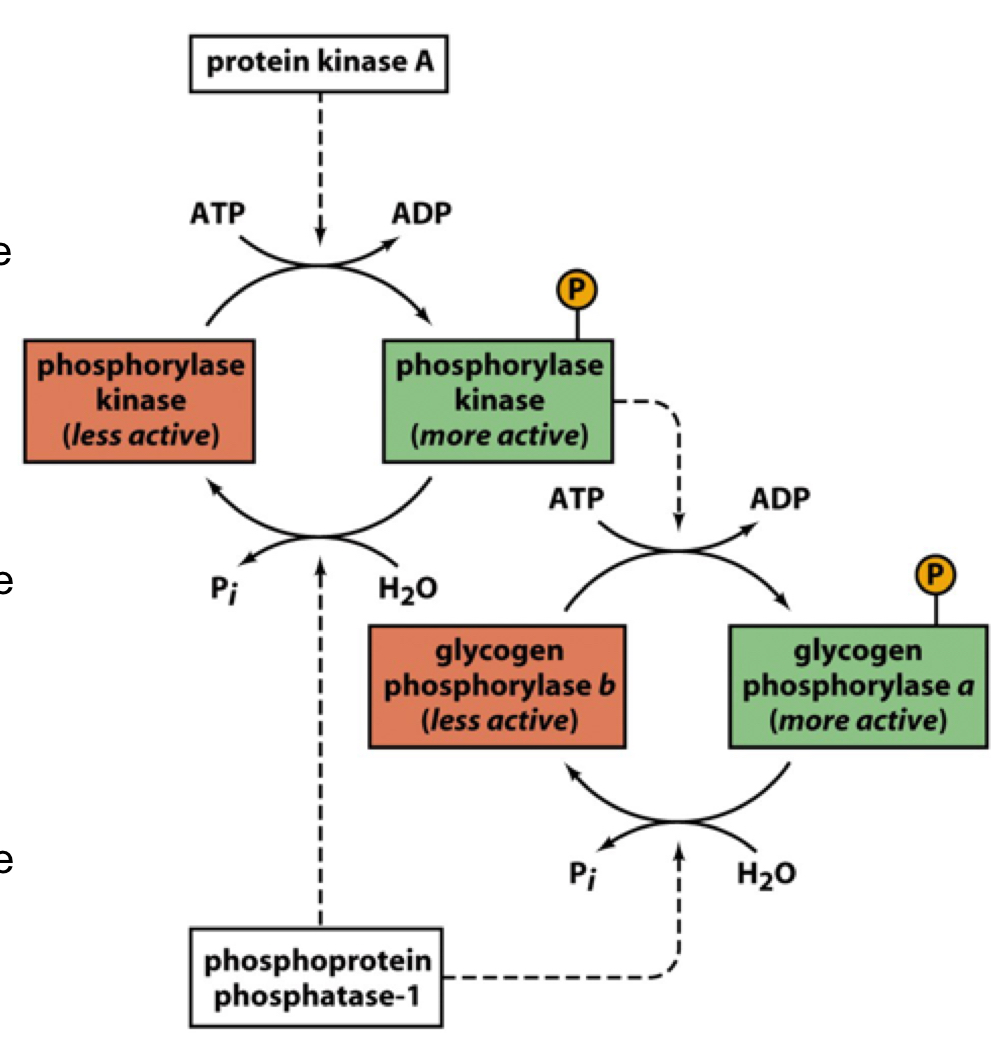
the role of protein kinase A
protein kinase A (PKA) activates phosphorylase kinase by phosphorylating it
activated phosphorylase kinase phosphorylates glycogen phosphorylase at Ser 14
protein phosphatase-1 (PP1) will dephosphorylate glycogen phosphorylase (lowering activity) and dephosphorylates phosphorylase kinase
activated phosphorylase kinase phosphorylates glycogen phosphorylase at Ser 14
protein phosphatase-1 (PP1) will dephosphorylate glycogen phosphorylase (lowering activity) and dephosphorylates phosphorylase kinase
47
New cards
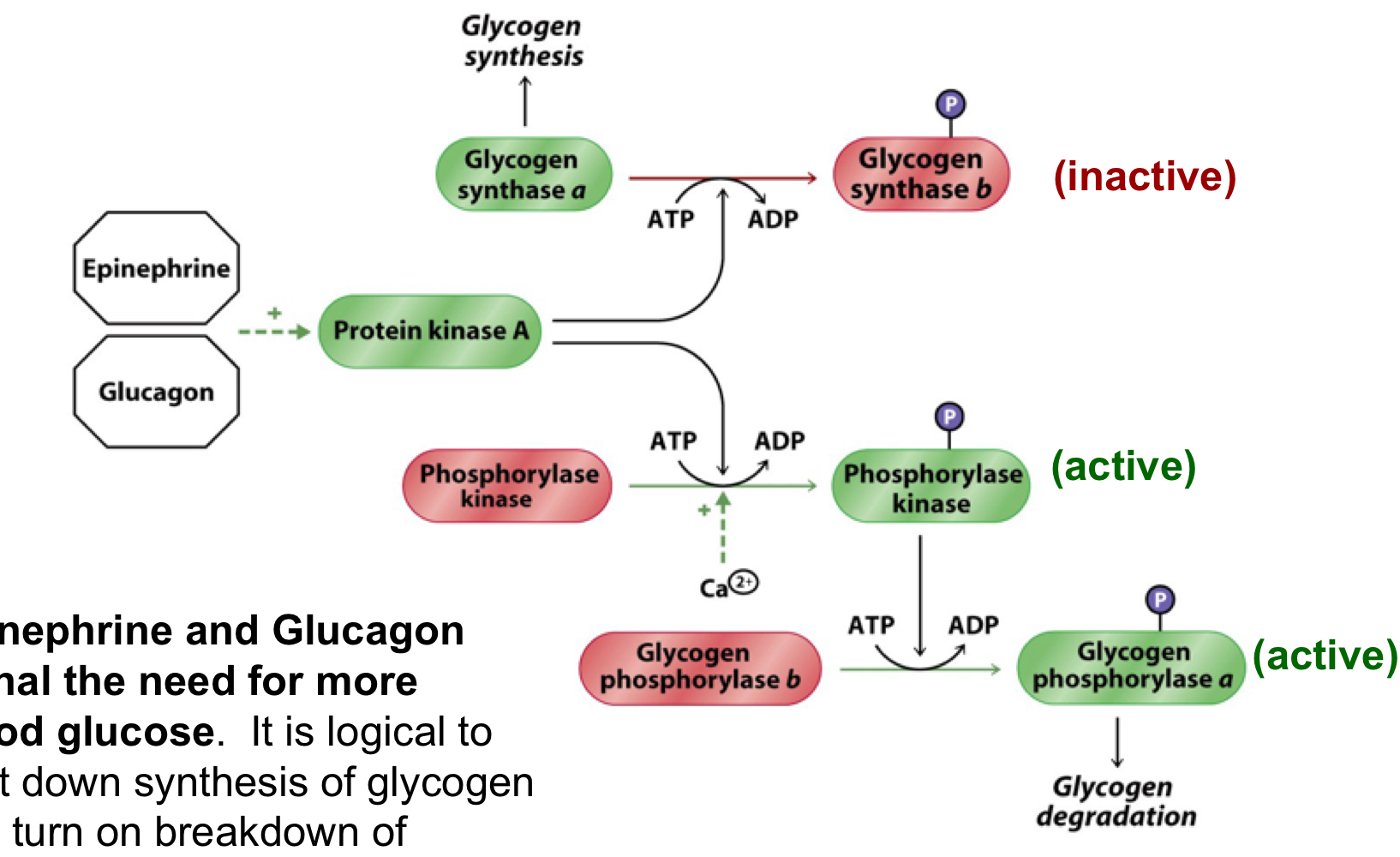
signal for more blood glucose
epinephrine and glucagon signal the need for more blood glucose
it’s logical to shut down synthesis of glycogen and turn on breakdown of glycogen to help restore blood glucose
activate PKA, which shifts glycogen synthase a to b (inactivating it), activates phosphorylase kinase, which then activates glycogen phosphorylase b by shifting it to a, causing glycogen degradation
it’s logical to shut down synthesis of glycogen and turn on breakdown of glycogen to help restore blood glucose
activate PKA, which shifts glycogen synthase a to b (inactivating it), activates phosphorylase kinase, which then activates glycogen phosphorylase b by shifting it to a, causing glycogen degradation
48
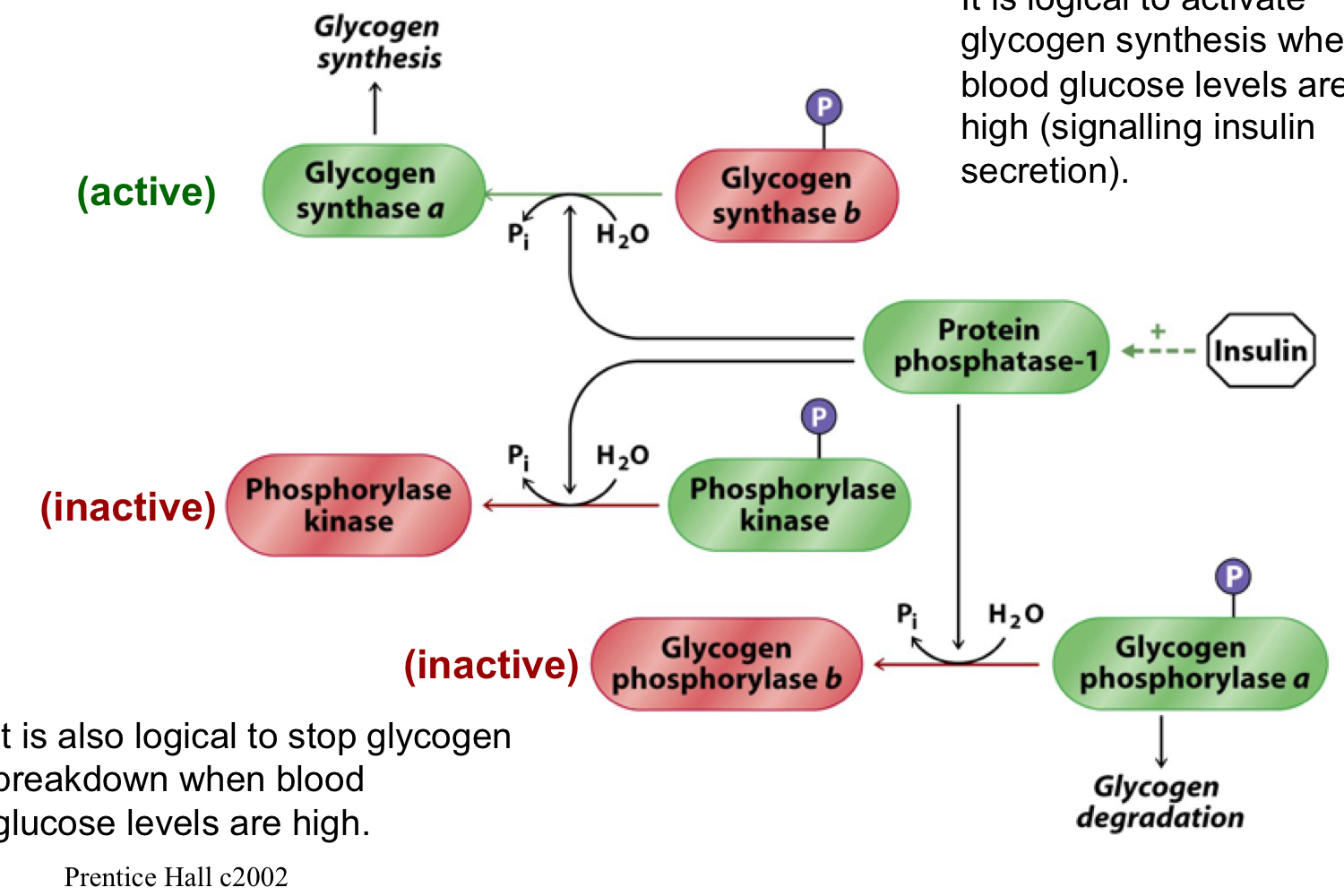
New cards
insulin
it’s logical to activate glycogen synthesis when blood glucose levels are high (signaling insulin secretion)
it’s also logical to stop glycogen breakdown when blood glucose levels are high
insulin activates protein phosphatase-1, which then activates glycogen synthase by shifting it to the a form, causing glycogen synthesis
protein phosphatase 1 also inactivates phosphorylase kinase and inactivates glycogen phosphorylase by shifting it to the b form (stopping glycogen breakdown)
it’s also logical to stop glycogen breakdown when blood glucose levels are high
insulin activates protein phosphatase-1, which then activates glycogen synthase by shifting it to the a form, causing glycogen synthesis
protein phosphatase 1 also inactivates phosphorylase kinase and inactivates glycogen phosphorylase by shifting it to the b form (stopping glycogen breakdown)
49
New cards
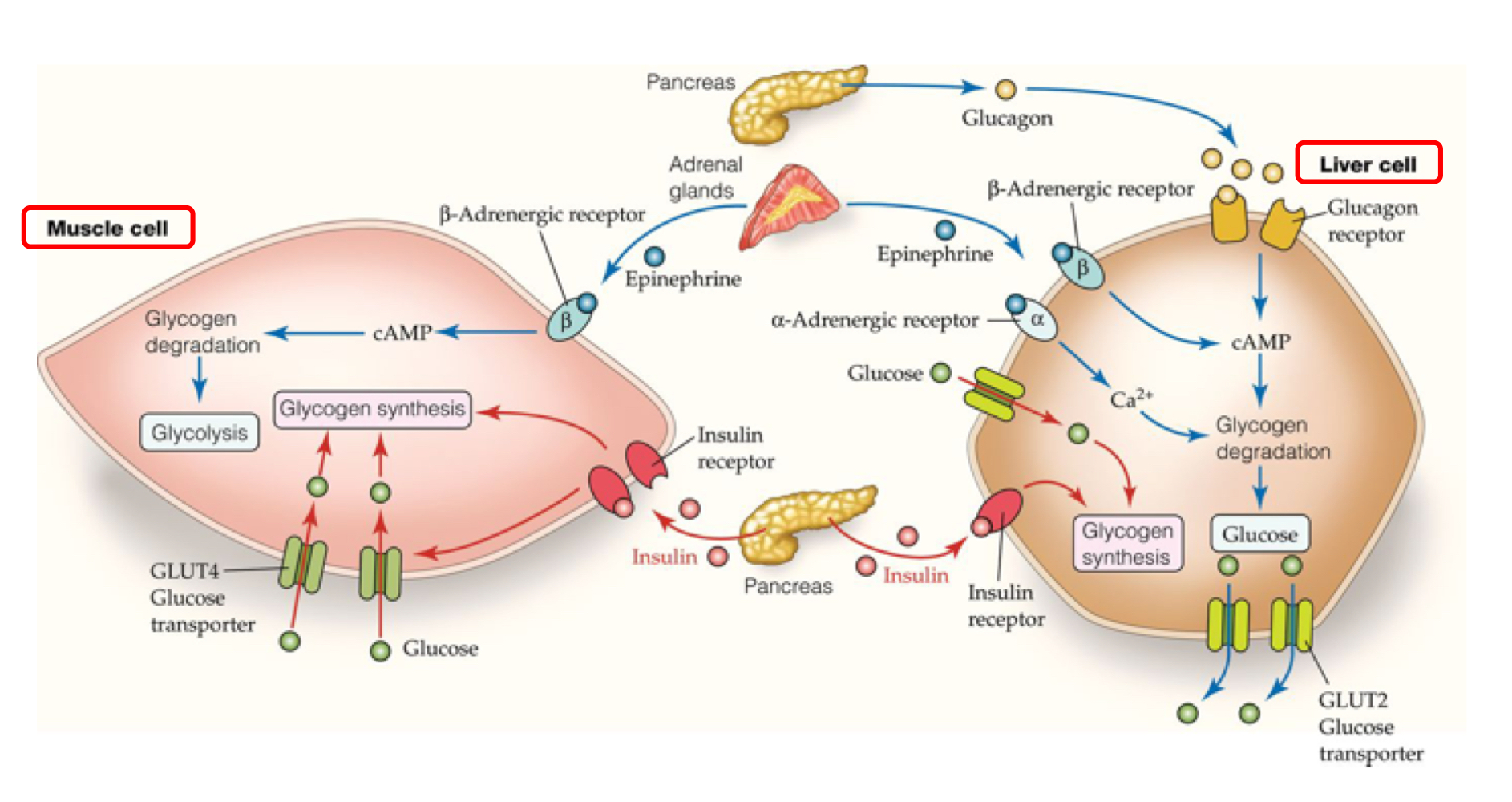
hormonal control of glycogen metabolism in muscle and liver cells
cAMP is used in both cells to activate glycogen degradation
in muscle cells, insulin from the pancreas binding simultaneously activates glucose transporters and glycogen synthesis
in liver cells insulin binding only activates glycogen synthesis, not glucose transporters
in muscle and liver cells, epinephrine binding to a β adrenergic receptor activates cAMP, causing glycogen degradation, causing glycolysis (in muscle) and creating glucose (in liver)
in liver cells, glucagon from the pancreas binding activates cAMP
muscle cells consume energy, while liver cells are involved in glucose metabolism and shuffling
in liver cells, α adrenergic receptors increase Ca2+, signaling for glycogen degradation
in muscle cells, insulin from the pancreas binding simultaneously activates glucose transporters and glycogen synthesis
in liver cells insulin binding only activates glycogen synthesis, not glucose transporters
in muscle and liver cells, epinephrine binding to a β adrenergic receptor activates cAMP, causing glycogen degradation, causing glycolysis (in muscle) and creating glucose (in liver)
in liver cells, glucagon from the pancreas binding activates cAMP
muscle cells consume energy, while liver cells are involved in glucose metabolism and shuffling
in liver cells, α adrenergic receptors increase Ca2+, signaling for glycogen degradation
50
New cards
which of the following enzymes is exclusively used in glycogen breakdown?
hexokinase
glucose-6-phosphatase
glycogen phosphorylase
glycogen synthase
phosphoglucomutase
hexokinase
glucose-6-phosphatase
glycogen phosphorylase
glycogen synthase
phosphoglucomutase
glycogen phosphorylase
51
New cards
overview of gluconeogenesis and glycolysis
steps in glycolysis that require new enzymes to bypass non-equilibrium reactions in glycolysis are shown with a green box
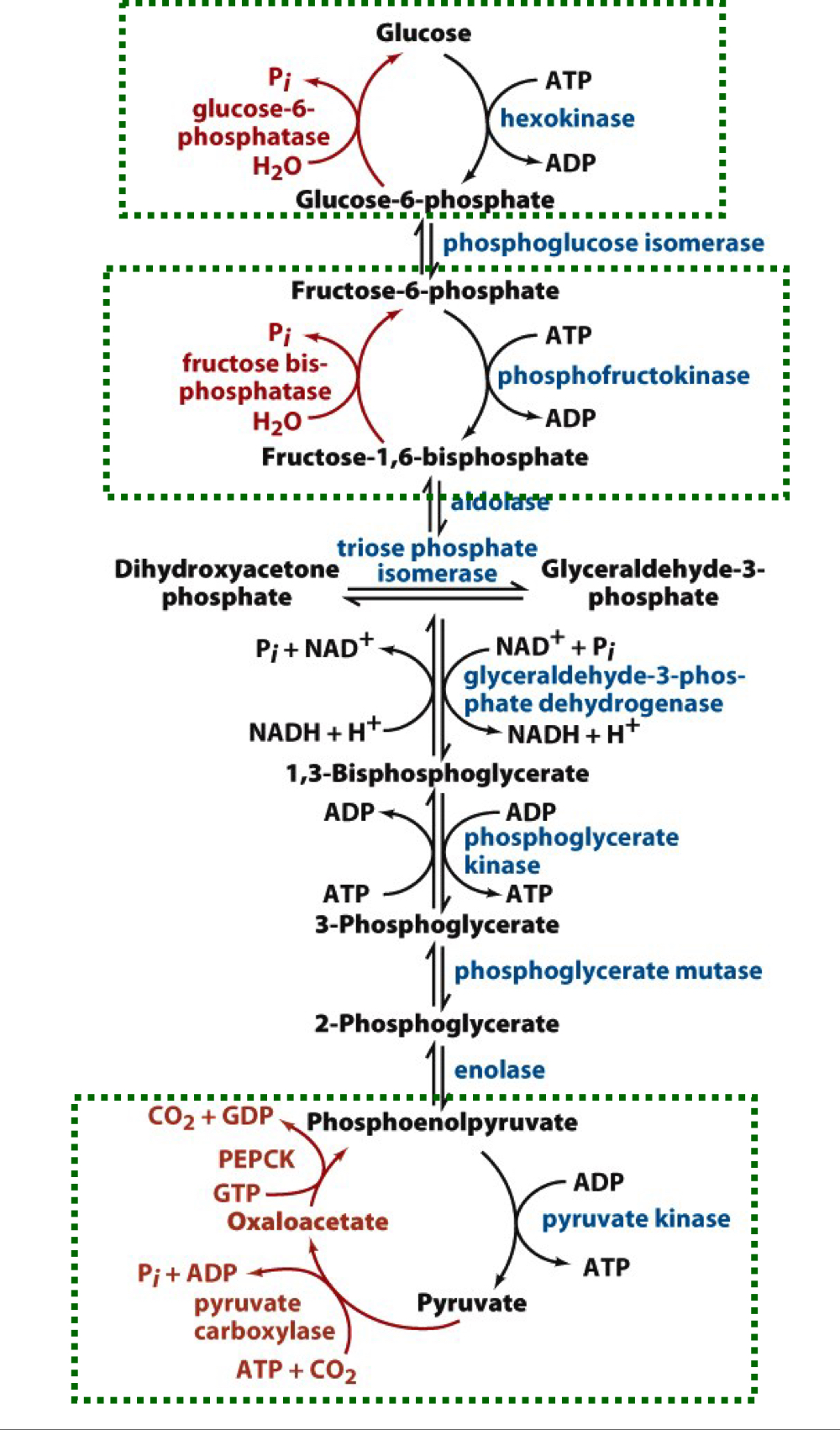
52
New cards
enzymes needed to bypass pyruvate kinase step in glycolysis
two enzymes (pyruvate carboxylase and PEP carbokinase) are needed in gluconeogenesis to bypass the highly irreversible step catalyzed by pyruvate kinase in glycolysis
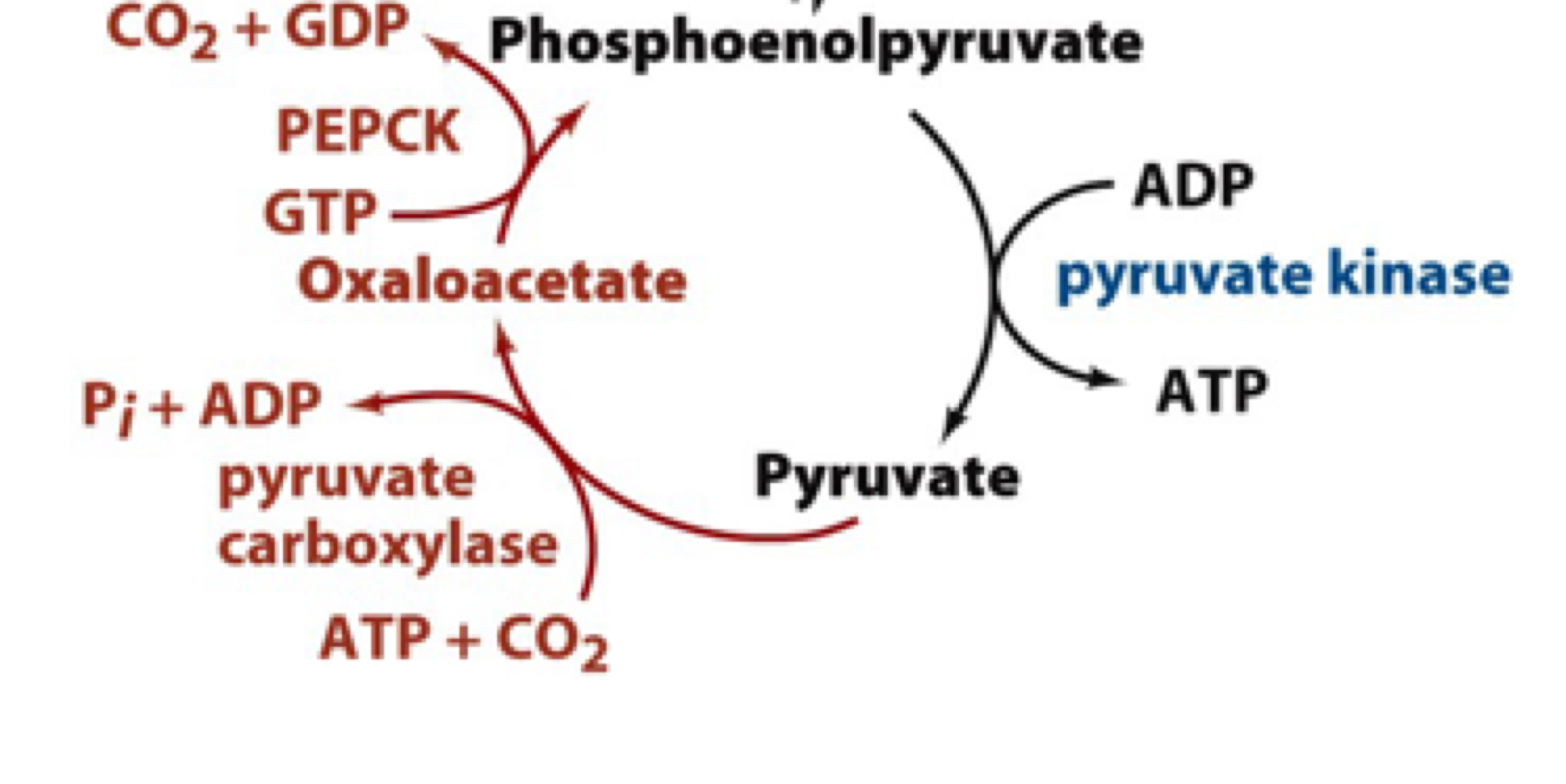
53
New cards
overall strategy of forming phosphoenolpyruvate from pyruvate and CO2
1. form oxaloacetate using ATP to drive this reaction (catalyzed by pyruvate carboxylase)
2. decarboxylation of oxaloacetate is energetically favorable and can help drive PEP formation. GTP is used as the phosphorylase group donor (catalyzed by PEP carboxykinase)
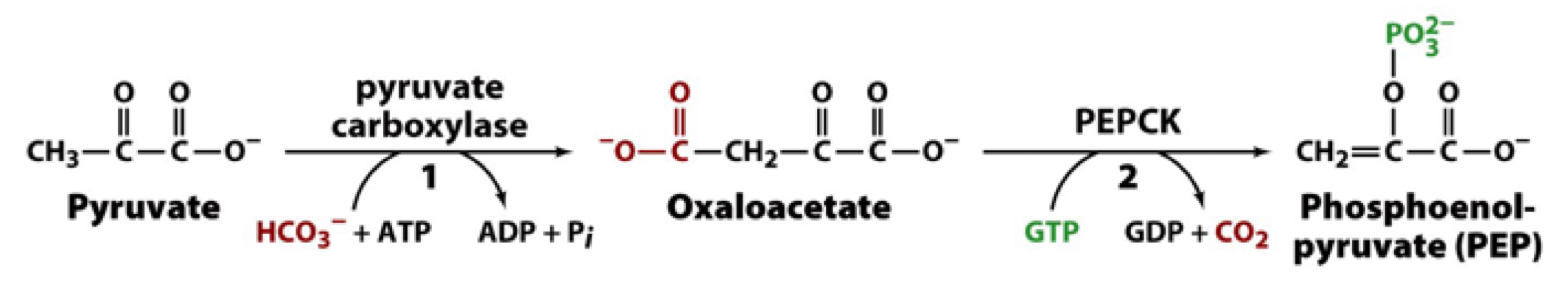
54
New cards
pyruvate carboxylase
PC catalyzes a metabolically irreversible reaction that fixes CO2 and forms oxaloacetate
PC is the first step in gluconeogenesis, but it’s also important to replenishment of the TCA cycle
PC is a mitochondrial enzyme that is allosterically activated by acetyl CoA. accumulation of acetyl CoA from fatty acid oxidation during fasting/starvation signals the liver to direct pyruvate to oxaloacetate for gluconeogenesis
PC uses biotin as a cofactor to carry CO2 in the form of carboxybiotinyl group
PC is the first step in gluconeogenesis, but it’s also important to replenishment of the TCA cycle
PC is a mitochondrial enzyme that is allosterically activated by acetyl CoA. accumulation of acetyl CoA from fatty acid oxidation during fasting/starvation signals the liver to direct pyruvate to oxaloacetate for gluconeogenesis
PC uses biotin as a cofactor to carry CO2 in the form of carboxybiotinyl group
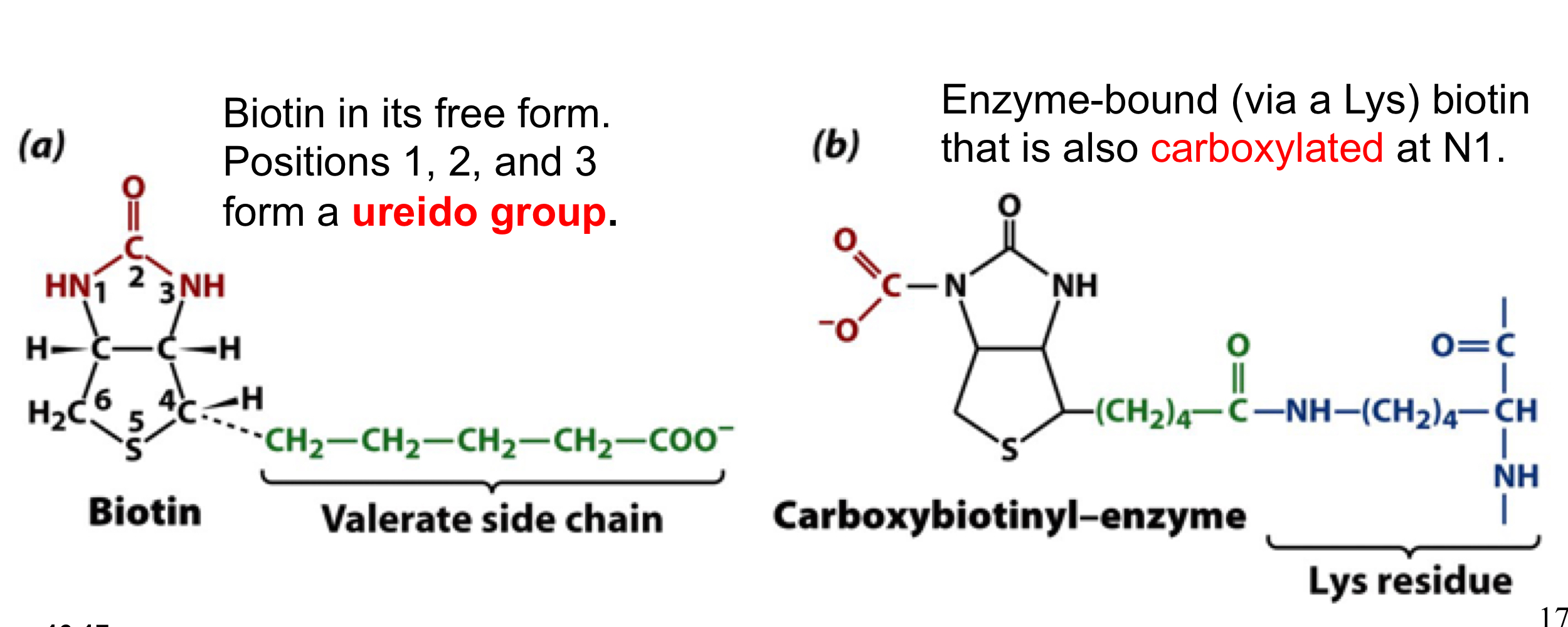
55
New cards
PC reaction phase I
cleavage of ATP drives formation of a carboxyphosphate intermediate. transfer of the CO2 group to biotin is exergonic (favorable)
this forms an activated biotin-bound carboxyl group that is a better donor than free bicarbonate
this forms an activated biotin-bound carboxyl group that is a better donor than free bicarbonate
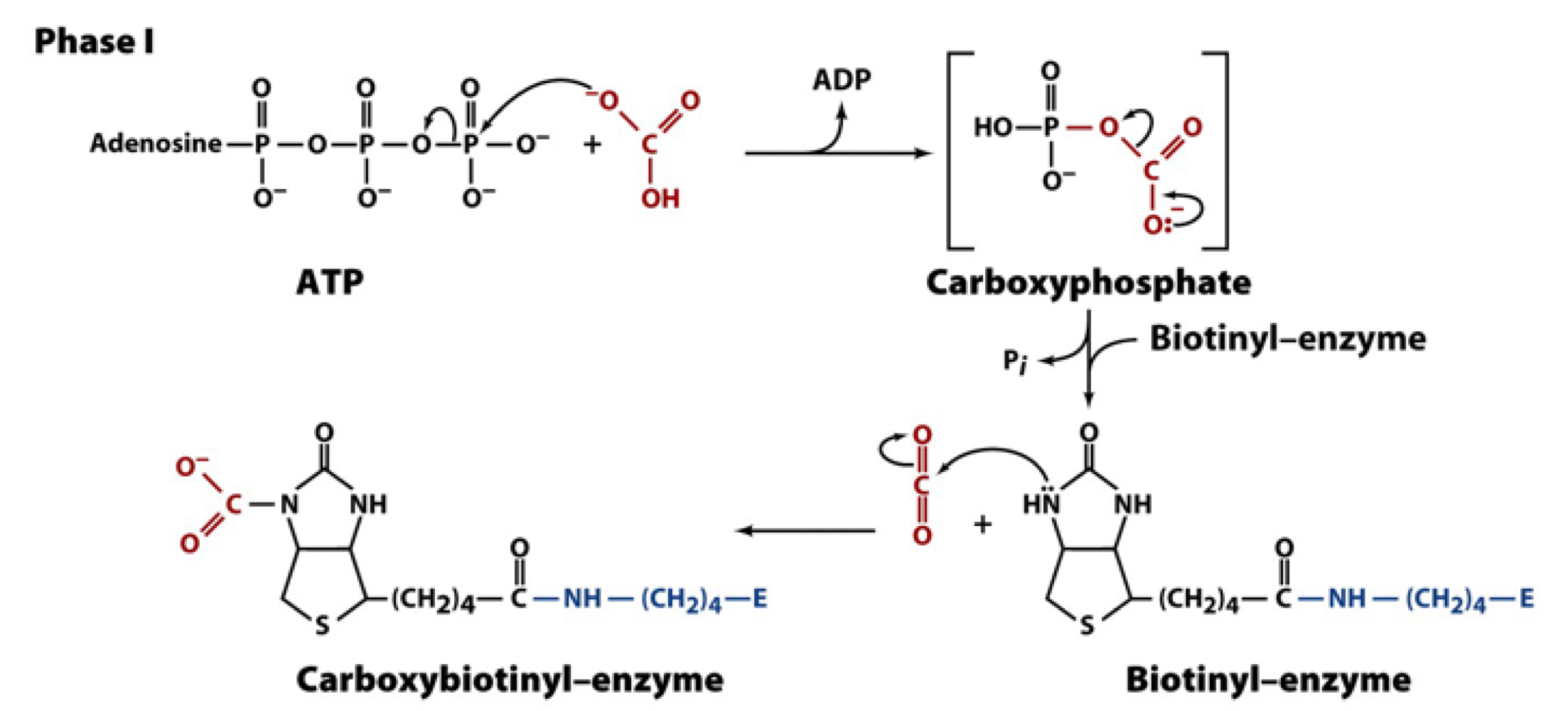
56
New cards
PC reaction phase II
the CO2 group is transferred to pyruvate as follows:
entry of pyruvate into the active site releases CO2 from biotin as the biotin accepts a proton from pyruvate
pyruvate (minus a proton) is now in an enolate form which carries out a nucleophilic attack on CO2
entry of pyruvate into the active site releases CO2 from biotin as the biotin accepts a proton from pyruvate
pyruvate (minus a proton) is now in an enolate form which carries out a nucleophilic attack on CO2
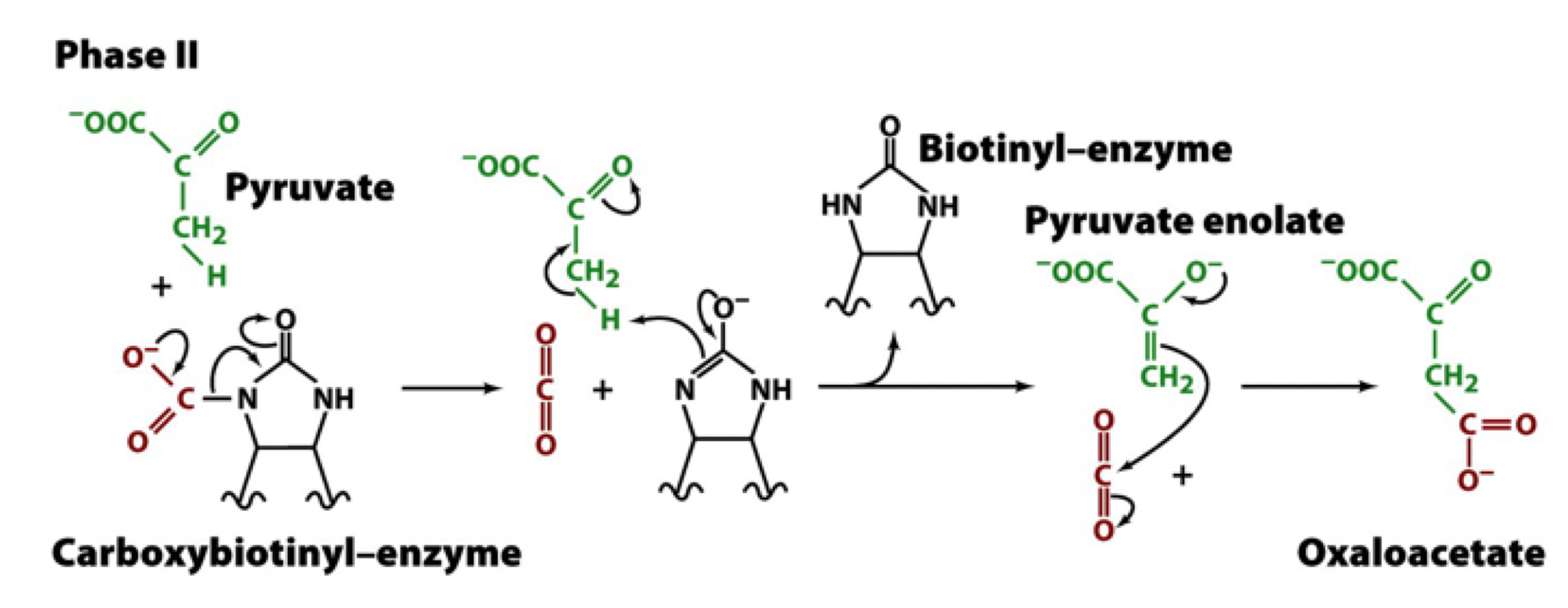
57
New cards
PEPCK reaction
the CO2 group integrated into pyruvate to make oxaloacetate is now released via a decarboxylation reaction, creating an enol form of the compound which is a good substate for phosphorylation by GTP. PEP is the product
oxaloacetate is an essential intermediate recognized by PEPCK
oxaloacetate is an essential intermediate recognized by PEPCK
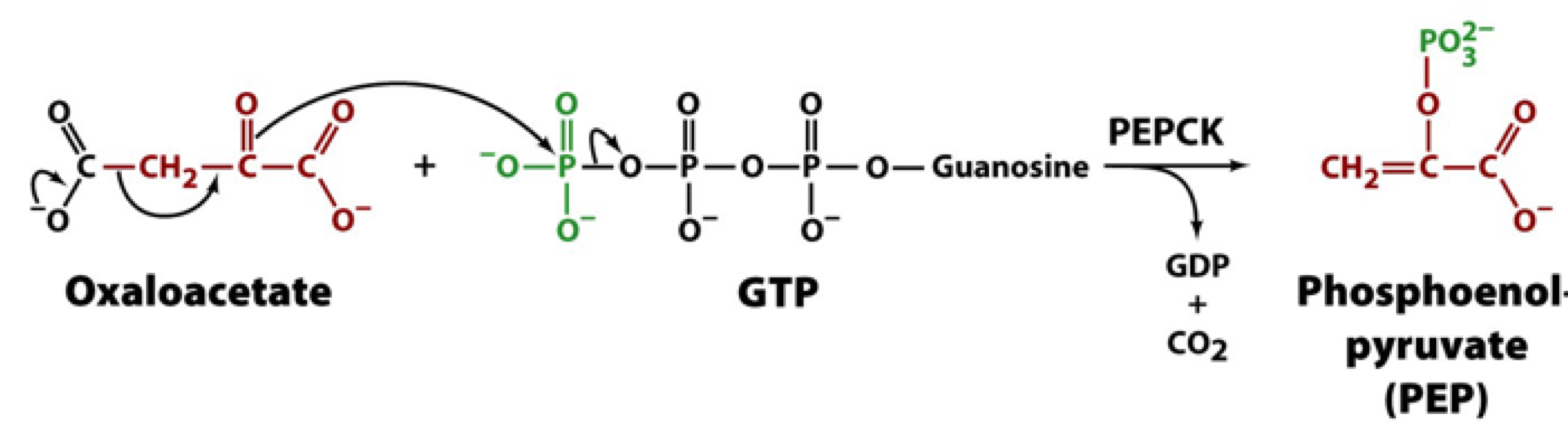
58
New cards
the triose phase of gluconeogenesis
after formation of phosphoenolpyruvate, the remaining triose stages are shared by glycolysis and gluconeogenesis (they can run in the reverse direction for gluconeogenesis)
all enzymes between PEP and DHAP/GAP are near equilibrium and flux can be pushed in the reverse direction by the production of PEP from the PC/PEPCK enzymes
also, pyruvate kinase would be down regulated under these conditions to avoid consumption of PEP in the opposite direction
all enzymes between PEP and DHAP/GAP are near equilibrium and flux can be pushed in the reverse direction by the production of PEP from the PC/PEPCK enzymes
also, pyruvate kinase would be down regulated under these conditions to avoid consumption of PEP in the opposite direction
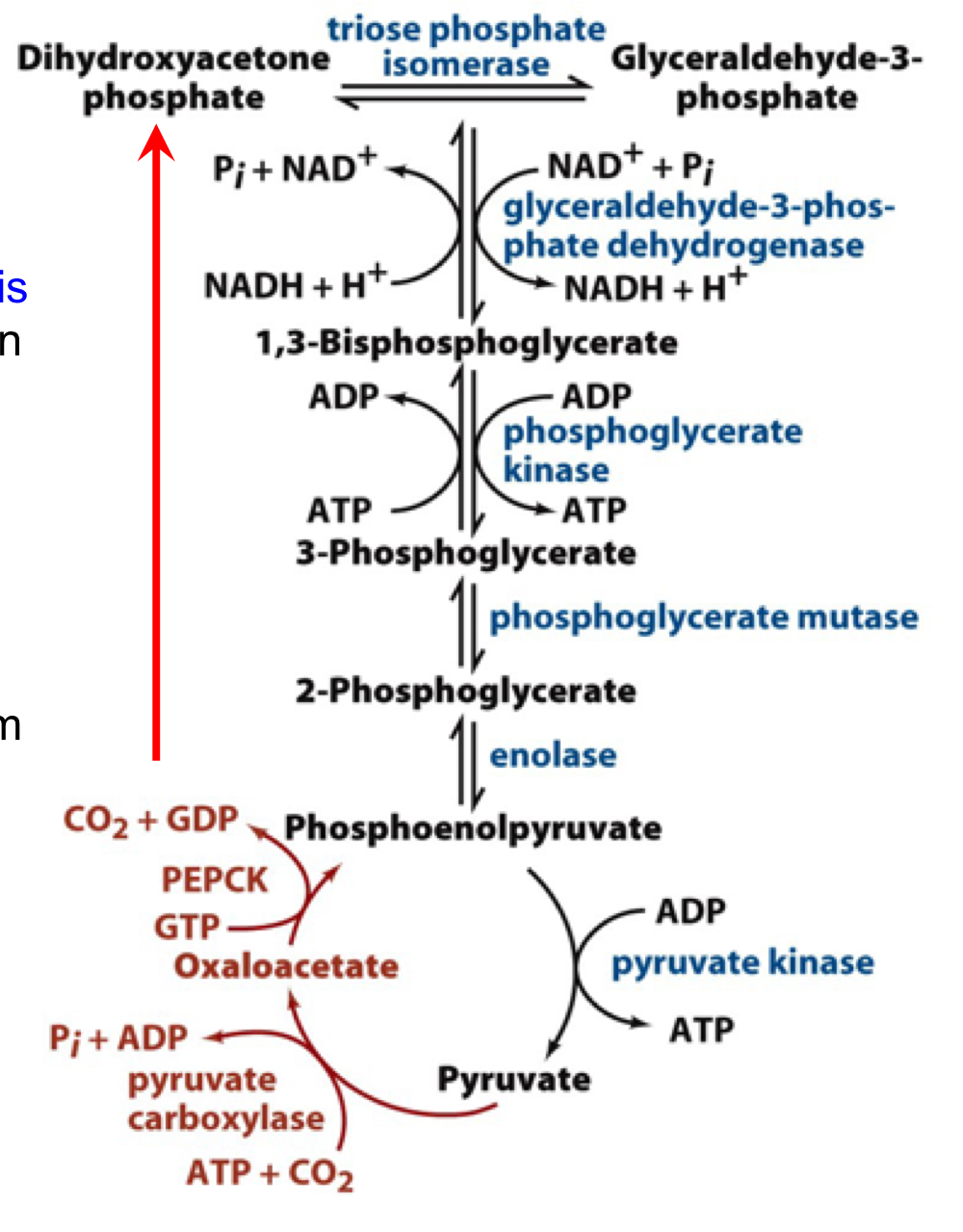
59
New cards
third reaction unique to gluconeogenesis
after formation of fructose-1,6-bisphosphate, a new enzyme must be used to bypass the energetically favorable PFK1 reaction
fructose-1,6-hisphosphatase is metabolically irreversible
fructose-1,6-hisphosphatase is metabolically irreversible
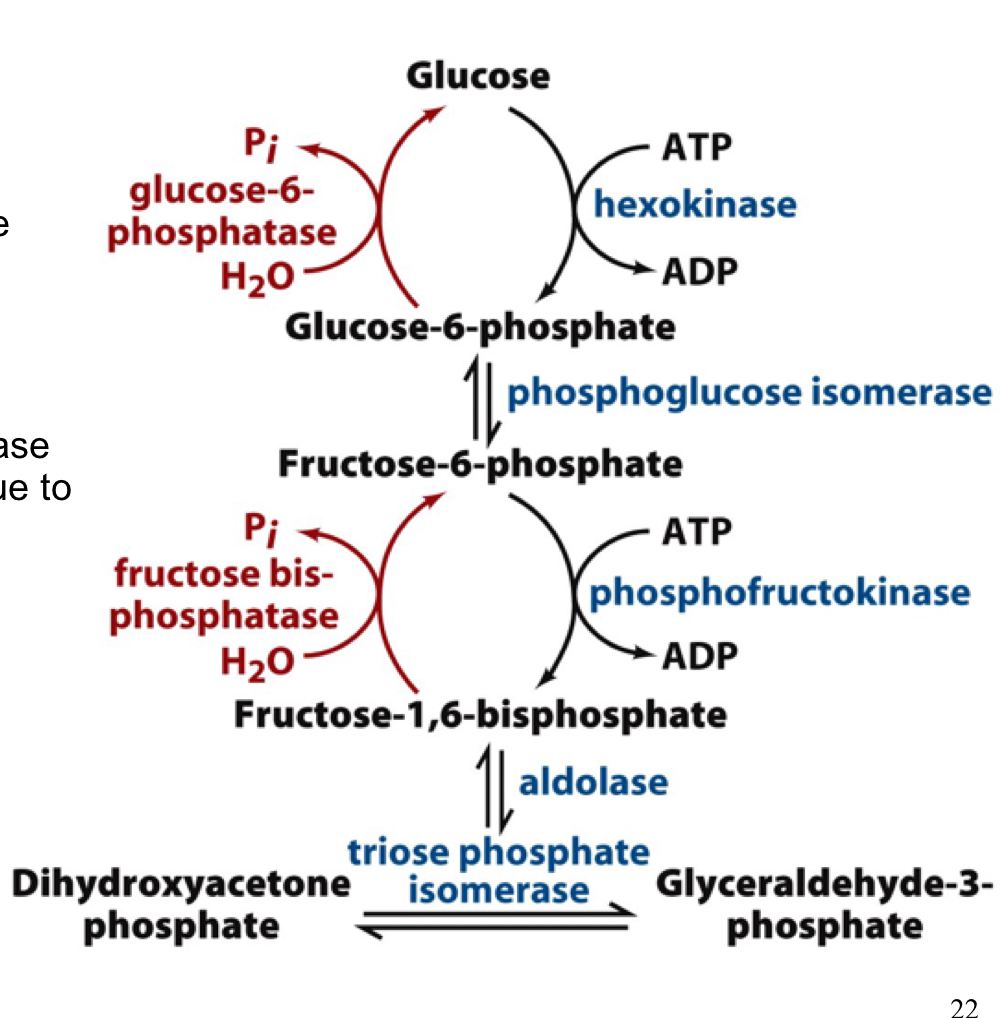
60
New cards
fructorse-1,6-bisphosphatase
hydrolysis of the phosphate ester provides a large negative ∆G, driving the reaction forward
F1,6BPase is allosterically inhibited by AMP and fructose 2,6-bisphosphate (F2,6BP)
PFK1 and F1,6BPase will not be active at the same time (reciprocal regulation in the liver)
F1,6BPase is allosterically inhibited by AMP and fructose 2,6-bisphosphate (F2,6BP)
PFK1 and F1,6BPase will not be active at the same time (reciprocal regulation in the liver)
61
New cards
glucose-6-phosphatase
the last of the gluconeogenesis reactions
produces free glucose, which is released from hepatocytes into the bloodstream to feed other organs/tissues
G6Pase is localized to the endoplasmic reticulum membrane and is most strongly expressed in the liver. it may transport the G6P into the ER where the active site can then hydrolyze the phosphate
the free glucose is exported from the ER through the cytoplasmic membrane where it is taken up by the bloodstream and transported to other tissues
produces free glucose, which is released from hepatocytes into the bloodstream to feed other organs/tissues
G6Pase is localized to the endoplasmic reticulum membrane and is most strongly expressed in the liver. it may transport the G6P into the ER where the active site can then hydrolyze the phosphate
the free glucose is exported from the ER through the cytoplasmic membrane where it is taken up by the bloodstream and transported to other tissues
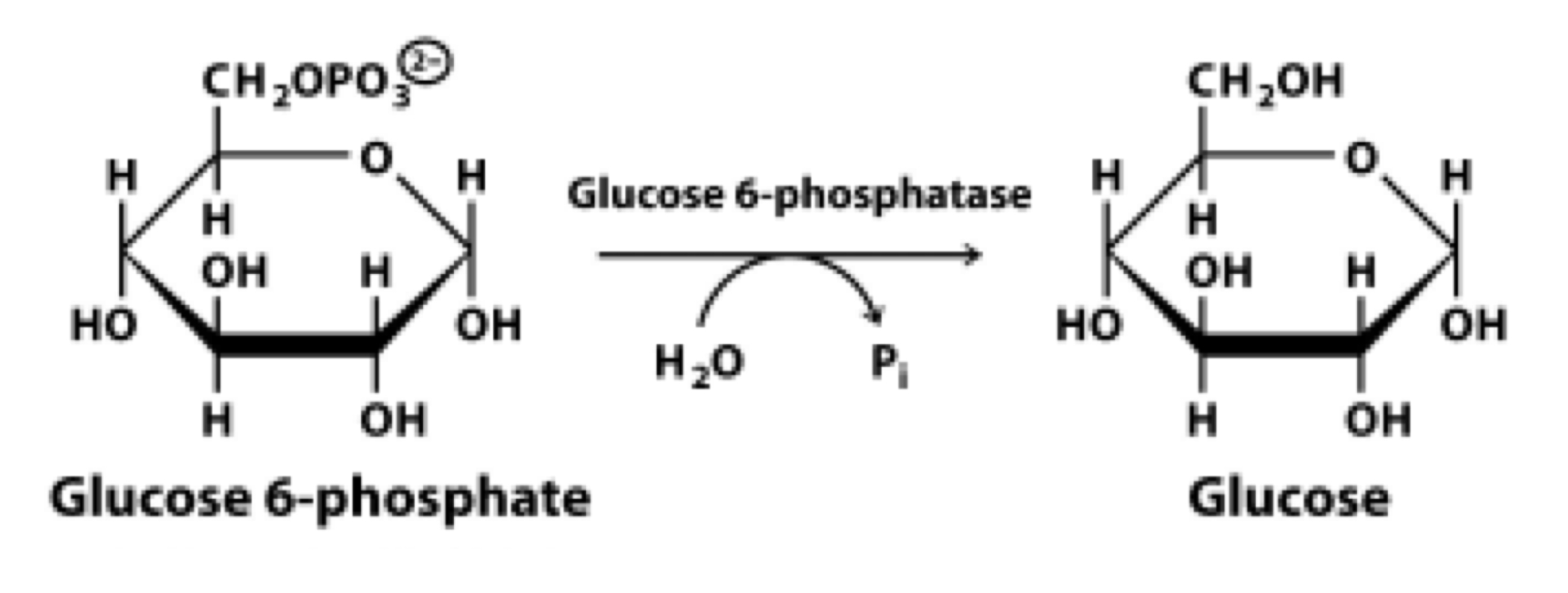
62
New cards
the three key irreversible steps for gluconeogenesis
1. pyruvate carboxylase + PEPCK
2. FBPase
3. glucose-6-phosphatase
63
New cards
net equation for gluconeogenesis
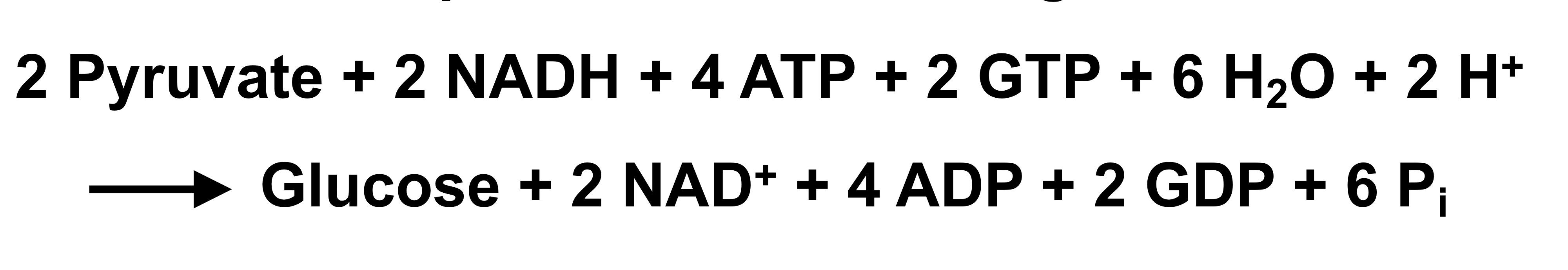
64
New cards
regulation of liver glycolysis and gluconeogenesis
generally, when one pathway is active, the opposing pathway is inactive, and vice versa
AMP and fructose-1,6-bisphosphate are inactivators for fructose-1,6-bisphosphatase (gluconeogenesis)
citrate and ATP are inactivators for phosphofructokinase-1
AMP and fructose-1,6-bisphosphate are activators for phosphofructokinase-1 (glycolysis)
acetyl CoA is an activator for pyruvate carboxylase (gluconeogenesis)
ATP and phosphorylation catalyzed by protein kinase A are inactivators for pyruvate kinase (glycolysis)
fructose-1,6-bisphosphate is an activator for pyruvate kinase (glycolysis)
AMP and fructose-1,6-bisphosphate are inactivators for fructose-1,6-bisphosphatase (gluconeogenesis)
citrate and ATP are inactivators for phosphofructokinase-1
AMP and fructose-1,6-bisphosphate are activators for phosphofructokinase-1 (glycolysis)
acetyl CoA is an activator for pyruvate carboxylase (gluconeogenesis)
ATP and phosphorylation catalyzed by protein kinase A are inactivators for pyruvate kinase (glycolysis)
fructose-1,6-bisphosphate is an activator for pyruvate kinase (glycolysis)
65
New cards
β-D-fructose-2,6-bisphosphate (F2,6P)
a second phosphofructokinase isoform (PFK-2) synthesizes fructose-2,6-bisphosphate (F2,6P), an important regulator of glycolysis and gluconeogenesis
F2,6P is not a glycolytic intermediate, but is solely a regulatory molecule
F2,6P activates PFK-1 (glycolysis) and inhibits FBPase (gluconeogenesis)
F2,6P is not a glycolytic intermediate, but is solely a regulatory molecule
F2,6P activates PFK-1 (glycolysis) and inhibits FBPase (gluconeogenesis)
66
New cards
PFK-2 and F-2,6-bisphosphatase
different activities of the same enzyme, which are interconvertible by phosphorylation and dephosphorylation
protein kinase A convertes PFK-2 to FBPase-2, decreasing levels of F2,6P, which decreases activity of PFK-1 by degrading the F2,6P activator and slows the rate of glycolysis
protein kinase A also downregulates pyruvate kinase (which slows glycolysis), decreases glycogen synthesis, and activates glycogen breakdown
the end result in the liver is that glucose is either made or released from glycogen so that it can be exported to the blood under fasting conditions
protein kinase A convertes PFK-2 to FBPase-2, decreasing levels of F2,6P, which decreases activity of PFK-1 by degrading the F2,6P activator and slows the rate of glycolysis
protein kinase A also downregulates pyruvate kinase (which slows glycolysis), decreases glycogen synthesis, and activates glycogen breakdown
the end result in the liver is that glucose is either made or released from glycogen so that it can be exported to the blood under fasting conditions
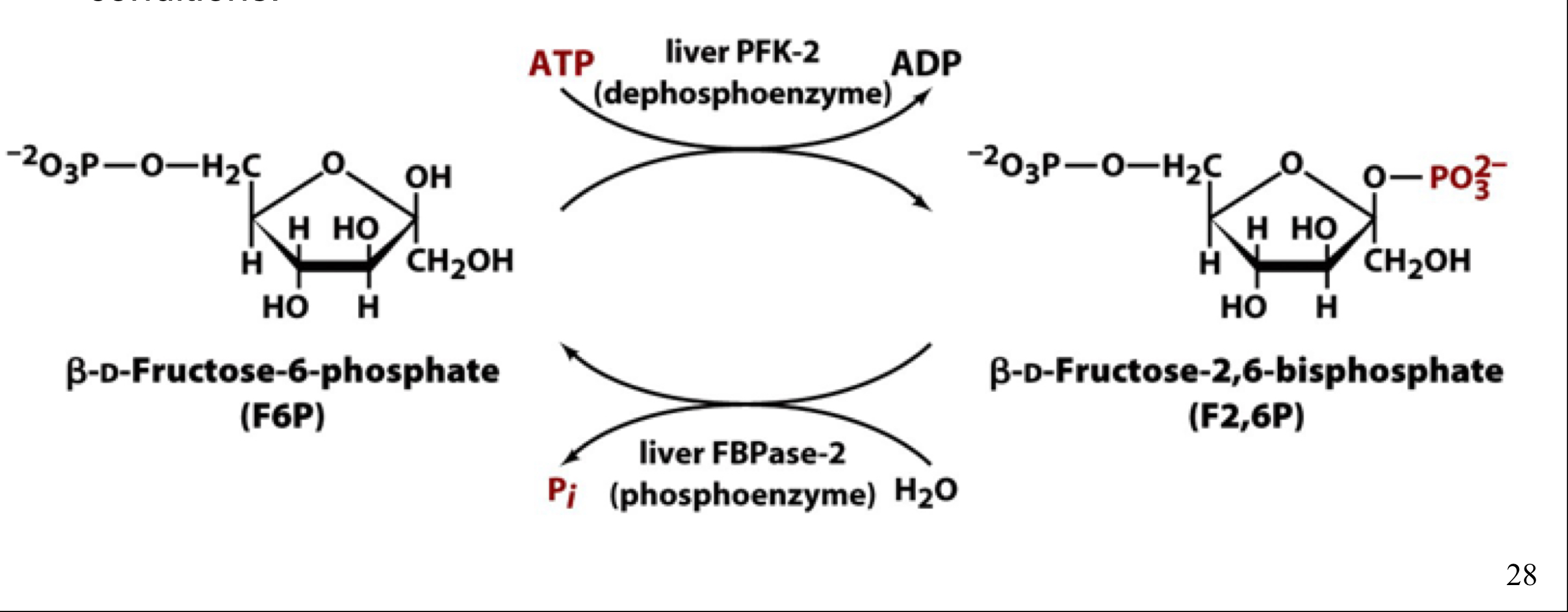
67
New cards
which of the following statements about glycogen is false?
it consists of glucose residues linked by α-1-6 and α-1-4 glycosidic bonds
the first step in glycogen synthesis is catalyzed by phosphoglucomutase
UDP-glucose is an activated compound that can donate a glucose molecule to a growing glycogen chain
the product of the glycogen phosphorylase reaction is glucose-6-phosphate
it consists of glucose residues linked by α-1-6 and α-1-4 glycosidic bonds
the first step in glycogen synthesis is catalyzed by phosphoglucomutase
UDP-glucose is an activated compound that can donate a glucose molecule to a growing glycogen chain
the product of the glycogen phosphorylase reaction is glucose-6-phosphate
the product of the glycogen phosphorylase reaction is glucose-6-phosphate
the product is glucose-1-phosphate
the product is glucose-1-phosphate
68
New cards
which of the following enzymes occurs in both glycolysis and gluconeogenesis?
3-phosphoglycerate kinase
hexokinase
glucose-6-phosphatase
phosphofructokinase-1
pyruvate kinase
3-phosphoglycerate kinase
hexokinase
glucose-6-phosphatase
phosphofructokinase-1
pyruvate kinase
3-phosphoglycerate kinase
69
New cards
under what metabolic conditions does gluconeogenesis typically occur?
starvation, fasting, and intense exercise
70
New cards
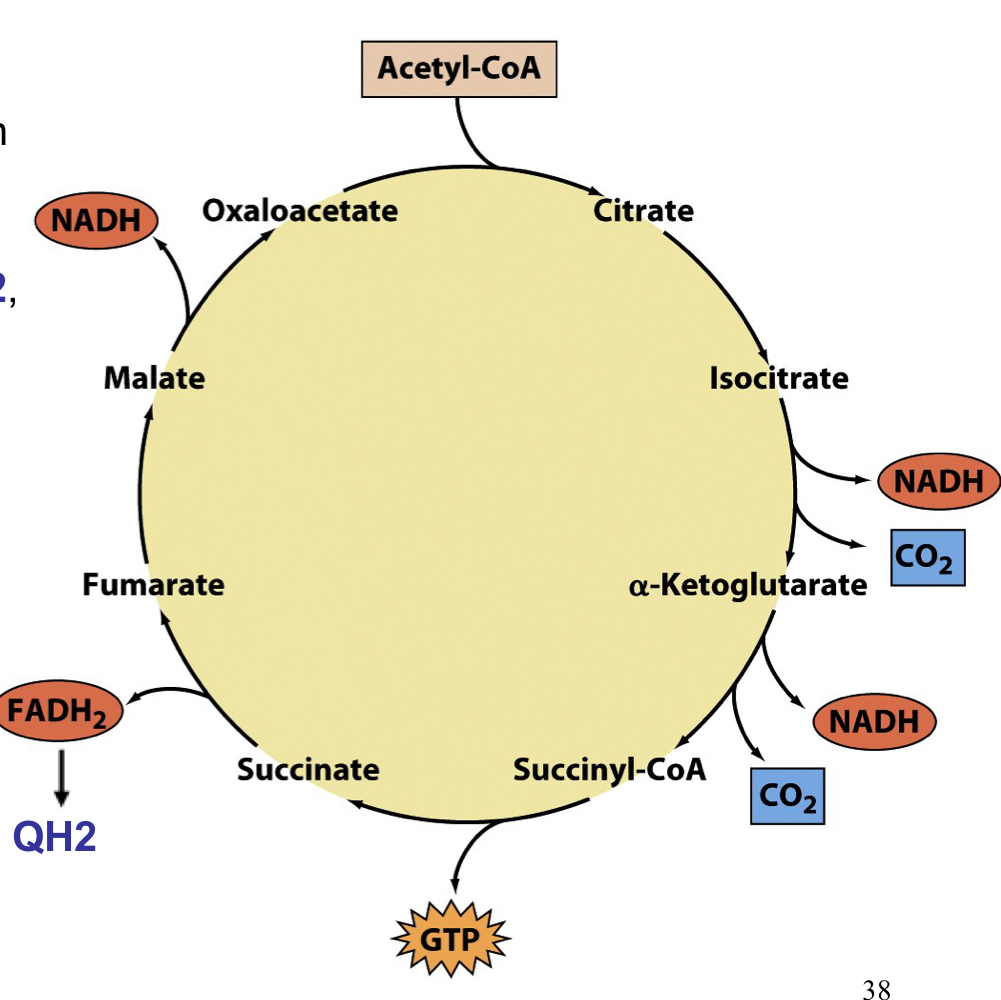
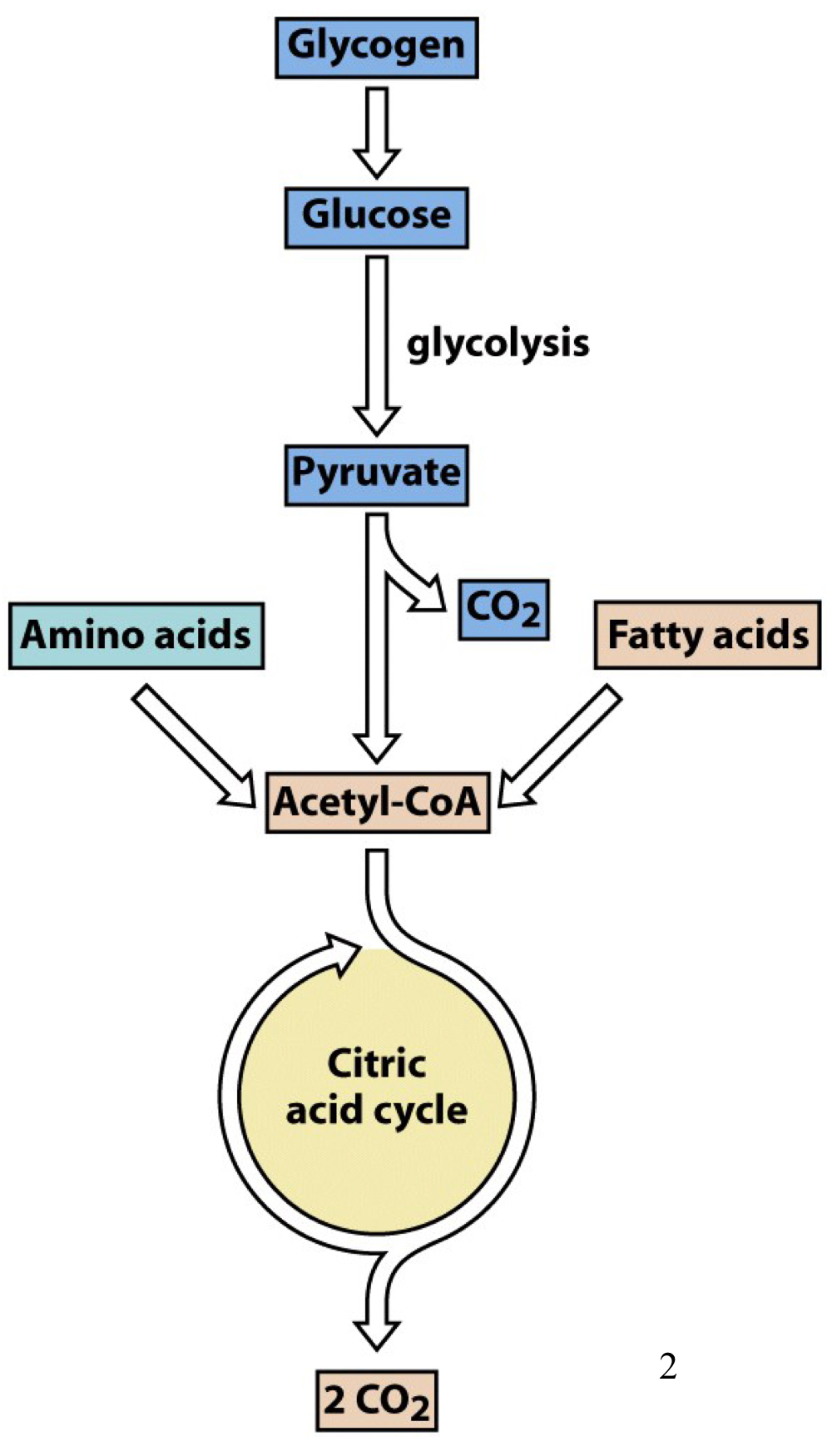
citric acid cycle overview
aka Krebs cycle or tri-carboxylic acid (TCA) cycle
not only a continuation of glycolysis, but rather a central pathway for energy production using a variety of sources, including amino acids and fatty acids
acetyl-CoA is the main common entry point into the citric acid cycle for all the various carbon sources
the ultimate fate of the carbon is to be oxidized all the way to CO2, with the electrons saved in the form of reduced cofactors that will be used for oxidative phosphorylation
main way for many metabolites to be oxidized to form CO2
not only a continuation of glycolysis, but rather a central pathway for energy production using a variety of sources, including amino acids and fatty acids
acetyl-CoA is the main common entry point into the citric acid cycle for all the various carbon sources
the ultimate fate of the carbon is to be oxidized all the way to CO2, with the electrons saved in the form of reduced cofactors that will be used for oxidative phosphorylation
main way for many metabolites to be oxidized to form CO2
71
New cards
full citric acid cycle
acetyl groups are fed into the cycle and completely oxidized. the oxaloacetate substrate used at the beginning is regenerated so only the 2 Caron acetyl group is consumed
all reactions occur in the mitochondria of eukaryotic cells so substrate/product transport is often required
acetyl-CoA is the high energy compound (thirster) that initiates the cycle
the 2 carbons that enter as acetyl-CoA are not immediately oxidized in the first round (those come from oxaloacetate) but will eventually become oxidized through multiple cycles
all reactions occur in the mitochondria of eukaryotic cells so substrate/product transport is often required
acetyl-CoA is the high energy compound (thirster) that initiates the cycle
the 2 carbons that enter as acetyl-CoA are not immediately oxidized in the first round (those come from oxaloacetate) but will eventually become oxidized through multiple cycles
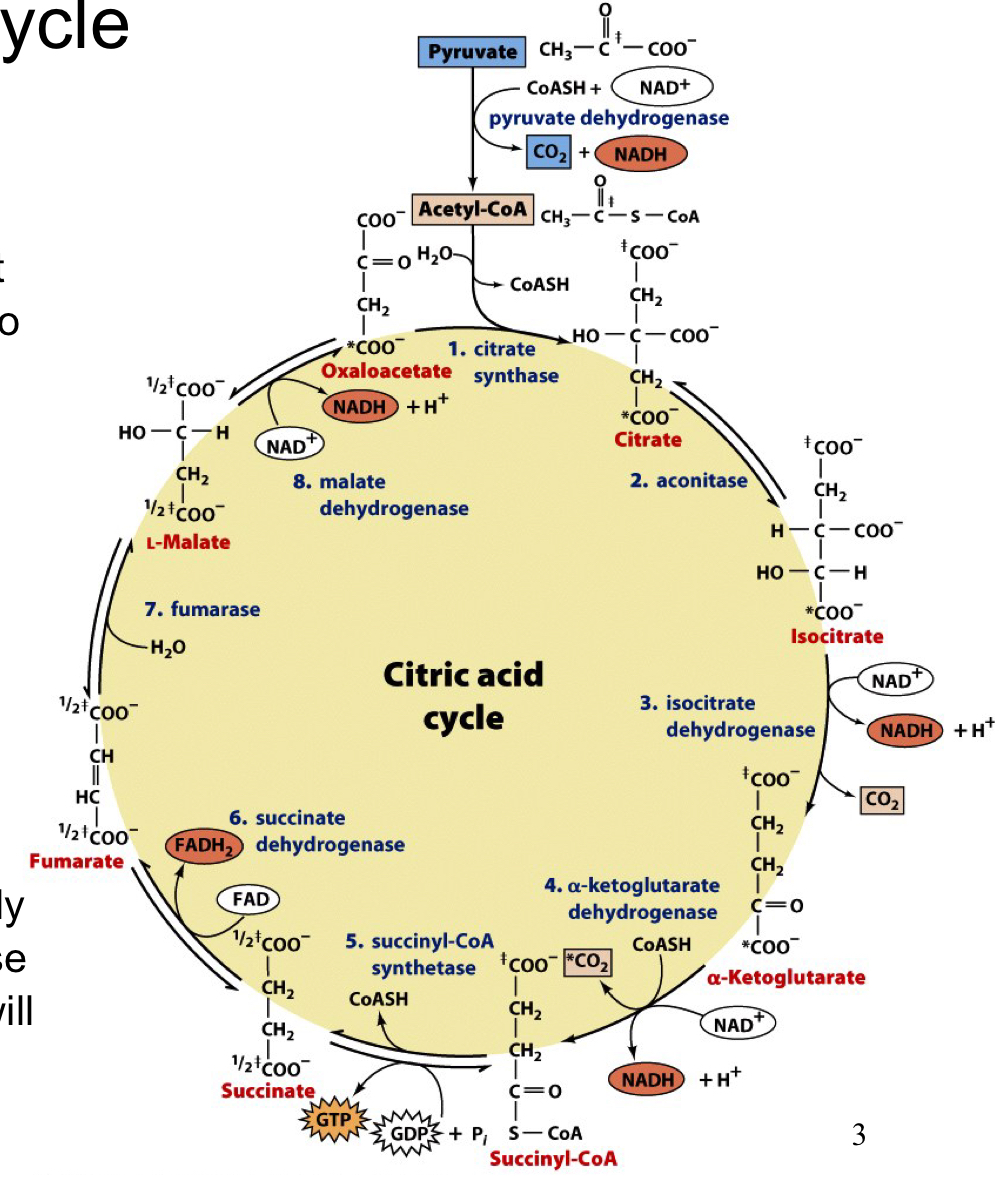
72
New cards
transporting pyruvate into the mitochondrion
pyruvate is formed in the cytosol by glycolysis and must be transferred to the mitochondria for oxidative catabolism (TCA cycle)
pyruvate translocate transports pyruvate into the mitochondria in support with H+
pyruvate translocate transports pyruvate into the mitochondria in support with H+
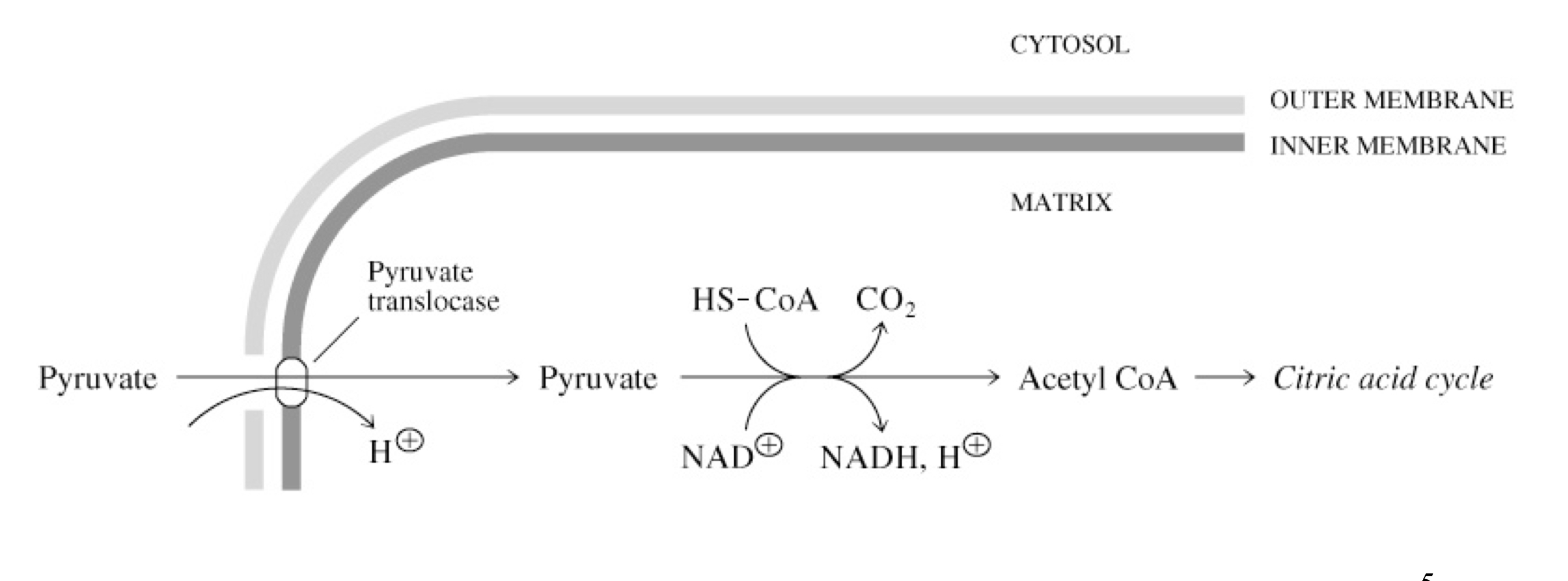
73
New cards
advantages of multi enzyme complexes over single enzymes
1. increased rate of movement between enzyme active sites (close proximity)
2. substrate channeling to reduce immediate side reactions
3. opportunities for coordinate control of all reactions carried out by the complex
faster, more efficient, fewer side products
74
New cards
enzymes that make up the pyruvate dehydrogenase (PDH) complex
E2: dihydrolipoamide acetyltransferase
E1: pyruvate dehydrogenase
E3: dihydrolipoamide dehydrogenase
E1: pyruvate dehydrogenase
E3: dihydrolipoamide dehydrogenase
75
New cards
the coenzymes and prosthetic groups of pyruvate dehydrogenase (cofactor, location, and function
cofactor: taming pyrophosphate (TPP)
\- location: bound to E1
\- function: decarboxylates pyruvate yielding a hydroxyethyl-TPP carbanion
cofactor: lipoic acid
\- location: covalently linked to a Lys on E2 (lipoamide)
\- function: accepts the hydroxyethyl carbanion from TPP as an acetyl group
cofactor: coenzyme A (CoA)
\- location: substrate for E2
\- function: accepts the acetyl group from lipoamide
cofactor: flavin adenine dinucleotide (FAD)
\- location: bound to E3
\- function: reduced by lipoamide
cofactor: nicotinamide adenine dinulceotide (NAD+)
\- location: substrate for E3
\- function: reduced by FADH2
\- location: bound to E1
\- function: decarboxylates pyruvate yielding a hydroxyethyl-TPP carbanion
cofactor: lipoic acid
\- location: covalently linked to a Lys on E2 (lipoamide)
\- function: accepts the hydroxyethyl carbanion from TPP as an acetyl group
cofactor: coenzyme A (CoA)
\- location: substrate for E2
\- function: accepts the acetyl group from lipoamide
cofactor: flavin adenine dinucleotide (FAD)
\- location: bound to E3
\- function: reduced by lipoamide
cofactor: nicotinamide adenine dinulceotide (NAD+)
\- location: substrate for E3
\- function: reduced by FADH2
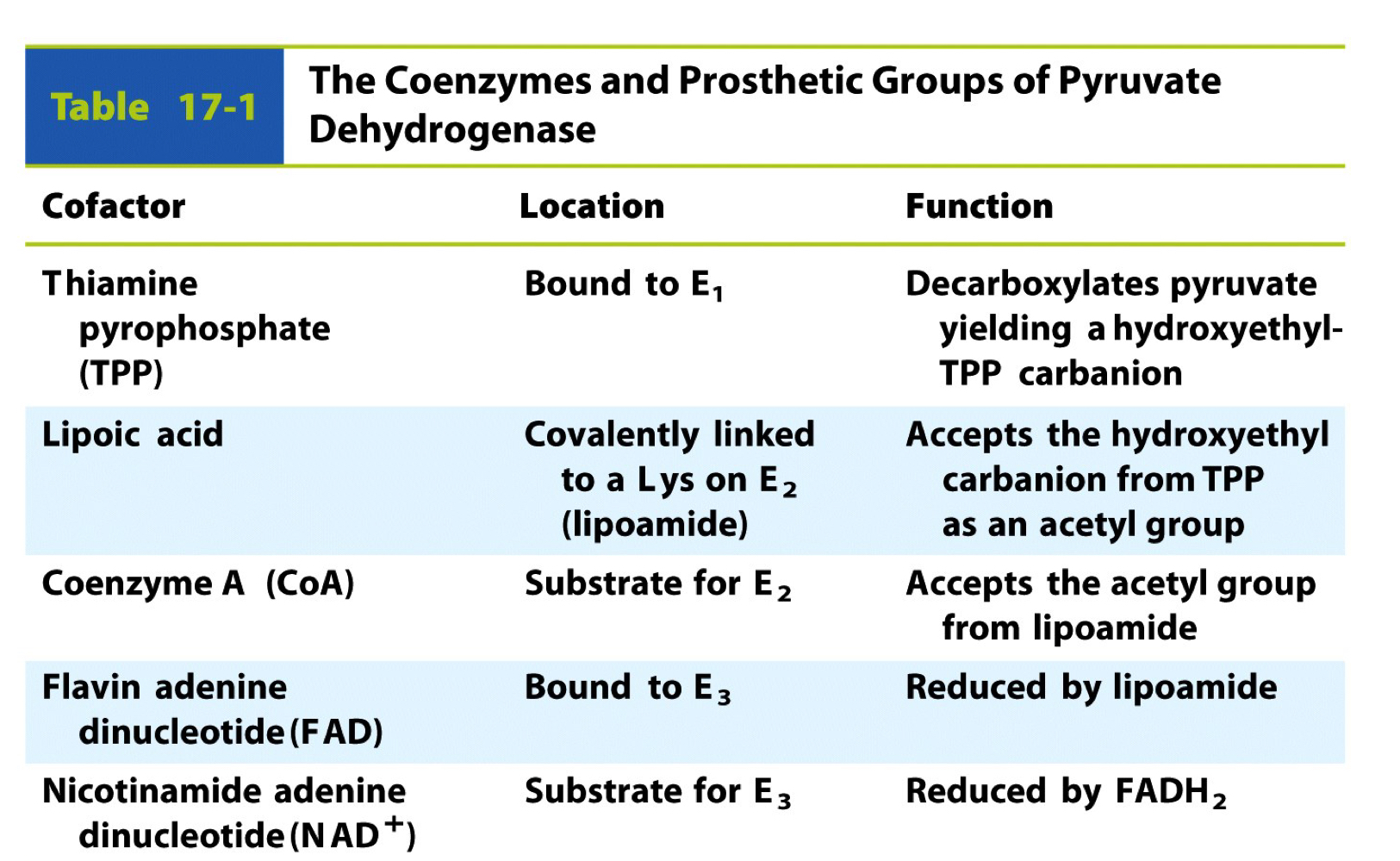
76
New cards
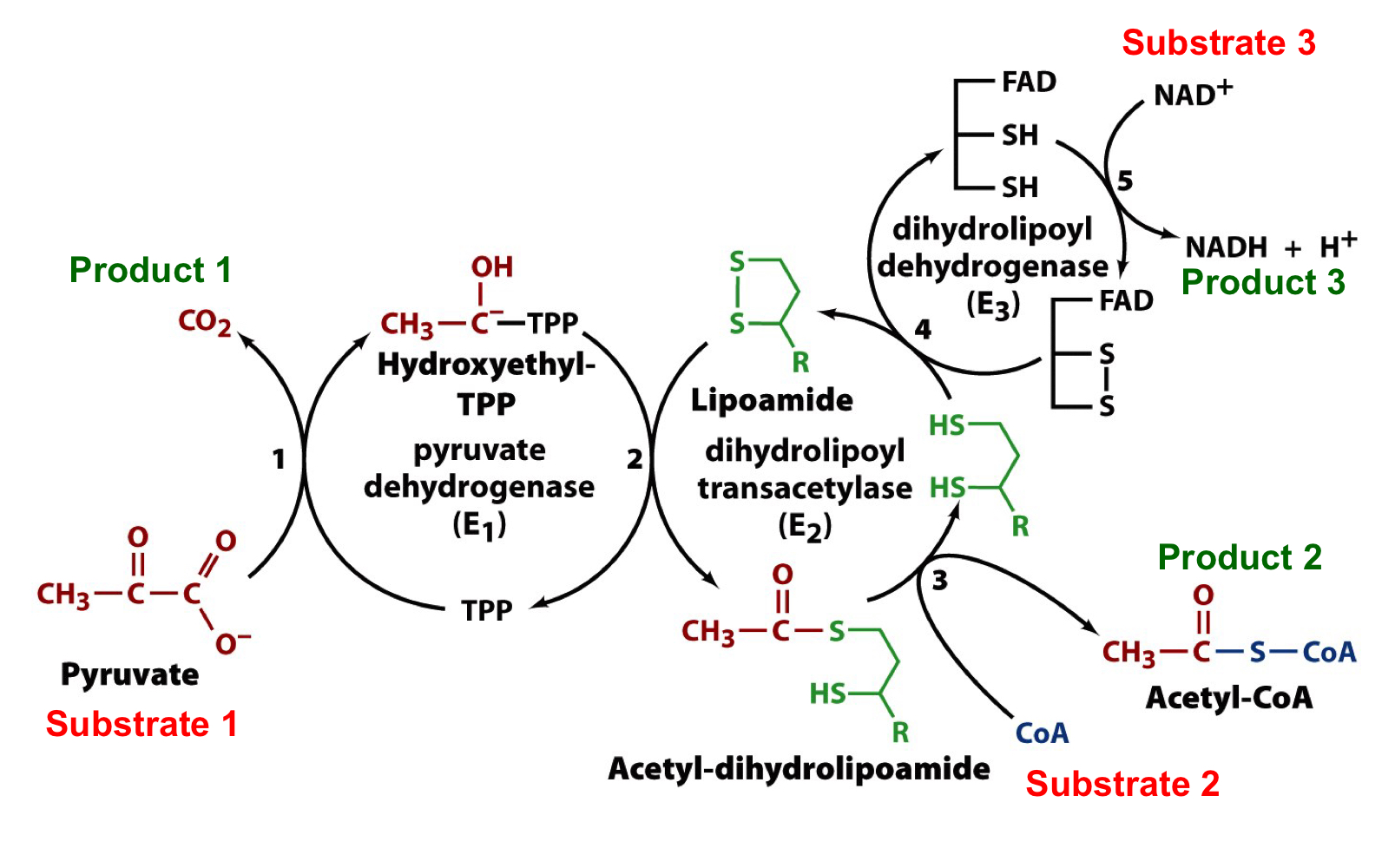
5 reactions carried out by the PDH complex
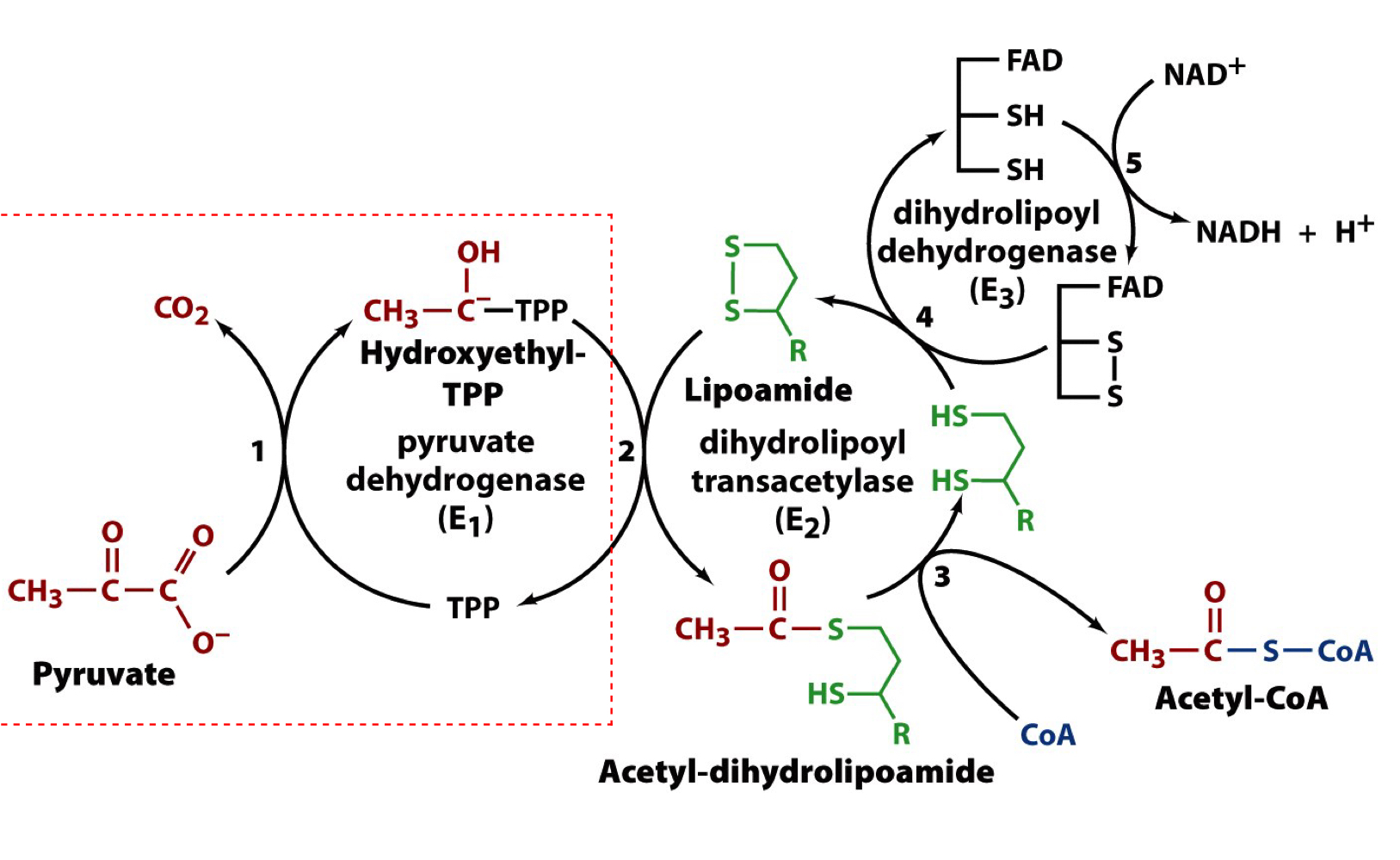
77
New cards
step 1 of the PDH complex
the first cofactor, TPP, carboxylates pyruvate, the first substrate, and forms the acetaldehyde product of TPP, HETPP
the C atom flanked by the S and N acts as a carbanion that attacks the carbonyl C of pyruvate leading to loss of CO2, the first product released in the overall reaction
the C atom flanked by the S and N acts as a carbanion that attacks the carbonyl C of pyruvate leading to loss of CO2, the first product released in the overall reaction
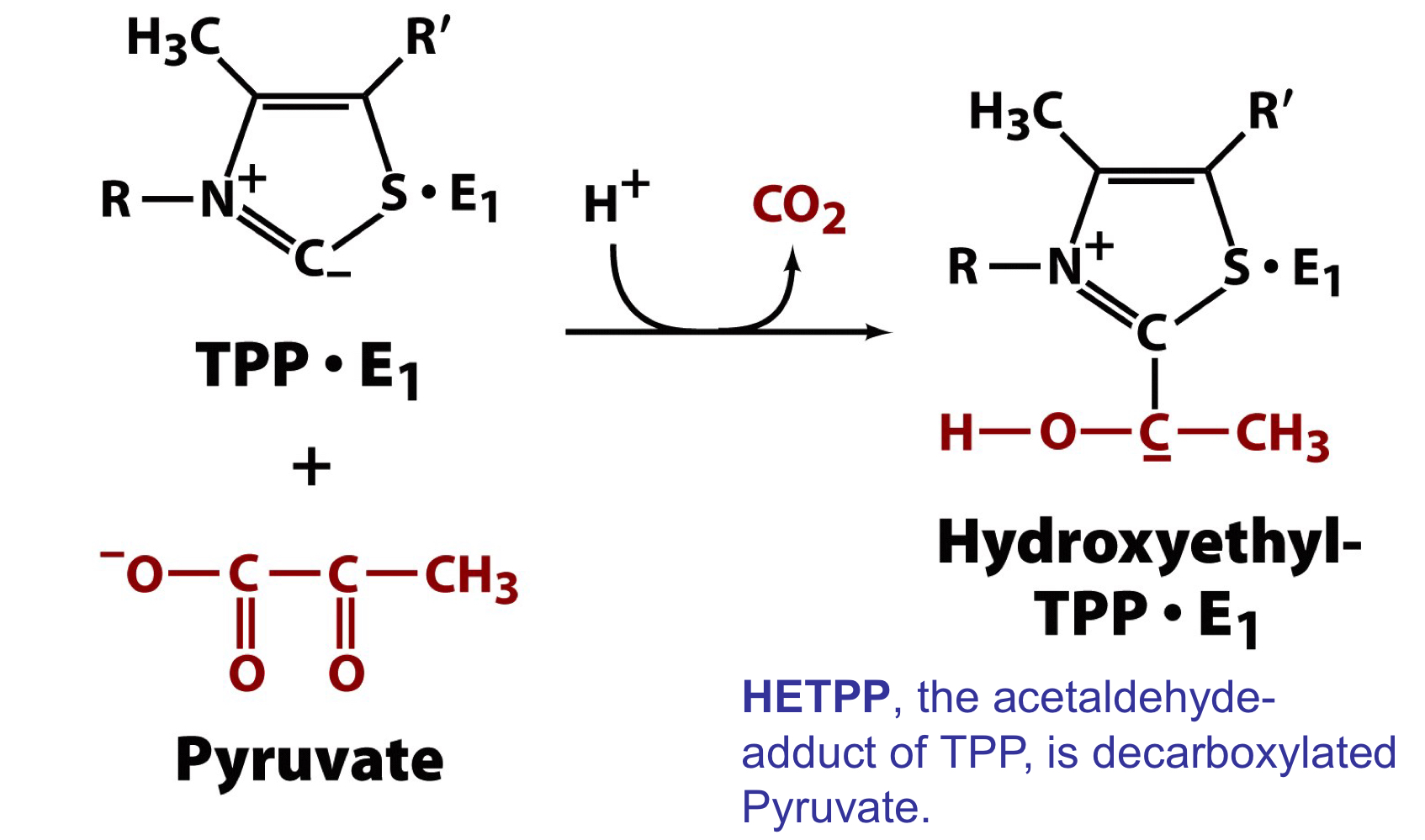
78
New cards
step 2 of the PDH complex
a second cofactor, lipoamide (a prosthetic group) is used as an oxidizing agent to convert the acetaldehyde-TPP adduct to the oxidized acetyl-dihydrolipoamide form
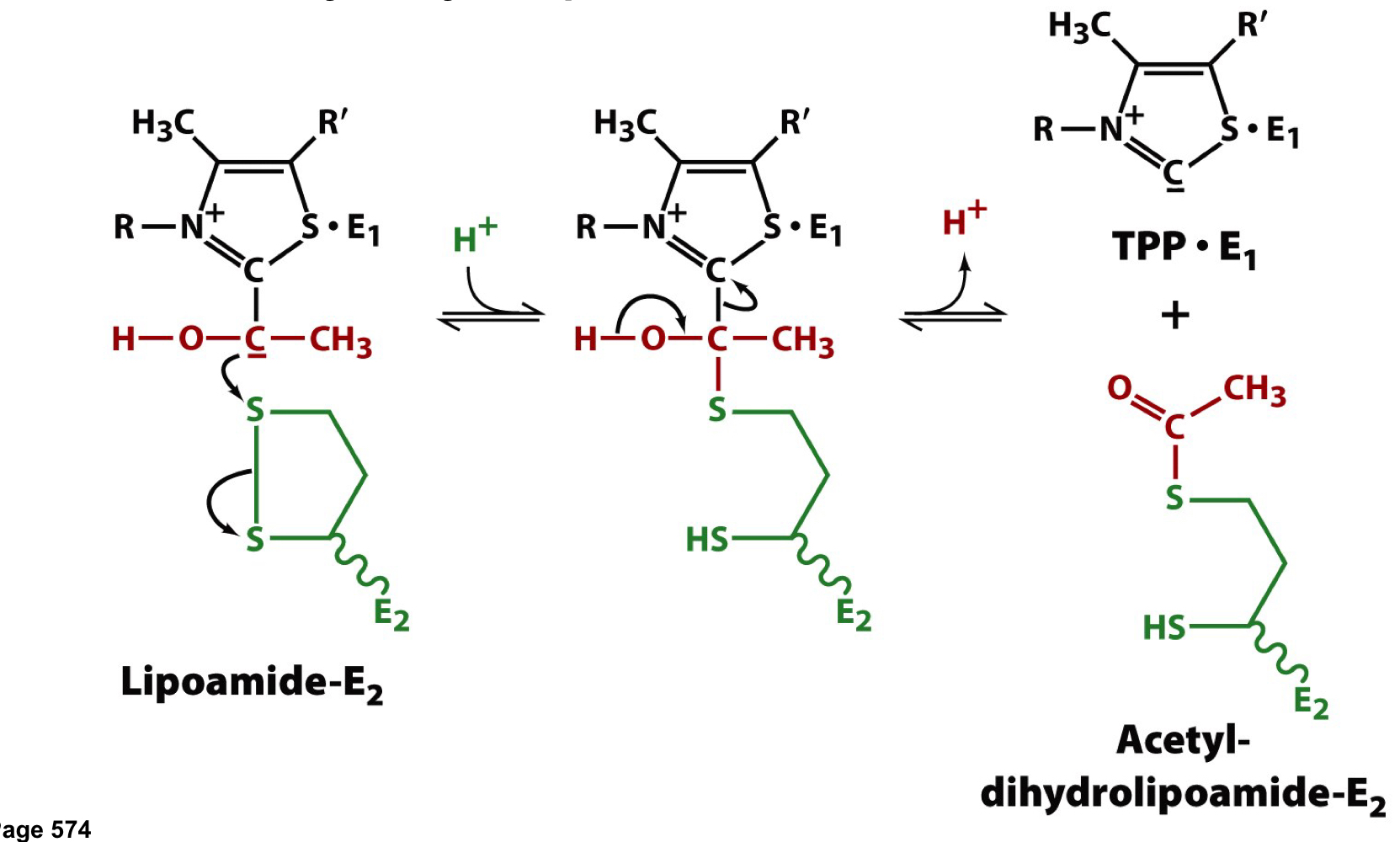
79
New cards
thioesters
energy rich compounds (acetyl CoA has a ∆Go = -31 kJ/mol)
CoA functions as a carrier of acetyl and other acyl groups in metabolism. the groups are bound to CoA via a thioester linkage
thioester bonds are formed to capture energy during metabolism. their hydrolysis can drive exergonic processes
CoA functions as a carrier of acetyl and other acyl groups in metabolism. the groups are bound to CoA via a thioester linkage
thioester bonds are formed to capture energy during metabolism. their hydrolysis can drive exergonic processes
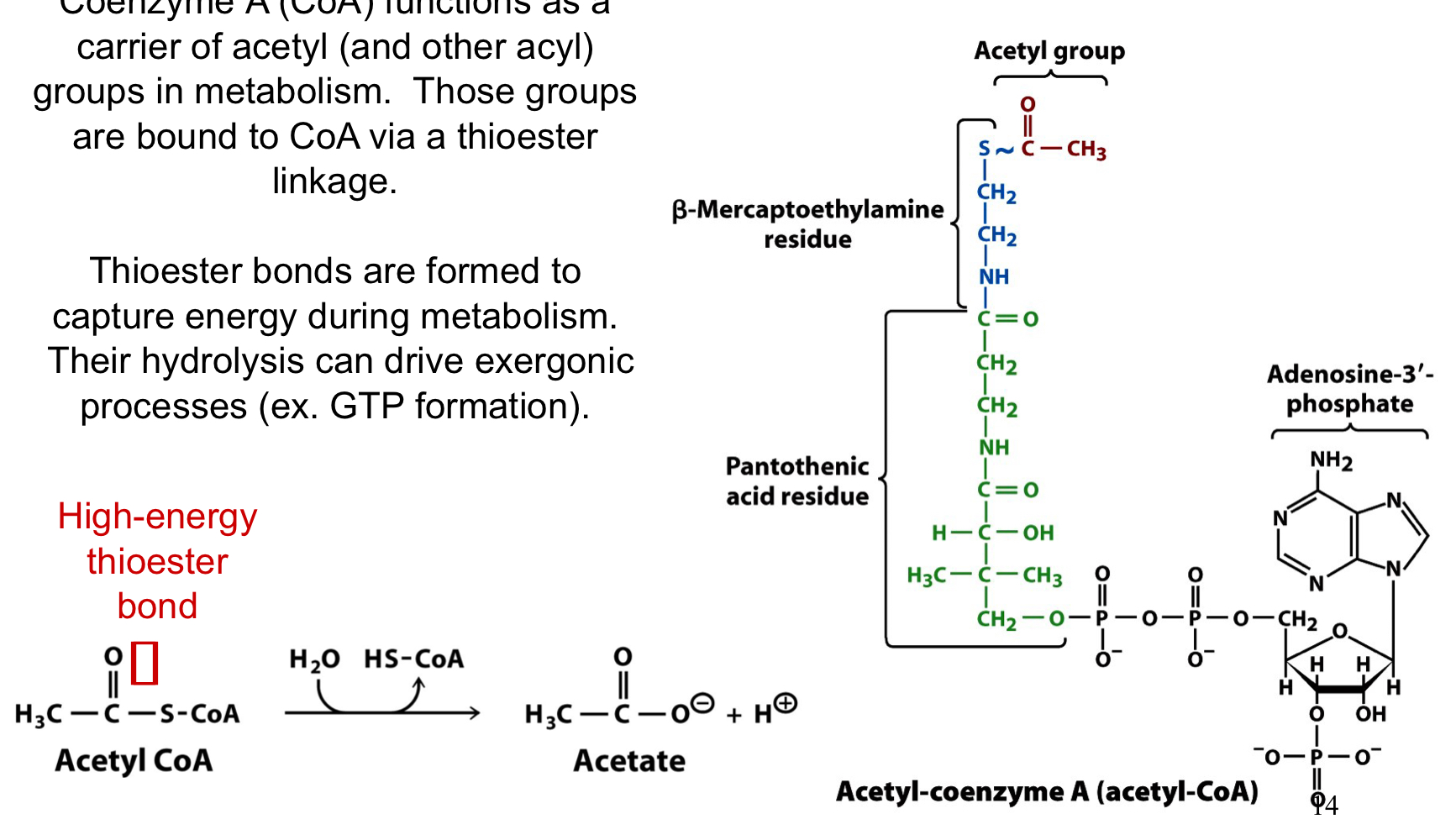
80
New cards
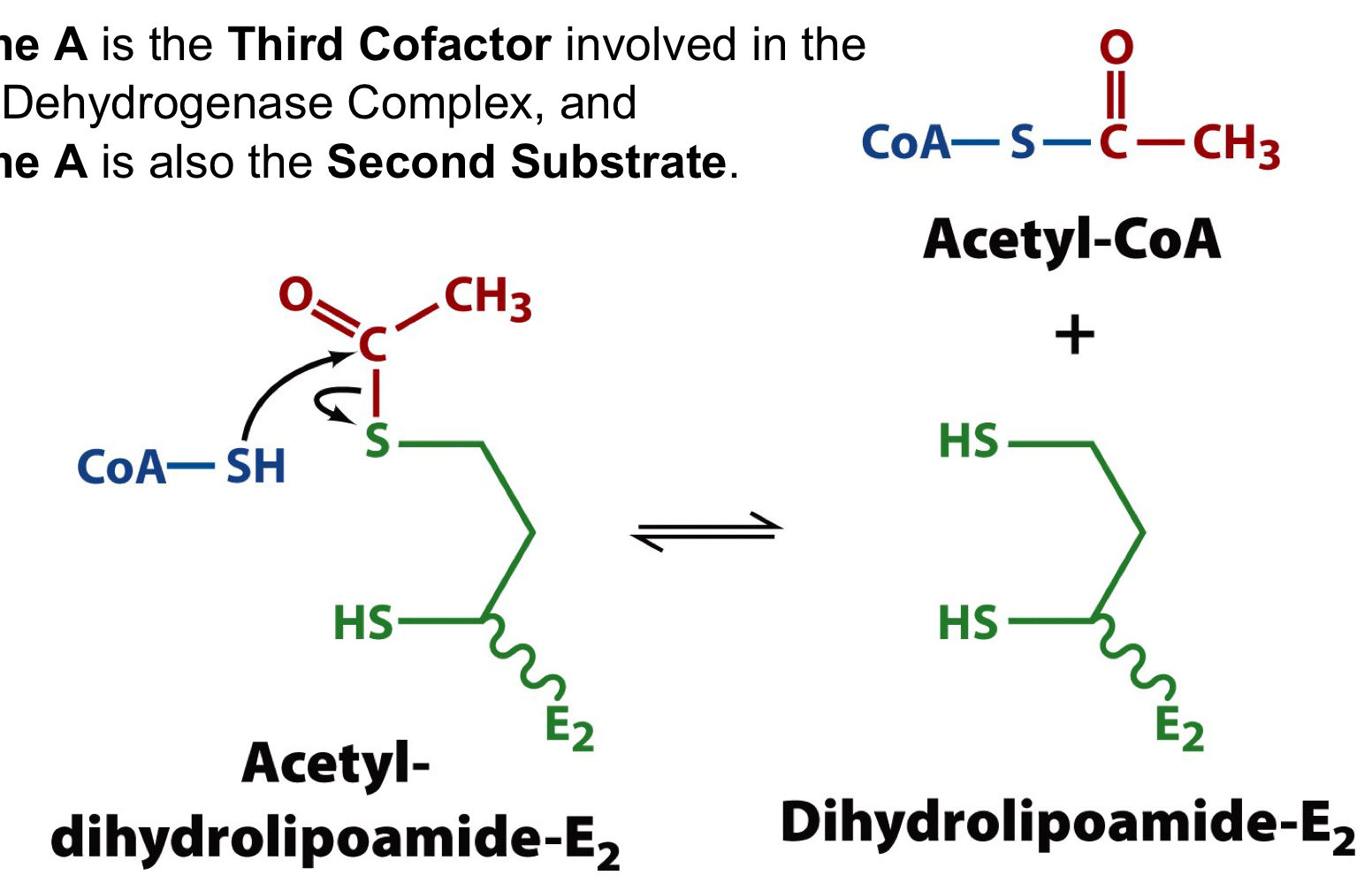
step 3 of the PDH complex
coenzyme A is the third cofactor and the second substrate
a disulfhydryl (reduced) form of lipoamide must be deoxidize back to the disulfide (oxidized) form for the enzyme complex to continue operating
a disulfhydryl (reduced) form of lipoamide must be deoxidize back to the disulfide (oxidized) form for the enzyme complex to continue operating
81
New cards
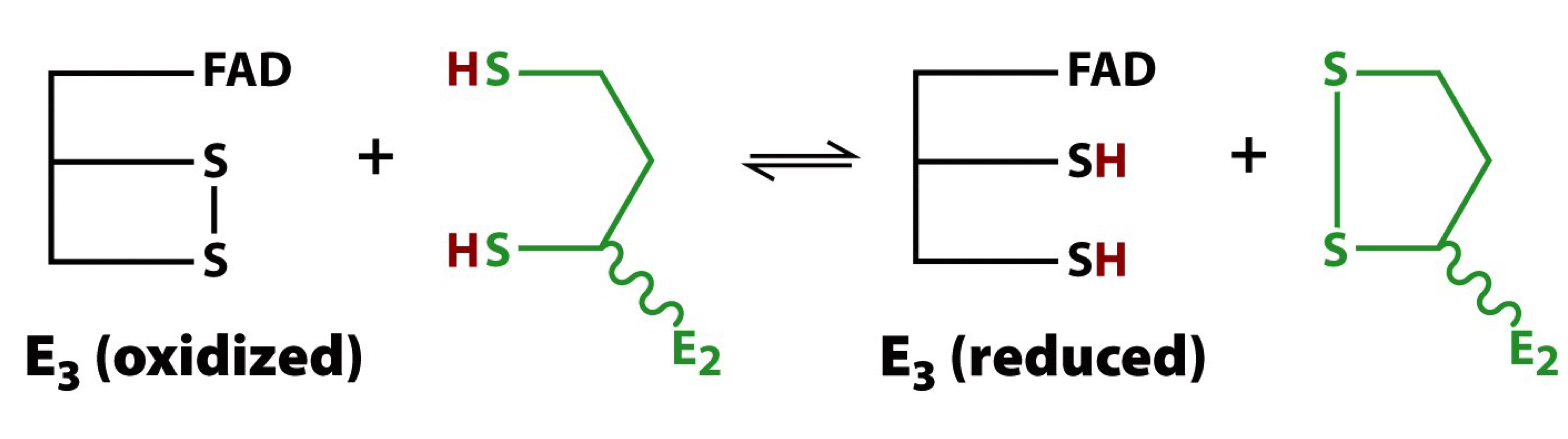
step 4 of the PDH complex
the disulfide group seres as the oxidant to convert reduced dihydrolipoamide back to the active oxidized disulfide form, lipoamide
disulfide bond exchange rxn
E3-disulfide must now be oxidized
disulfide bond exchange rxn
E3-disulfide must now be oxidized
82
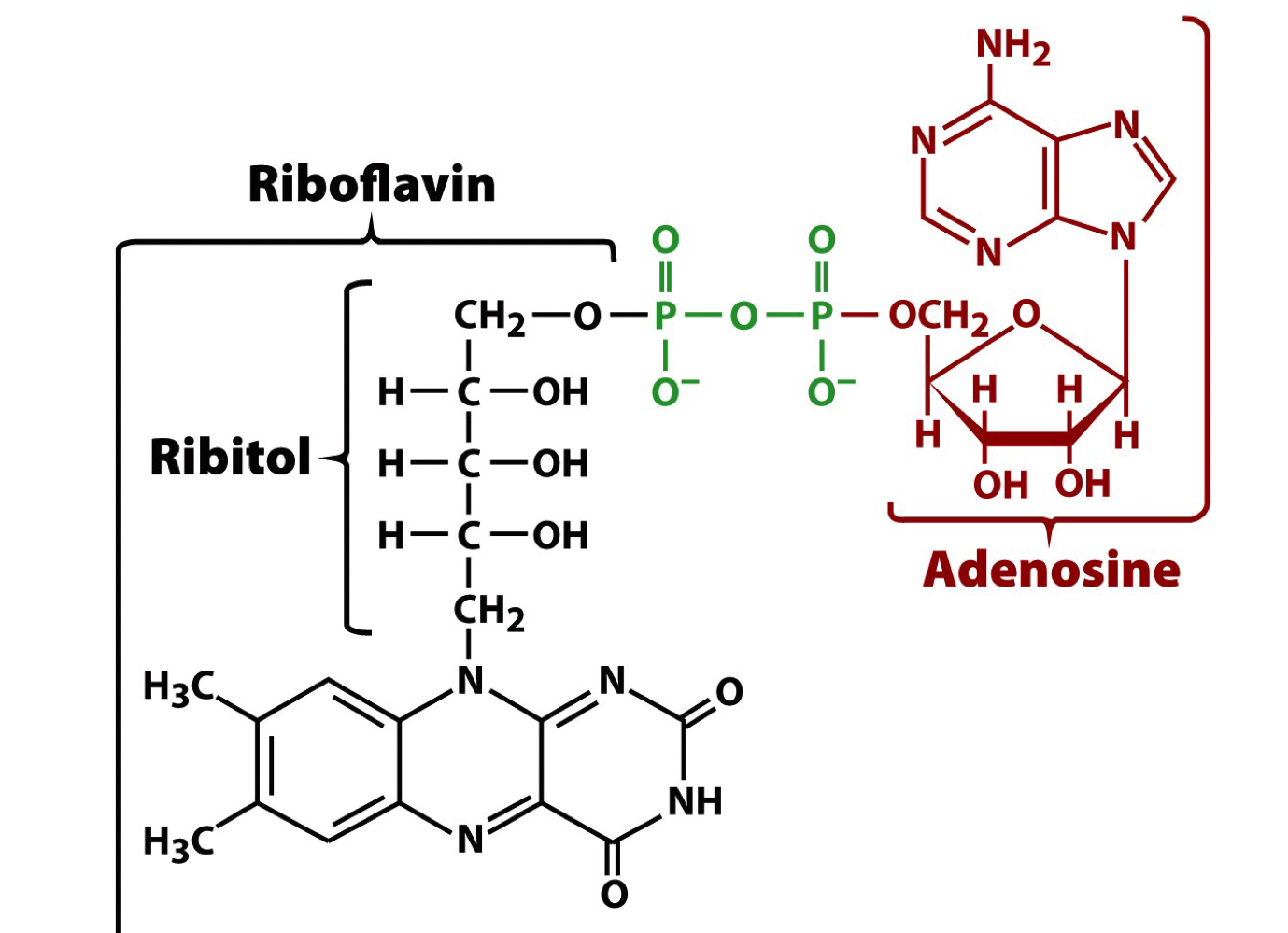
New cards
FAD
flavin adenine dinucleotide is another major electron carrier
the conjugated ring system can accept 1 or 2 e-
very useful for shuttling electrons between other redox active cofactors
allows for deoxidation of disulfide bonds
the conjugated ring system can accept 1 or 2 e-
very useful for shuttling electrons between other redox active cofactors
allows for deoxidation of disulfide bonds
83
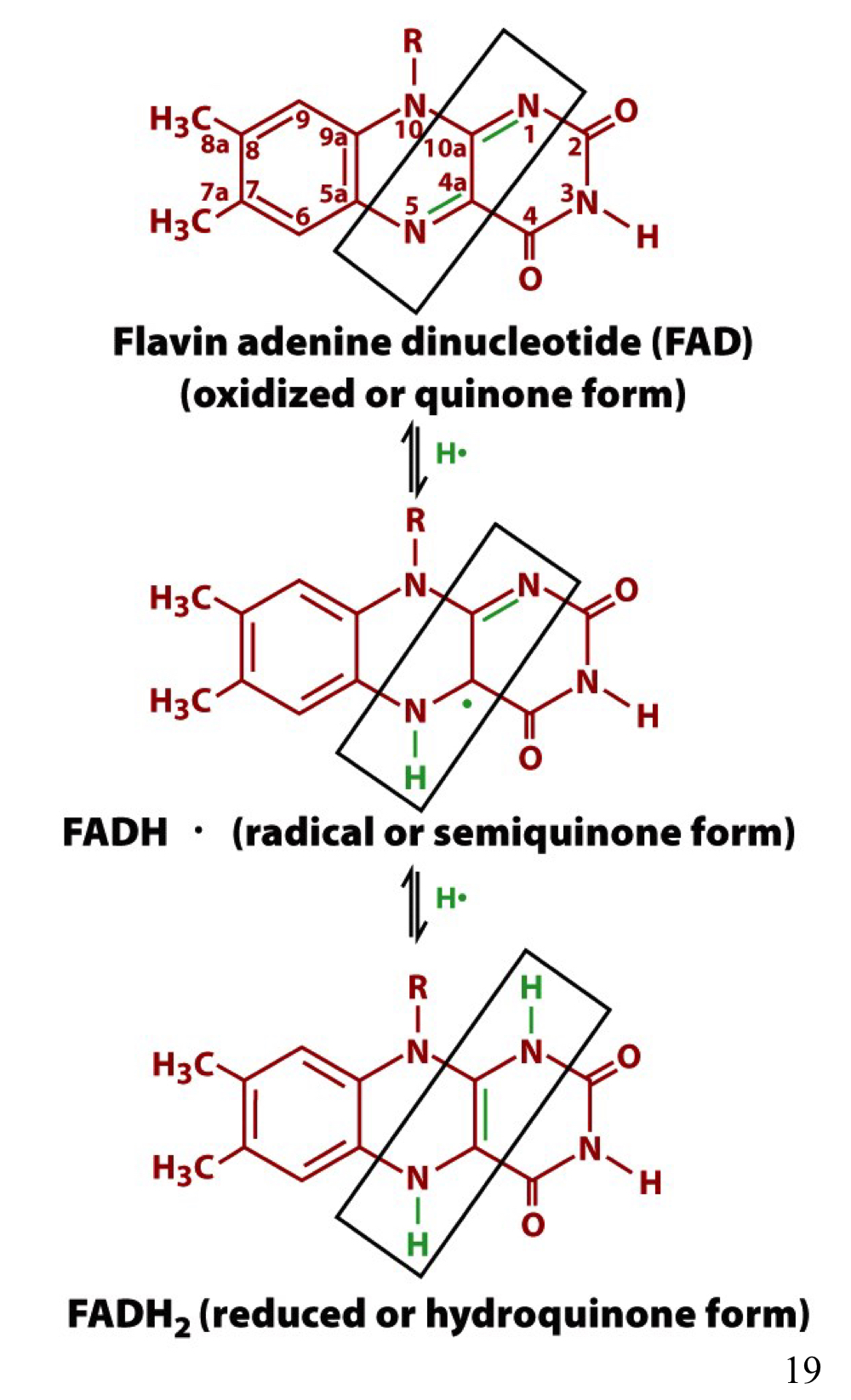
New cards
different electronic states of the conjugated ring system in FAD
2 e- transfers can also occur (bypass semiquinone)
84
New cards
step 5 of the PDH complex
the FAD group of E3 oxidizes the reduced dithiols of E2 forming FADH2
FAD is the fourth cofactor involved. this prosthetic group serves as the oxidant to convert reduced dithiols back to the active oxidized disulfide form
E3-FAD becomes reduced to E3-FADH that immediately passes the electrons on to NAD+ to eventually form NADH (third product of PDH)
FAD is the fourth cofactor involved. this prosthetic group serves as the oxidant to convert reduced dithiols back to the active oxidized disulfide form
E3-FAD becomes reduced to E3-FADH that immediately passes the electrons on to NAD+ to eventually form NADH (third product of PDH)
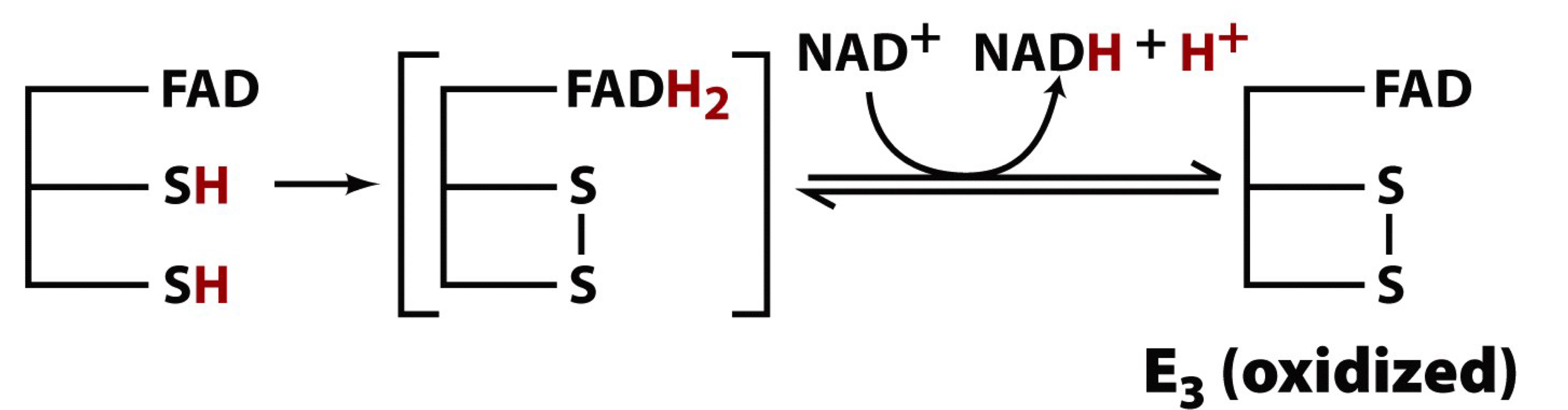
85
New cards
lipoyllsyl arm in the PDH complex
the lipoyllsyl arm physically swings between active sites on all three enzymes of the PDH complex
its flexibility and length are critical for the substrate channeling between enzymes
its flexibility and length are critical for the substrate channeling between enzymes
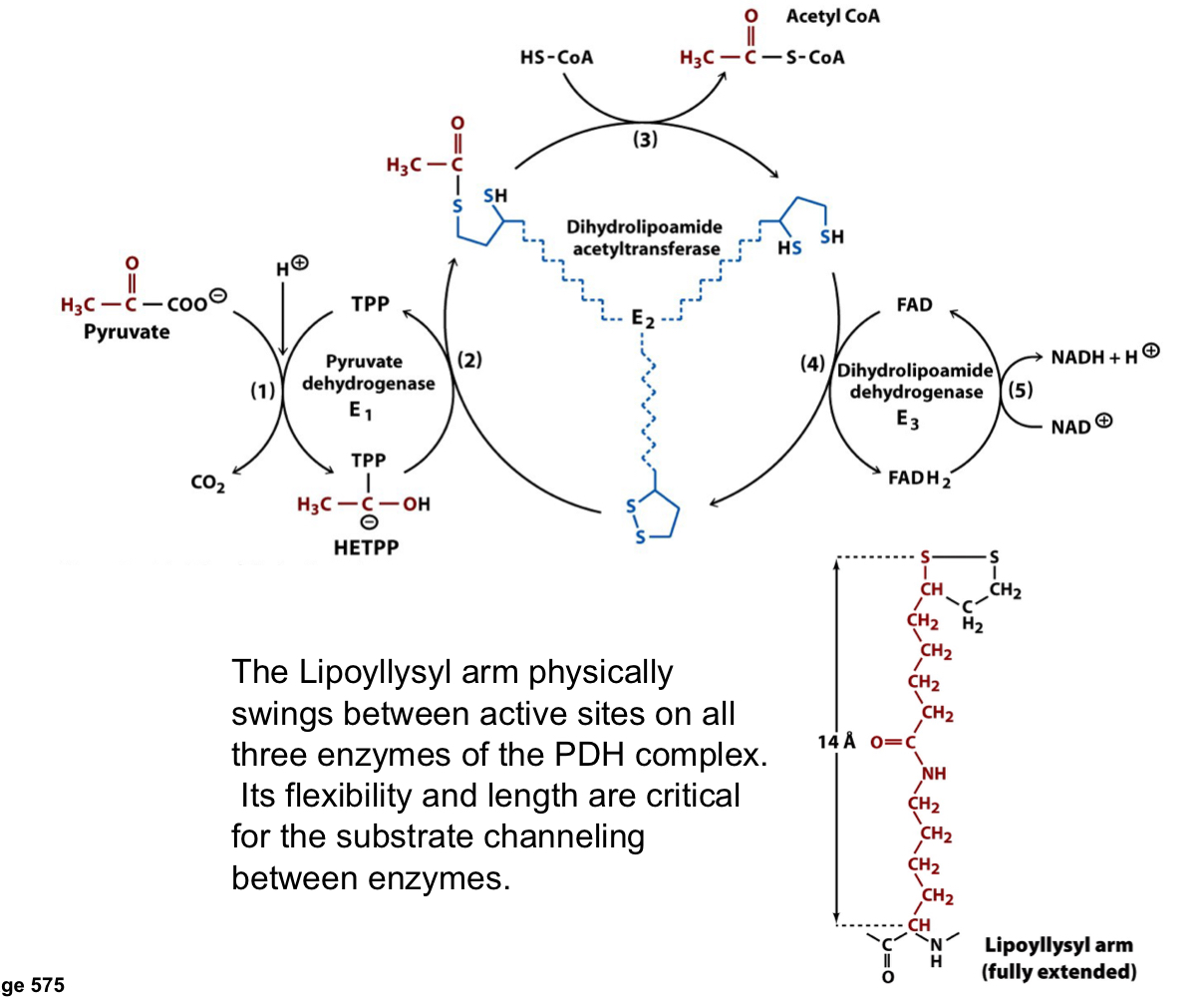
86
New cards
substrates and products of the PDH complex
products: CO2, acetyl-CoA, and NADH + H+
substrates: pyruvate, CoA, and NAD+
substrates: pyruvate, CoA, and NAD+
87
New cards
which coenzyme is required by E1 of the pyruvate dehydrogenase complex for catalytic activity?
thiamine phosphate
88
New cards
reaction 1 of the TCA cycle
citrate synthase
citrate is formed from acetyl CoA and oxaloacetate
the high free energy of the thioester of acetyl CoA drives this, the first metabolically irreversible reaction of the TCA cycle
the only TCA cycle reaction in which c-c bond formation occurs
citrate contains a tertiary alcohol that cannot be oxidized under biological conditions
citrate is formed from acetyl CoA and oxaloacetate
the high free energy of the thioester of acetyl CoA drives this, the first metabolically irreversible reaction of the TCA cycle
the only TCA cycle reaction in which c-c bond formation occurs
citrate contains a tertiary alcohol that cannot be oxidized under biological conditions
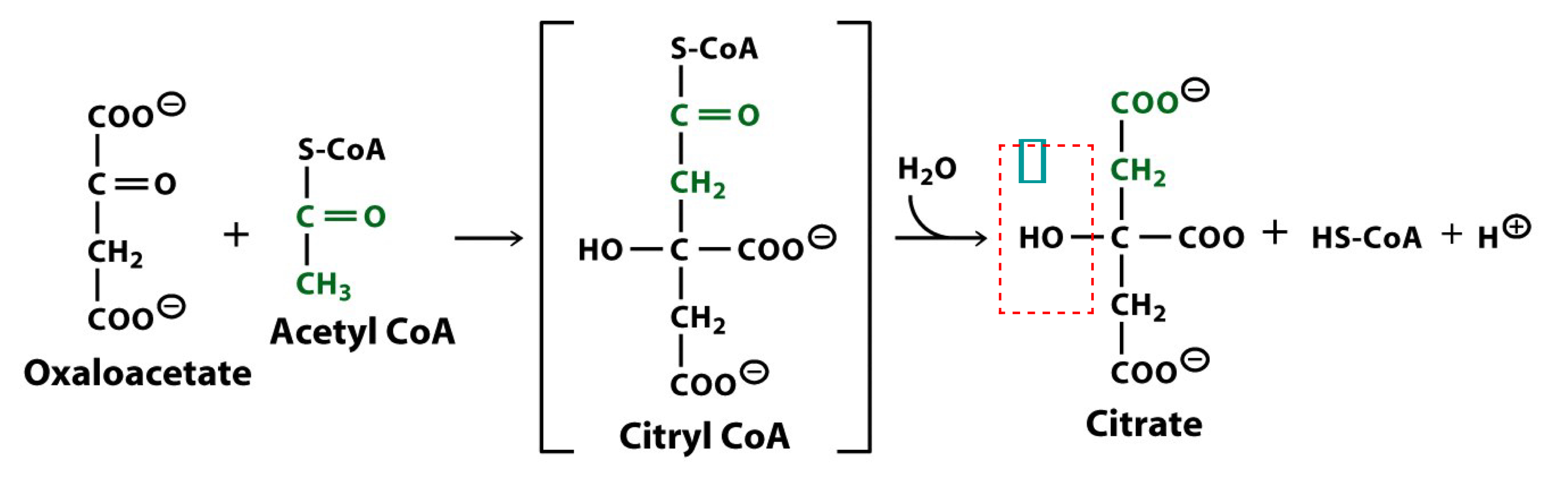
89
New cards
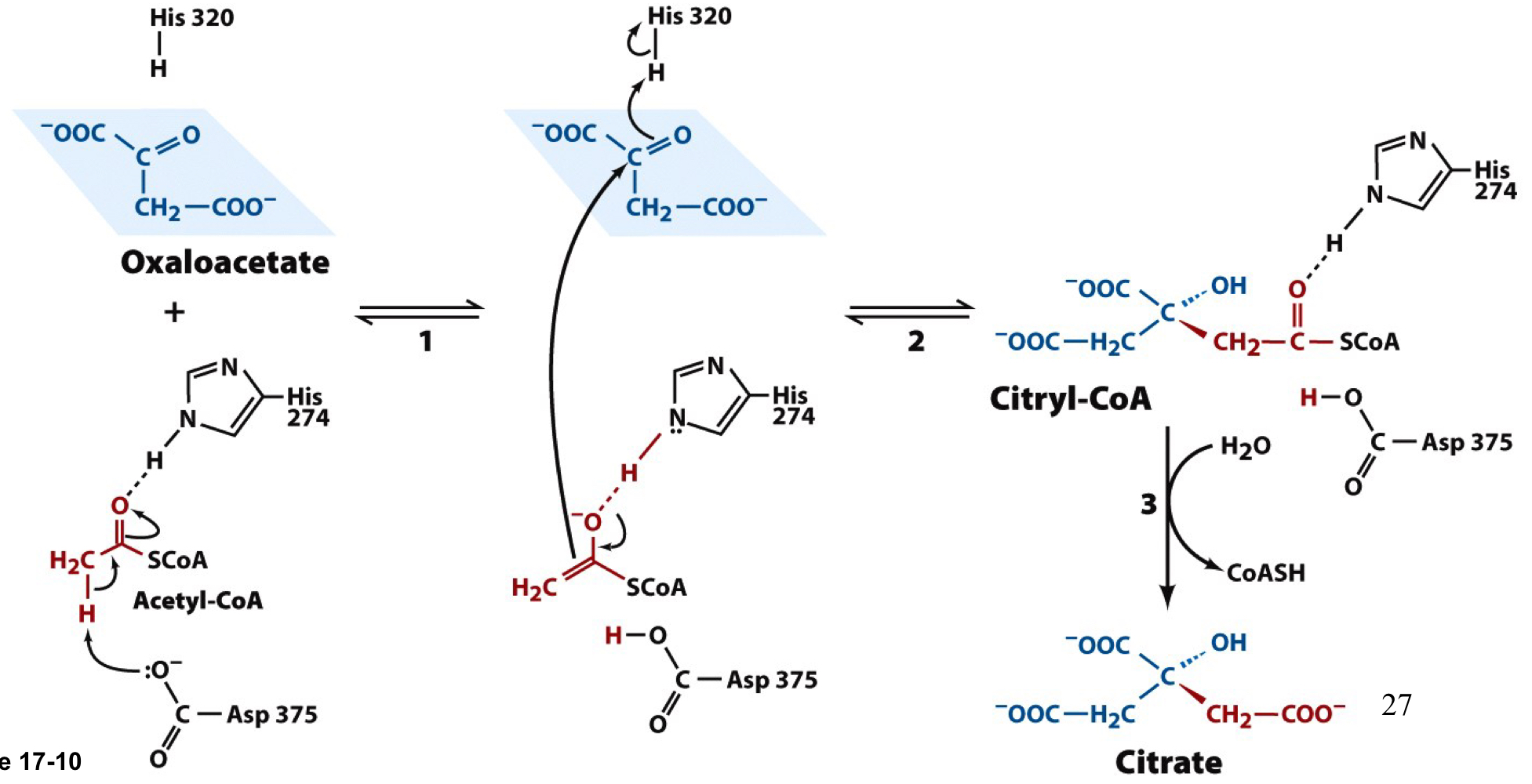
citrate synthase steps
1. Asp375 deprotonates acetyl-CoA at the methyl group, generating an enroll that is stabilized by hydrogen bonding to His274
2. acetyl-CoA enolate attacks oxaloacetate while His320 donates a proton to the oxaloacetate carbonyl, forming citryl-CoA
3. citryl-CoA is hydrolyzed to citrate and CoA with a ∆Go’=-31.5 kJ/mol
90
New cards
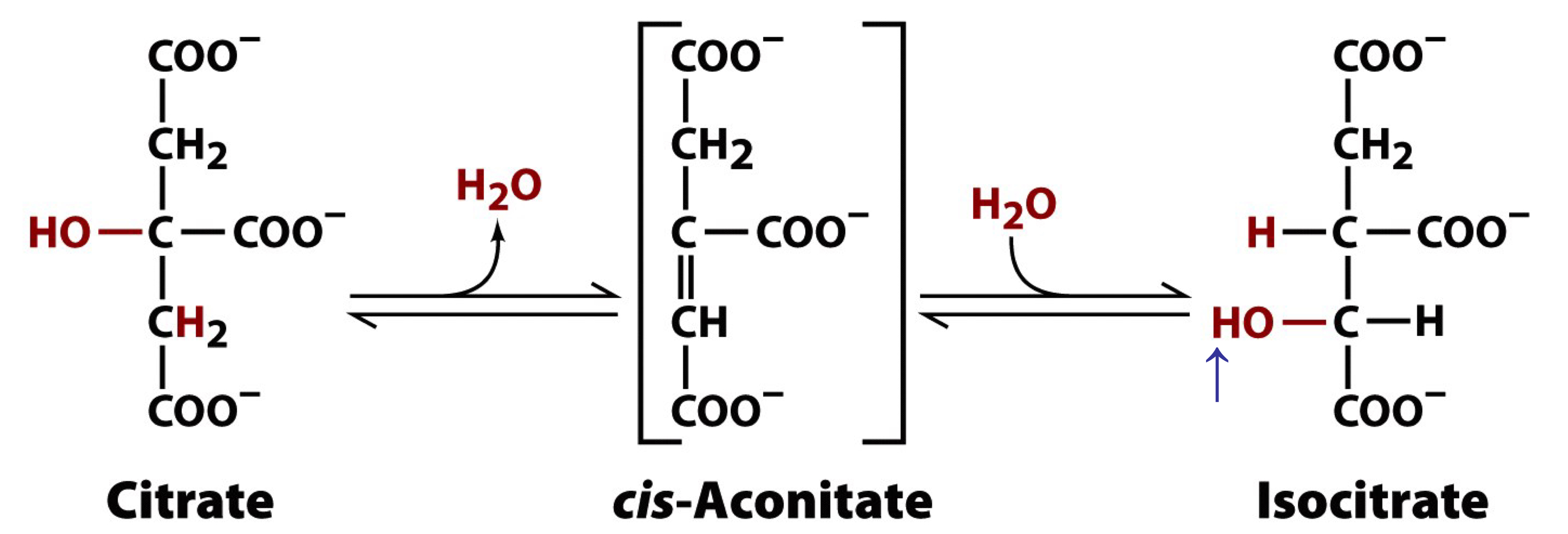
reaction 2 of the TCA cycle
aconitase
aconitase removes H2O from citrate to form the C=C bond of cis-aconitase
stereospecific re-addition of H2O to cis-aconitase forms 2R,3S-isocitrate
isocitrate contains a secondary alcohol that is susceptible to biological oxidation
metabolically reversible
aconitase removes H2O from citrate to form the C=C bond of cis-aconitase
stereospecific re-addition of H2O to cis-aconitase forms 2R,3S-isocitrate
isocitrate contains a secondary alcohol that is susceptible to biological oxidation
metabolically reversible
91
New cards
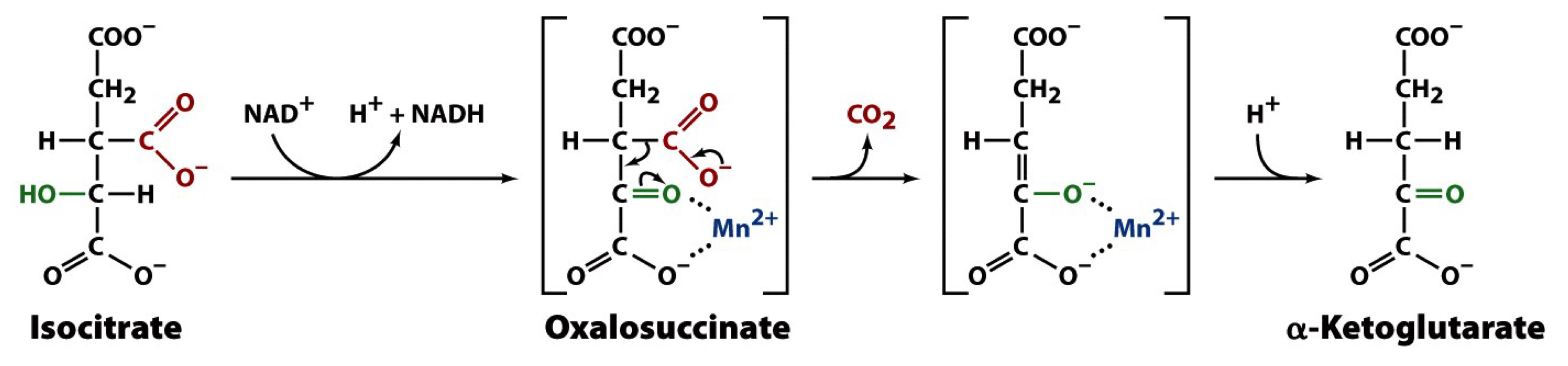
reaction 3 of the TCA cycle
isocitrate dehydrogenase
2 hydrogen atoms are removed from the secondary alcohol of isocitrate, forming an α-keto group
the intermediate in this dehydrogenation reaction is enzyme-bound oxalosuccinate, an α-keto tricarboxylic acid that spontaneously converts to α-KG releasing CO2 via a β-elimination reaction (assisted by Mn2+ or Mg2+ that polarize the new carbonyl)
the second metabolically irreversible reaction of the TCA cycle
the first of four oxidation-reduction reactions
2 hydrogen atoms are removed from the secondary alcohol of isocitrate, forming an α-keto group
the intermediate in this dehydrogenation reaction is enzyme-bound oxalosuccinate, an α-keto tricarboxylic acid that spontaneously converts to α-KG releasing CO2 via a β-elimination reaction (assisted by Mn2+ or Mg2+ that polarize the new carbonyl)
the second metabolically irreversible reaction of the TCA cycle
the first of four oxidation-reduction reactions
92
New cards
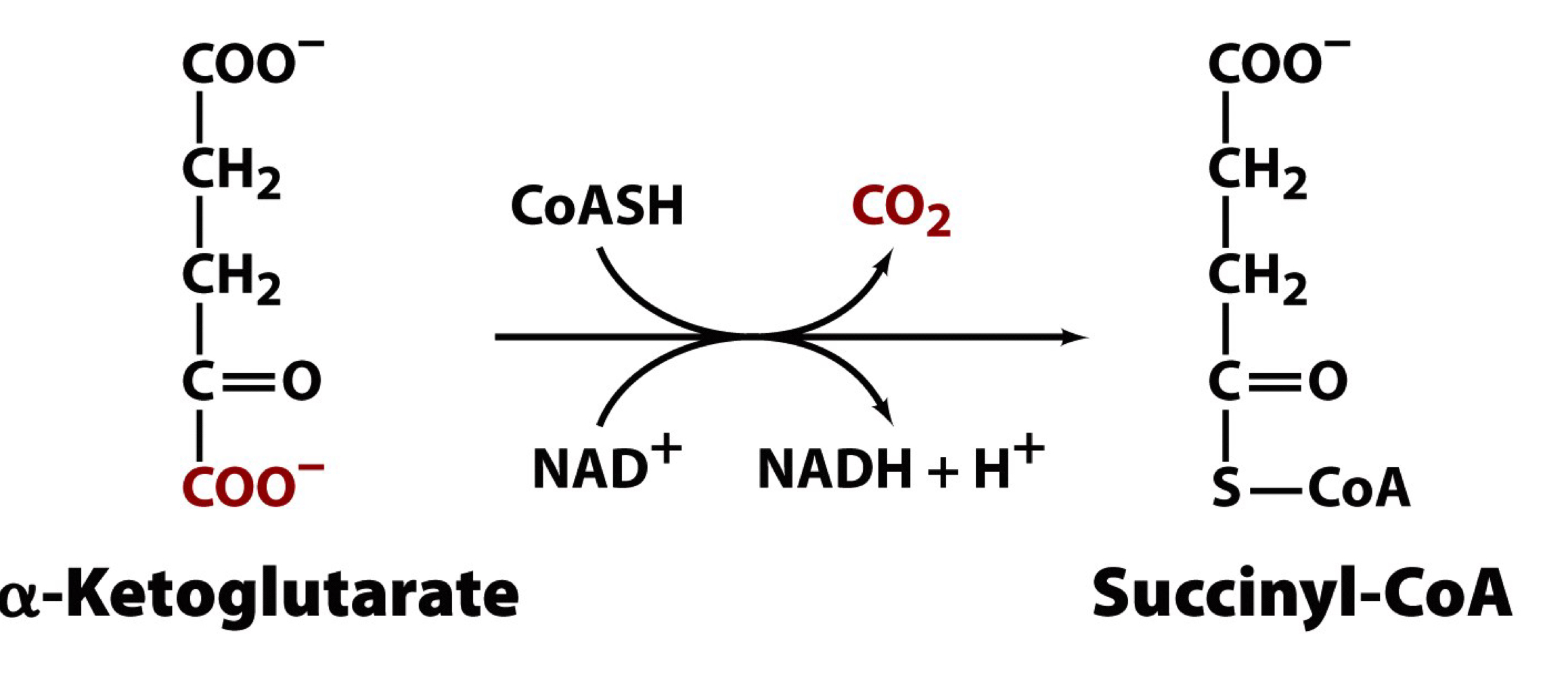
reaction 4 of the TCA cycle
α-ketoglutarate dehydrogenase complex
α-ketoglutarate is an α-keto acid
oxidative decarboxylation of α-ketoglutarate proceeds by a reaction similar to that of the pyruvate dehydrogenase complex (similarity in substrates and products between the two dehydrogenase reactions)
KDH and PDH are very similar enzyme complexes
this is the third metabolically irreversible reaction
the product, succinyl-CoA, is a high energy thioester that participates in a substrate level phosphorylation in the next reaction
α-ketoglutarate is an α-keto acid
oxidative decarboxylation of α-ketoglutarate proceeds by a reaction similar to that of the pyruvate dehydrogenase complex (similarity in substrates and products between the two dehydrogenase reactions)
KDH and PDH are very similar enzyme complexes
this is the third metabolically irreversible reaction
the product, succinyl-CoA, is a high energy thioester that participates in a substrate level phosphorylation in the next reaction
93
New cards
reaction 5 of the TCA cycle
succinyl-CoA synthetase
free energy of the thioester bond of succinyl CoA is conserved as GTP
succinyl-CoA synthase was called “thiokinase: because it conserves energy of a thioester to form GTP, a nulceotide triphosphate
1. succinyl-CoA reacts with free phosphate (pI0 to form succinyl-phosphate and CoA
2. the phosphorylase group is then transferred to a His residue in the enzyme, forming 3-phospho-His
1. the phosphorylase group is then transferred to GDP, forming GTP (substrate level phosphorylation)
free energy of the thioester bond of succinyl CoA is conserved as GTP
succinyl-CoA synthase was called “thiokinase: because it conserves energy of a thioester to form GTP, a nulceotide triphosphate
1. succinyl-CoA reacts with free phosphate (pI0 to form succinyl-phosphate and CoA
2. the phosphorylase group is then transferred to a His residue in the enzyme, forming 3-phospho-His
1. the phosphorylase group is then transferred to GDP, forming GTP (substrate level phosphorylation)
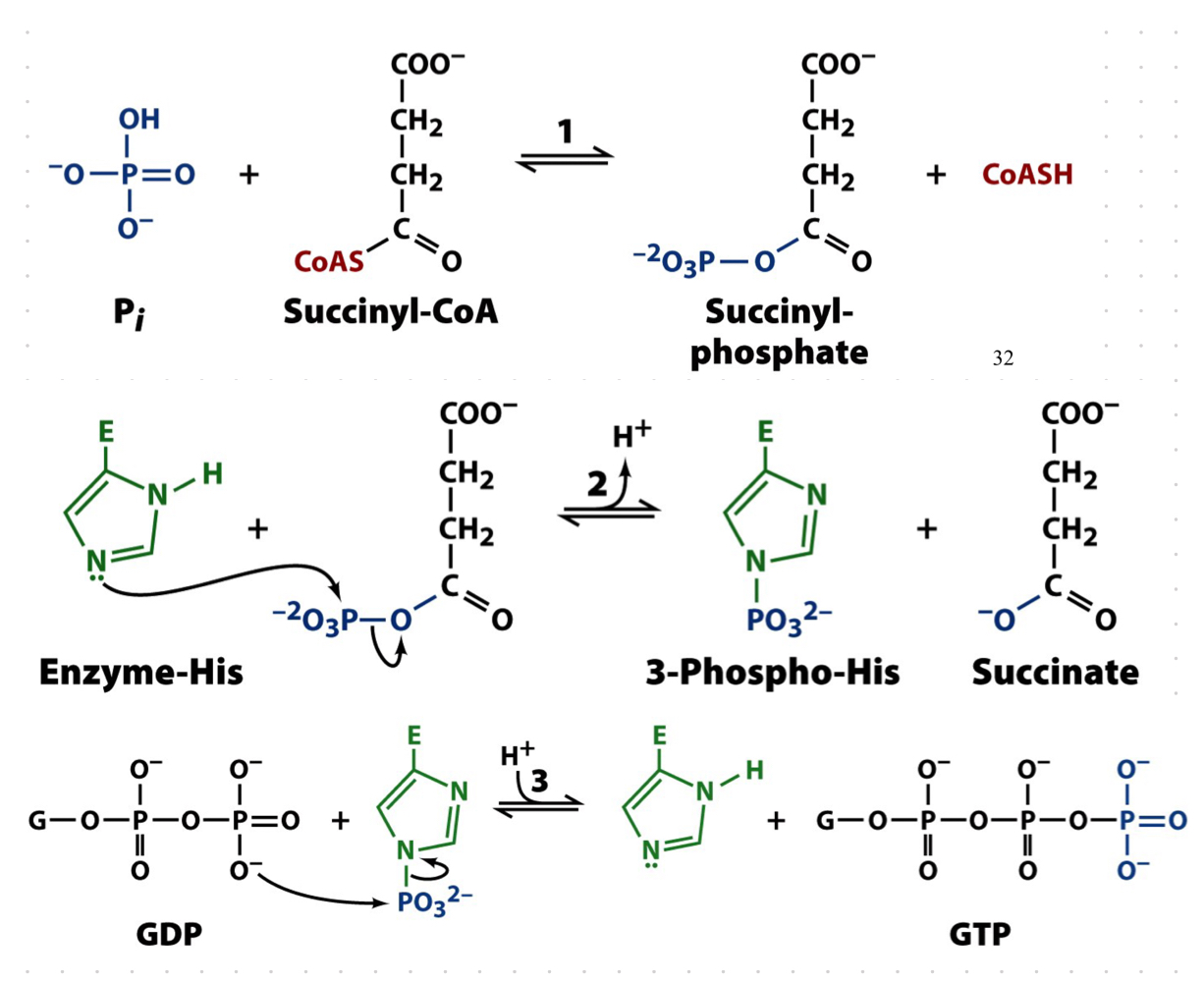
94
New cards
reaction 6 of the TCA cycle
succinate dehydrogenase complex
metabolically reversible
metabolically reversible
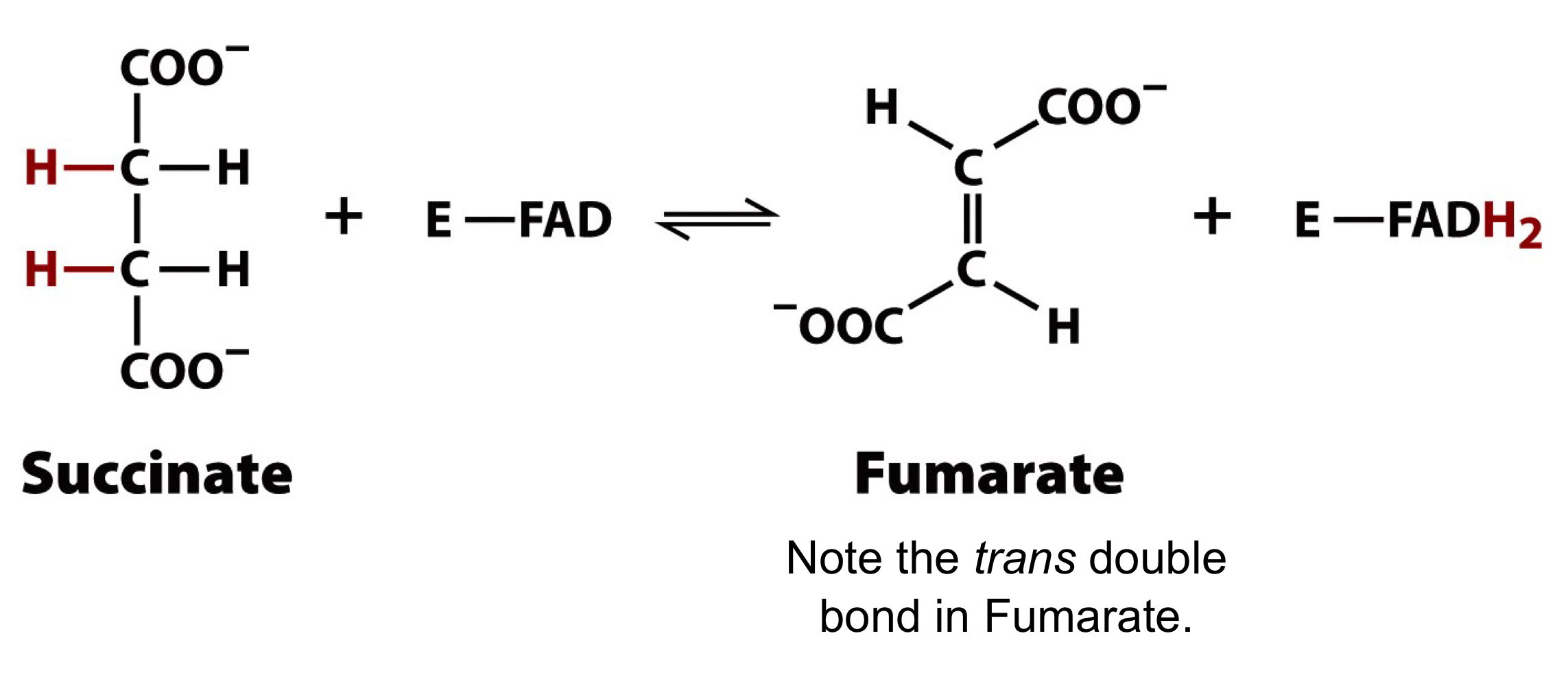
95
New cards
characteristic features of FAD-dependent dehydrogenases
the substrate has adjacent-CH2 groups
the removal of 2 Hs yields a trans double bond
the removal of 2 Hs yields a trans double bond
96
New cards
the SDH complex
succinate dehydrogenase is a complex of several polypeptides and contains an FAD prosthetic group (bound covalently by a His) which serves as the primary oxidizing agent
the FAD prosthetic group then transfers the electrons through iron-sulfur clusters to Q, forming QH2, a mobile lipophilic redox agent
the SHD complex is located in the inner mitochondrial matrix
the FAD prosthetic group then transfers the electrons through iron-sulfur clusters to Q, forming QH2, a mobile lipophilic redox agent
the SHD complex is located in the inner mitochondrial matrix
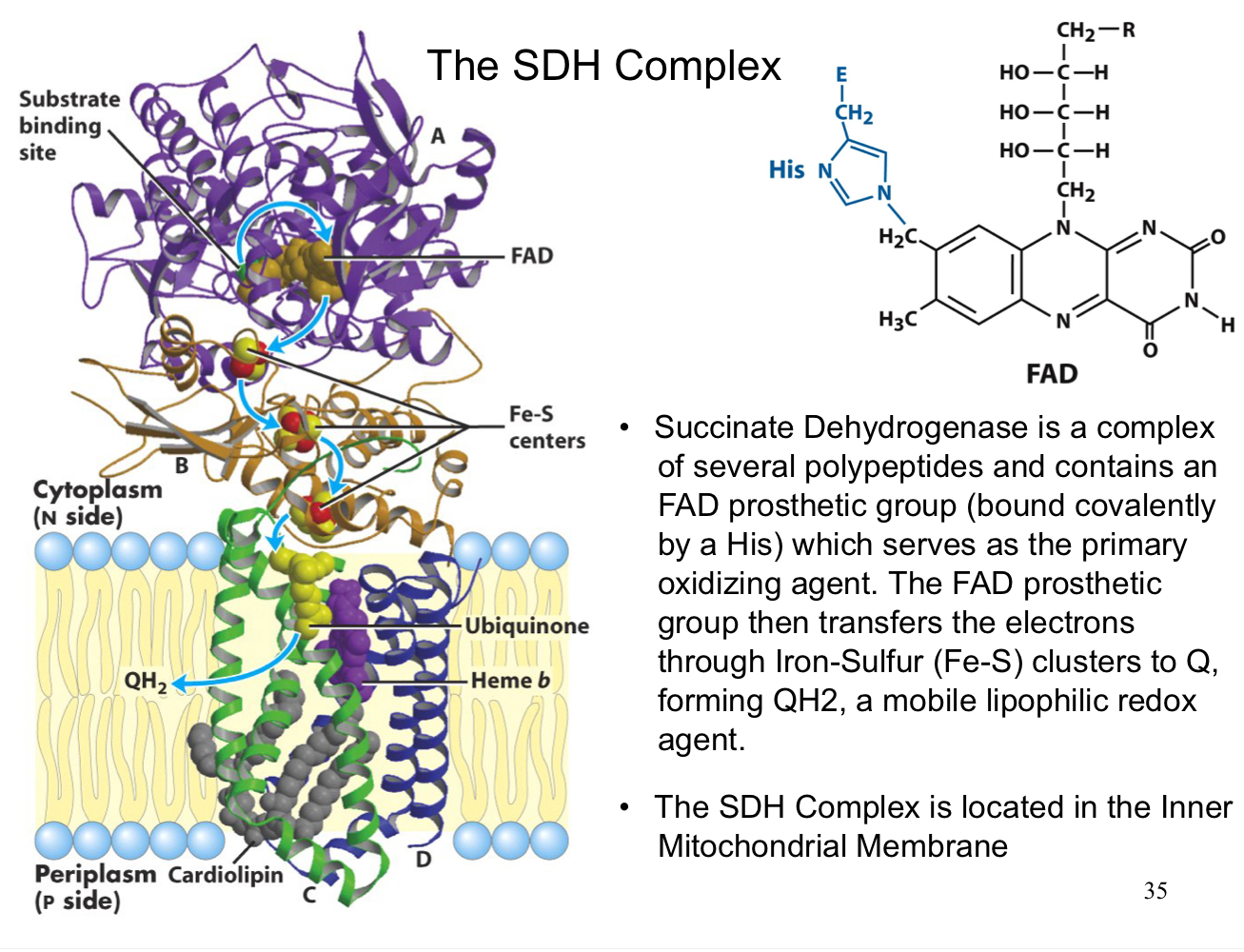
97
New cards
reaction 7 of the TCA cycle
fumarase
stereospecific addition of water to the trans double bond of fumarate forms L-malate
metabolically reversible
stereospecific addition of water to the trans double bond of fumarate forms L-malate
metabolically reversible
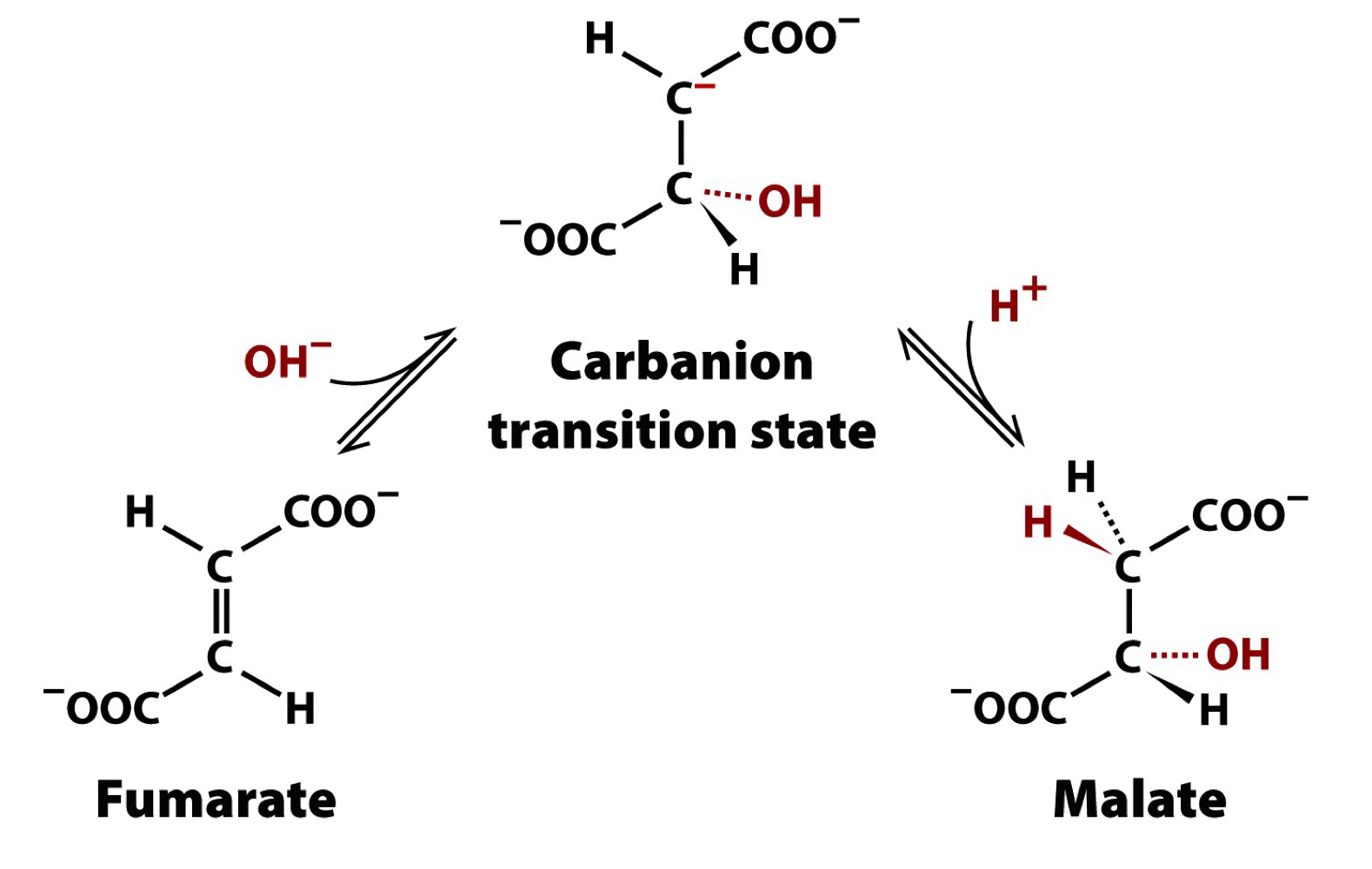
98
New cards
reaction 8 of the TCA cycle
malata dehydrogenase
uses NAD+ to oxidize the secondary alcohol of malate to a ketone group, producing NADH and forming oxaloacetate, regenerating the acceptor molecule
uses NAD+ to oxidize the secondary alcohol of malate to a ketone group, producing NADH and forming oxaloacetate, regenerating the acceptor molecule
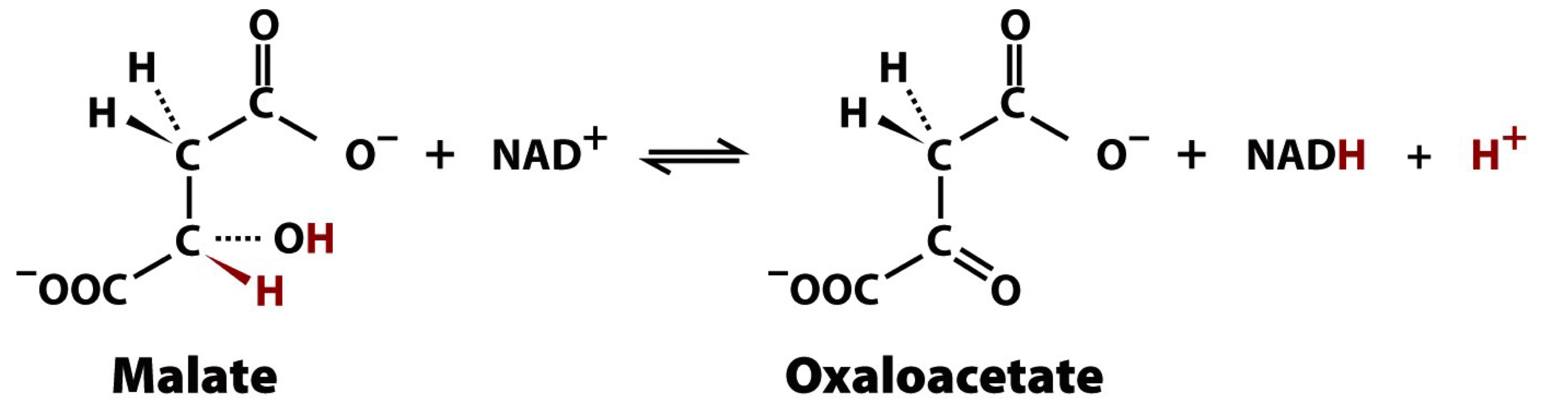
99
New cards
limiting substrate for the TCA cycle
oxaloacetate
at equilibrium reaction 8 lies far to the left, the level of L-malate is much higher than the level of oxaloacetate (∆Go’=+29.7 kJ/mol)
the citrate synthase reaction has to be exergonic to overcome the low concentrations of oxaloacetate in the cell
at equilibrium reaction 8 lies far to the left, the level of L-malate is much higher than the level of oxaloacetate (∆Go’=+29.7 kJ/mol)
the citrate synthase reaction has to be exergonic to overcome the low concentrations of oxaloacetate in the cell
100
New cards
energy conservation in the TCA cycle
energy is conserved in the form of reduced coenzymes: 3 NADH, 1 FADH2, and one nucleotide triphosphate (GTP)
NADH and QH2 are reoxidizes in the electron transport scheme and produce much more ATP by oxidative phosphorylation
NADH and QH2 are reoxidizes in the electron transport scheme and produce much more ATP by oxidative phosphorylation