Chem fundamentals (unit 1)
1.1 The study of chemistry
Matter: physical material; anything with mass that takes up space
Pure substances: matter that has a ==definite composition,== one that does not change, and has distinct properties. They can ==only be separated by chemical reactions==
- Elements: pure substances that cannot be decomposed into simpler substances.
- Atoms: smallest building block of matter. Each element is composed of 1 type of atom
- ex: C
- Molecules: ==2+ atoms,== can be same or different
- ex: O2, H2O
- Compounds: pure substances ==composed of 2+ different elements==. They can only be separated chemically
- Ex: H2O
1.2 Classification of Matter
Property: a characteristic to recognize/distinguish types of matter
- Physical properties: can be observed without changing the identity or composition of matter. They are the result of IMFAs between structures (melting point, refractive index, color)
- Intensive: properties that are ==independent of quantity== (boiling point, odor)
- Extensive: properties that are ==dependent on quantity== (volume, mass)
- Chemical properties: observed by destroying substance, they result from chemical reactions
Mixtures: a combo of 2+ pure substances. Each substance maintains its own properties. Mixtures can be separated into its pure substances
- Homogeneous: mixtures that are uniform throughout. The components are ==evenly distributed.== They look pure but aren’t since they’re not chemically combined.
- Solution: small particles, don’t scatter light
- ex: copper sulfate (aq) or brass
- Colloid: have large particles, scatter light
- ex: milk
- Heterogenous: Mixtures that aren’t evenly distributed (granite, wood)
- Suspension: you can see layers
1.3 Properties of Matter
Physical change: changes physical appearance, not composition
- same substance before and after change
- ex: ice→ water (state change)
Chemical change: substance→different substance
Separation of mixtures
- Distillation: process depends on the boiling points to form gases
- NaCl + H2O: when you boil it, the water evaporates, leaving salt behind
- Chromatography: depends on the differing size and polarity of substances to adhere to surfaces of solids to separate mixtures
- Separating chlorophyll pigments in leaves
- Filtration: mix of solids and liquid that’s poured through filter paper. The liquid passes through, leaving the solid in the filter paper
- ex: coffee
2.1: Atomic theory of matter
Dalton’s atomic theory:
Each element is composed of extremely small particles (atoms)
All atoms of a given element are identical to each other
- All O2 atoms are the same, all N2 atoms are the same
Atoms of 1 element can’t be changed into atoms of different elements by chemical reactions.
- O2 can’t turn into N2
Compounds are formed when atoms of more than 1 element chemically combine.
- O + N (elements)→NO (compound)
Law of conservation of mass: matter isn’t created or destroyed, just rearranged
Law of constant composition/definite proportion: g==iven compounds always have same elements in the same proportion.== The ratios are fixed
- H2O is always 2:1 ratio of H:O
Law of multiple proportions: compounds with different ratios of the same atoms are different
- H2O is different from H2O2
2.2 discovery of atomic structure
Democritus: made first atomic model in 400 BC
- proposed that all matter is made up of atoms (small, solid, indivisible particles)
- Model: ball

Dalton: determined that each element is made up of atoms, created atomic theory
- model: ball (same as above image)
Thompson: through cathode ray tube experiments, determined that there are negatively charged electrons
because electrons contribute a small fractions of atom’s mass, they are small
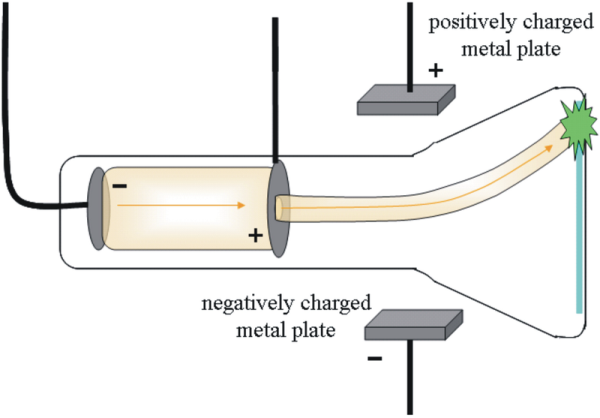
Cathode ray: A glass tube is pumped with air and when high voltage is applied to the electrodes. radiation occurs between them. The radiation (cathode rays, stream of - charged particles) originate at the anode and travel to the cathode. The rays are the same regardless of the cathode material.
He constructed a cathode ray tube w/ a hole in the anode where the cathode ray can pass. Because the electron is negative, the electric field deflects rays in 1 direction. This allowed him to calculate 1.76 x 10^8 Coulombs/g for the ratio of electron charge: mass
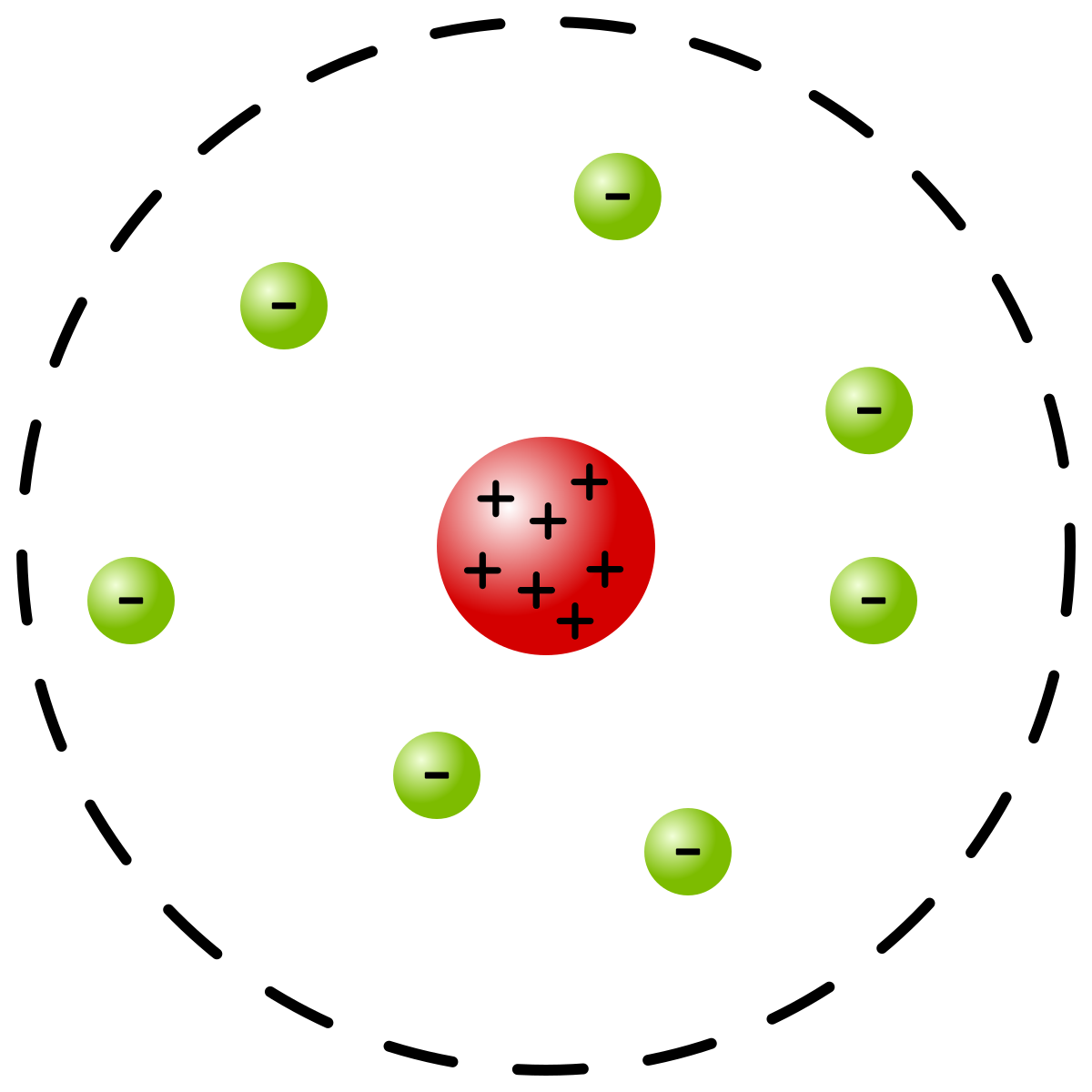
Rutherford: through his gold foil experiment, he discovered protons and the nucleus
- Most of the atom’s mass comes from dense + nucleus and most of the volume is empty space (electron cloud)
- Bombarded gold foil with alpha particles---majority passed through with no deflection. Very few particles were deflected-----proved that there’s a small but highly charged nucleus
Chadwick: through nuclear bombardment, he found the neutron
Radioactivity: 3 types of radiation (alpha, beta, gamma)
Beta (-1) are high speed electrons bent towards + end
Alpha (+2) bent towards - end
Gamma (0) are high energy waves, no particles or charge bent towards no end, unaffected
2.3: modern view of atomic structure
Nucleus: contains protons and neutrons with an overall + charge.
- very small and dense (1x10^-15 m)
Electron cloud: contains negatively charged electrons
- almost no mass but most of atom volume (1-5 x10^-10 m)
Angstrom: 1x10^-10m=100pm
Atomic mass unit (amu)=1.66054x10^-24 g
Subatomic particles
Proton: +1, 1.0073amu
Electron: -1, 5.486x10^-4amu
Neutron: 0, 1.0087amu
Isotopic Notation
A: mass number, protons+neutrons
- Isotopes: same # protons but different # neutrons, differing mass numbers
Z: atomic number, just protons, used to identify element
q: charge, #protons vs electrons 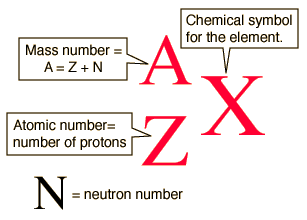
2.4: atomic weights
1amu=1.66054x10^-24 g
1g=6.02x10^23 amu
Atomic mass: the number from the periodic table is dependent on isotopic abundance
- Σ (isotope mass)(isotope abundance)
Mass spectrometer:
- Get atoms into gas phase and convert them into ions (cations)
- When gas phase cations made, they’re accelerated towards negative grid
- Only a narrow beam of ions can pass
- Beam passes through magnet poles that deflect ions
- Ions separated into their masses (isotopes) ---smaller mass goes first
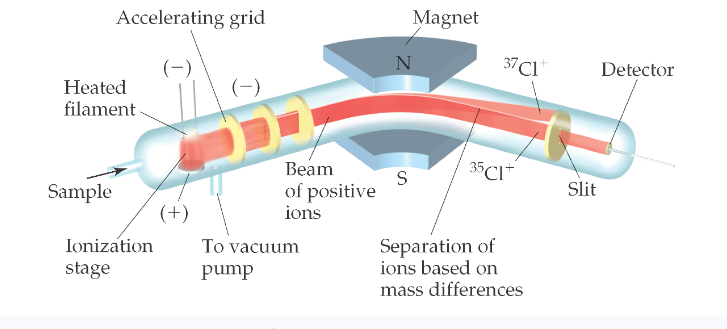
Mass spectroscopy: uses spectrometer to determine the mass of an element/molecule
Provides mass of ions and relative abundance, allows us to calculate atomic mass
converts atoms/molecules into anions to measure mass and abundance in electric field. The beam of ions passes through poles of a magnet which deflects them on a curved path. The extent of deflection depends on the mass. Ions are sorted according to their mass
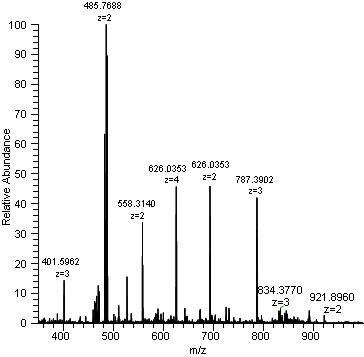
Ex: 9/16 atoms have a mass of 70, 6/16 have a mass of 72, 1/16 have mass of 74
AM=70(9/16)+72(6/16)+74(1/16)=71amu
2.5 Periodic Table
 Periods: the rows
Periods: the rows
Groups: the columns
- Group 1: alkali metals
- Group 2: alkaline earth metals
- Group 6: chalcogens
- Group 7: halogens
- Group 8: noble gases
2.6 Molecules, molecule compounds
Diatomic: molecules made of 2 atoms naturally (H, O, N, F, Cl, Br, I)
Molecular formula vs empirical formula: molecule is the actual # of atoms in a molecule while empirical is the smallest ratio
- Molecular: H2O2 Empirical: HO
Structural formula: drawing that shows how the atoms are joined but doesn’t show geometry
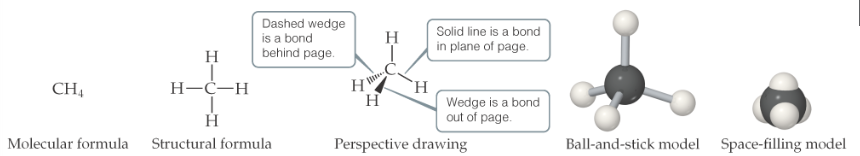
2.7 Ions, ionic compounds
ion: when an atom isn’t neutral, it either lost/gained electrons
- cation: positive ion, lost electrons
- anion: negative ion, gained electrons
- polyatomic ion: atoms joined as one molecule with an overall charge
ionic compound: cations and anions in an alternating crystal lattice. Always in empirical form
- formula unit: lowest whole # ratio of cations:anions
3.3 Formula weights
Formula weight: the sum of each atomic weight in a substance
- FW of H2O: 2(1.008)+1(16)=18.016 g
==%== ==composition: the mass contributed by each element==
- % comp= #atoms of element (atomic weight) /formula weight x100
Ex: calculate the % composition of C, H, and O for C12H22O11
- C: 12(12.01)/342.296 x100=42.10%
- H: 22(1.008)/342.296 x100=6.48%
- O: 11(16)/342.296 x100=51.42%
Ex: calculate % composition of phosphorus in calcium phosphate Ca3(PO4)2
- P: 2(30.97)/310.18 x100=19.96%
3.4: Avogadro’s number
Mole: 6.02x10^23
Calculate the # of H atoms in 0.350 mol of C6H12O6
| 0.350 mol C6H12O6 | 12 mol H | 6.02x10^23 atoms |
|---|---|---|
| 1 mol C6H12O6 | 1 mol H |
=2.53x10^24 H atoms
Molar mass: moles of different elements have different masses
- 1 mole of Cl=35.45 amu but 1 mole of Au is 197amu
Calculate # moles of glucose in 5.380g sample
| 5.380g C6H12O6 | 1 mole glucose |
|---|---|
| 180.156 g glucose |
= 0.0298 moles of glucose
Calculate mass of 0.4333 moles of Ca(NO3)2
| 0.4333 moles Ca(NO3)2 | 164.1 g Ca(NO3)2 |
|---|---|
| 1 mole Ca(NO3)2 |
=71.06g Ca(NO3)2
5.23 g sample of glucose. Find # molecules
| 5.23 g glucose | mole glucose | 6.02x10^23 molecules |
|---|---|---|
| 180.156 g glucose | mole |
=1.75x10^22 molecules glucose
==3.5 Empirical formulas==
Empirical formula determination--Given percentages of each element
- Base calculation on 100.g of compound. It’s easier
- Determine # moles of each element for 100.g of compound
- Divide each mole value by the smallest mole value to get the ratio
- Multiply by the integer to get a whole number formula
Ex: adipic acid has 46.32% C, 43.84% O, 6.85% H
- C: 46.32g →4.107 moles C O: 43.84g→2.74 moles O H: 6.85g →6.78 moles H
- C: 4.107moles C/2.74 moles=1.50 O:2.74moles/2.74=1.00 H: 6.78moles H/2.74=2.50
- Since not whole numbers, multiply everything by 2 →C3H5O2
Ex: Compound is 74% Hg and 26% Cl by mass. Find empirical
- 74g Hg and 26g Cl
- 74g Hg(1mole Hg/200.6g Hg)=0.369 moles Hg
- 26g Cl(1mole Cl/35.45g Cl)=0.733 moles Cl
- Hg:0.369moles Hg/0.369 =1 Hg
- Cl: 0.733moles Cl/0.369=2 Cl
- Empirical: HCl2
Ex: ascorbic acid is 40.92% C, 4.58% H, and 54.50% O. Find empirical formula
- 40.92g C, 4.58g H, 54.50g O
- 40.92g C(1mole C/12.01gC)=3.407 moles C
- 4.58gH(1mole H/1.008g H)=4.54 moles H
- 54.50g O(1 mole O/16g O)=3.406 moles O
- C: 3.407g/3.406=1 C
- H: 4.54 moles H/3.406=1.33 H
- O: 3.406g O/3.406=1
- Empirical: C3H4O3
finding the molecular formula from empirical formulas
==whole # multiple=molecular weight/empirical formula weight==
- find formula mass of empirical formula
- Find molar mass of the substance
- substance molar mass/empirical molar mass
- Multiply the empirical formula by that integer
Ex: empirical formula of adipic acid is C3H5O2 and the molar mass is 146g/mole. What is the molecular formula?
- 146 g molar mass/73.07 g formula mass =2
- 2(C3H5O2)= C6H10O4
Ex: C3H4O3 has an experimental mass of 76g.
- 88.062g C3H4O3 /76g=2
- 2(C3H4O3)=C6H8O6
Ex: C3H4 has an experimental mass of 121 amu, what’s molecular formula?
- 121amu/40.062amu=3
- 3(C3H4)=C9H12
Combustion analysis to find empirical formula
- Use mass of CO2 to find the amount of C in organic substance
- Use mass of H2O to find amount of H in organic substance
- If there’s oxygen in the organic, subtract Cmass and Hmass to get Omass by itself
- Once you have masses of each element, proceed like before, get mole substance ratios
Ex: menthol is composed of C, H, and O. A 0.1005g sample combusts, producing 0.2829g CO2 and 0.1159g H2O. What is the empirical formula if the molar mass of the organic is 156g/mole
- 0.2829g CO2(mole CO2/44.01g CO2)(mole C/mole CO2)(12.01g C/mole C)=0.07715g C
- 0.1159g H2O(mole H2O/18.016g H2O)(2 moles H/mole H2O)(1.008g H/mole H)=0.01288g H
- 0.1005-0.0771-0.01288=0.01047g O
- C: 0.07715g→0.006429 moles C
- H: 0.01288g→0.01288 moles H
- O: 0.01047g→0.0006544 moles O
- C: 0.006429moles C/0.0006544=10 moles of C
- H: 0.01288moles/0.0006544=20 moles of H
- O: 0.0006544/0.0006544=1 mole of O
- Empirical formula C10 H20 O
Ex:Isopropyl alcohol has C, H, O. The combustion of 0.255g alcohol produces 0.561g CO2 and 0.306g H2O
- 0.561g CO2(mole CO2/44g CO2)(1 mole C/mole CO2)(/12.01g C/mole C)=0.1531g C
- 0.306g H2O(mole H2O/18.016g H2O)(2 mole H/mole H2O)(1.008g H/mole H)=0.0342g H
- 0.255-0.1531-0.0342=0.0677 g O
- 0.1531g C (mole C/12.01g C)=0.0127moles C
- 0.0342g H(mole H/1.008g H)=0.0339 moles H
- 0.0677g O(mole O/16g O)=0.00423 moles O
- 0.0127moles C/0.00423=3 Carbon
- 0.0339 moles H/0.00423=8 Hydrogen
- Empirical=C3 H8 O
3.6 Quantitative Info from Balanced Equations
Coefficients: the relative # of molecules in a reaction
- Ex: 2H2 +O2 →2H2O shows 2 molecules of H2 reacting with 1 molecule of O2 to form 2 molecules H2O
Stoichiometrically equivalent quantities: used to convert between quantities
- 1.57 mole O2 reactions w H2 to form H2O, how many moles H2 used?
- 1.57mole O2 x 2mole H2/mole O2 =3.14 mole H2
- Determine how many g of H2O produced: C6H12O6 +6O2 →6CO2 +6H2O
- 1.00g glu x mole glu/180.156g glu x 6 mole H2O/mole glu x 18.016g H2O/mole H2O=0.600g H2O
3.7 Limiting Reactants
Limiting Reactant: the reactant that’s used up first. Once it’s gone, reaction can’t continue
- N2 +3H2 →2NH3, 3moles N2, 6 moles H2
- 3 mole N2 x 2mole NH3/mole N2 =6mol NH3
- 6mole H2 x2mol NH3/3mole H2=4 mol NH3
- LR: H2, RIE: N2, N2 left over: 1 mole
Theoretical and Percent Yields
Theoretical yield: quantity of product calculated to form when all LR is consumed (100% completion, no error)
Experimental yield: amount of product actually obtained (will always be less due to error)
Percent yield: how much you got exp/theo x100%
Percent error: how far you were exp-theo/theo x100%
4.5 Concentrations of Solutions
Concentration: amt of solute dissolved in given quantity of solvent
- more solute=more concentrated
Molarity: concentration of moles/L
- Calculate M of solution with 23.4g Na2SO4 to make 125ml
- 23.4g Na2SO4/0.125L =1.32M Na2SO4
- What’s M of each ion in 0.025M Ca(NO3)2
- 0.025mol Ca(NO3)2/L x mole Ca2+/mole Ca(NO3)2=0.025mol Ca2+
- 0.025mol Ca(NO3)2/L x 2mole NO3-/mole Ca(NO3)2 =0.050 mol NO3-
- 0.200M HNO3 solution. Calculate moles in 2.0 L
- 2.0L solution x 0.200mole HNO3/1L =0.40 moles HNO3
Dilution: adding water to make concentration lower
- C1V1 = C2V2
1. Want 250 ml of 0.100 M CuSO4 by diluting 1.00 M CuSO4
- C1= 1.00 M, V1=? C2=0.100M, V2=0.250L
- V1=(0.100M)(0.250L)/1.00M =.0250L or 25 ml of CuSO4 used
2. How many mL of 3.0M H2SO4 for 450mL 0.10M H2SO4
- C1=3.00M, V1=? C2=0.10M, V2=0.450L
- V1=(0.10M)(0.450L)/3.00M = 0.015 L or 15mL used
4.6 Solution Stoichiometry and Chem Analysis
If we know the solute M, we can used M and V to determine # moles
- How many g of Ca(OH)2 to neutralize 25.0mL of 0.100 HNO3 Ca(OH)2 +2HNO3 →Ca(NO3)2 +2H2O
- 0.025 L x 0.100 mole NO3/L x mole Ca(OH)2/2mole HNO3 x 74.096g/mole Ca(OH)2 =0.092 g Ca(OH)2
Titrations: combining solution with unknown concentration w reagent of known concentration (standard sol)
Equivalence point: where stoich equivalent quantities are brought together
- Indicator: dye that changes color as passing equivalence point
1. 45.7mL of 0.5 M H2SO4 req. to neutralize 20.0mL of NaOH sol. What is M of NaOH?
- H2SO4 + 2NaOH →2H2O +Na2SO4
- 45.7mL H2SO4 x L/1000ml x 0.5 mole H2SO4/L =0.229 moles H2SO4
- 0.229 moles H2SO4 x 2mole NaOH/1mole H2SO4 =0.046 moles NaOH
- 0.046 moles NaOH/20ml x 1000ml/L =2.285 M NaOH