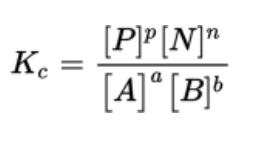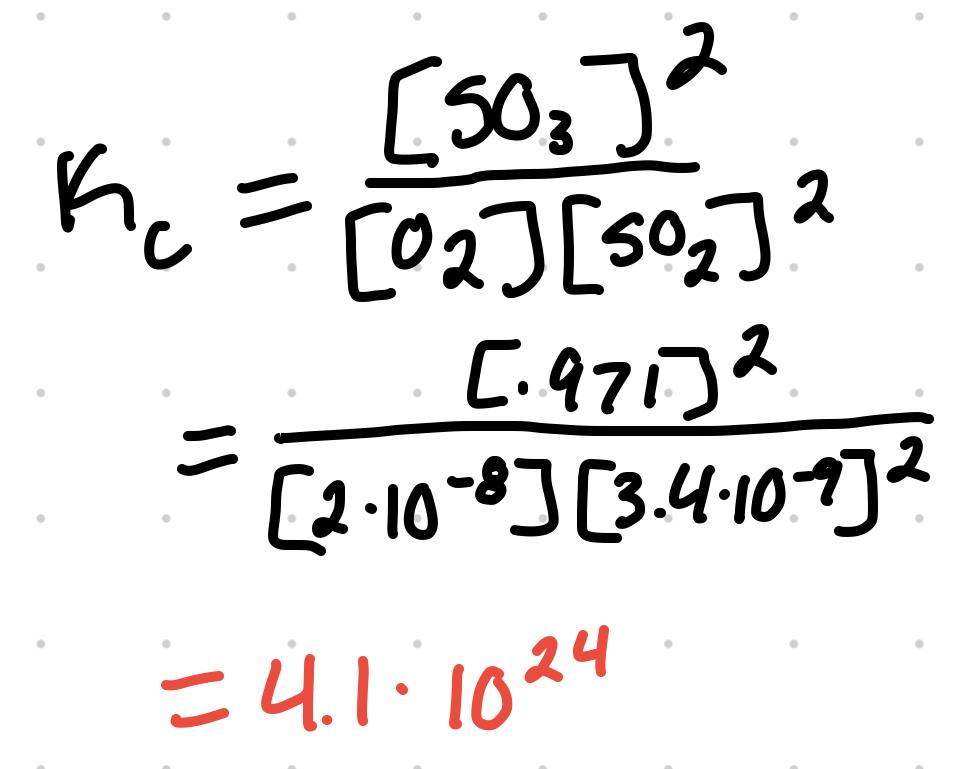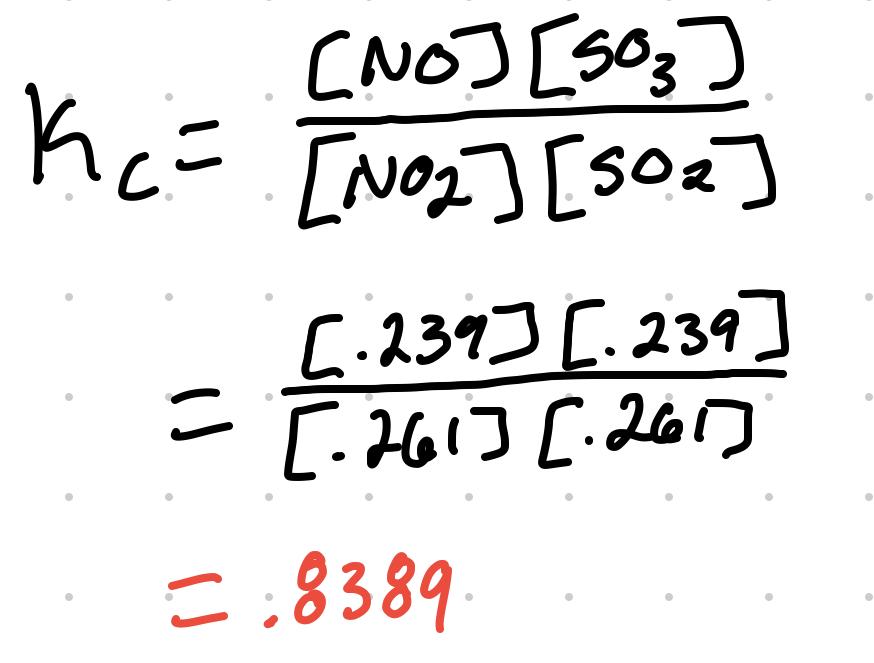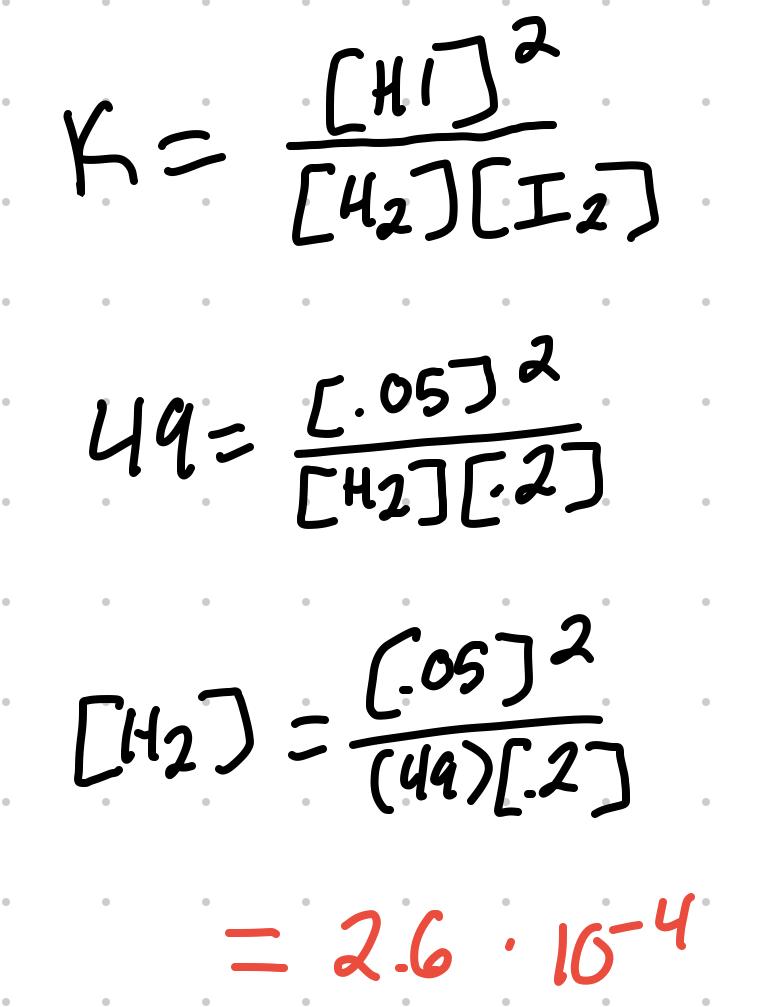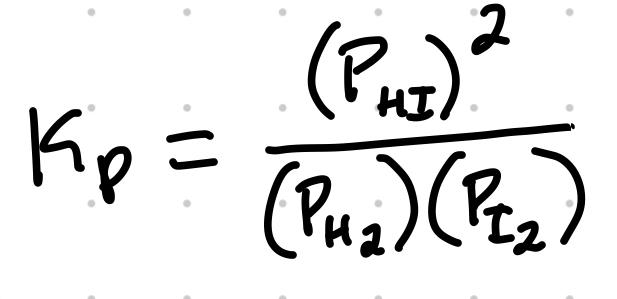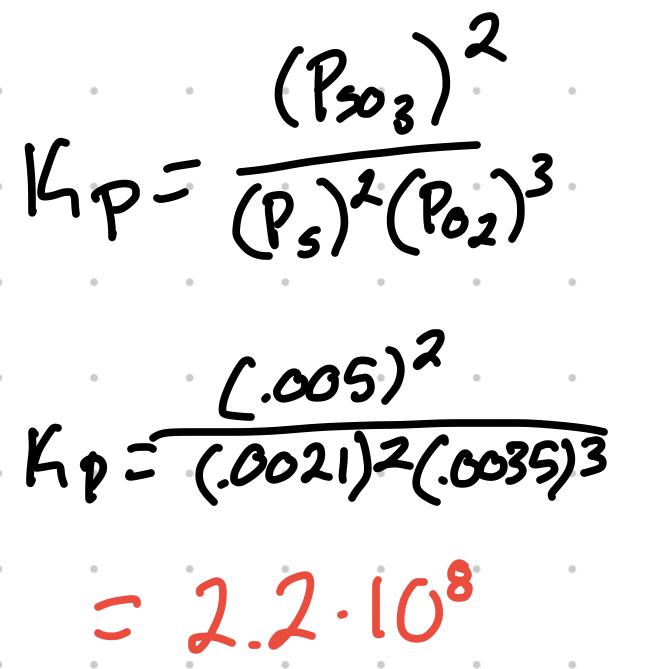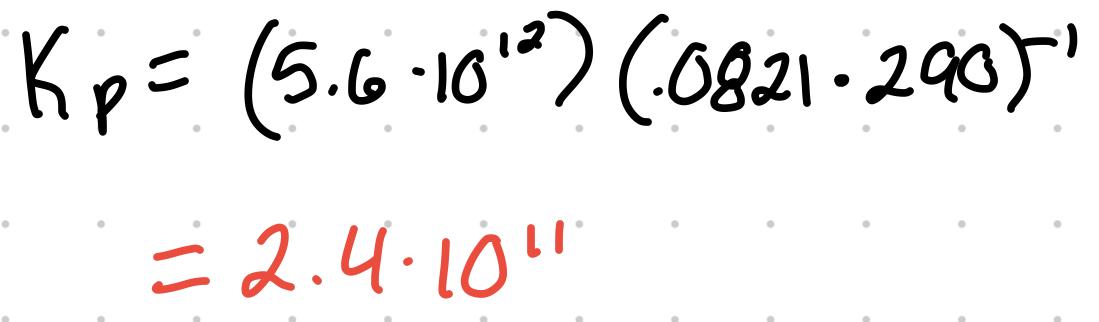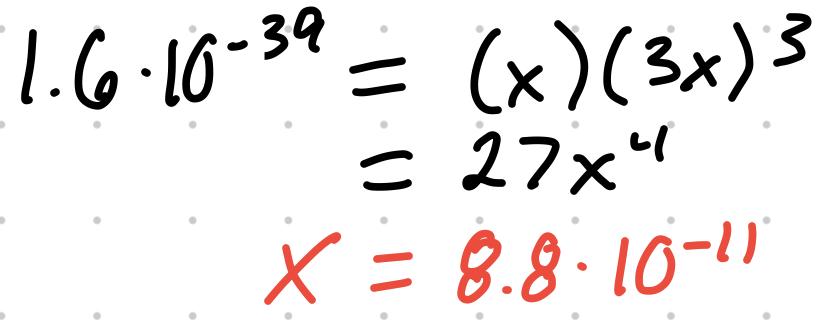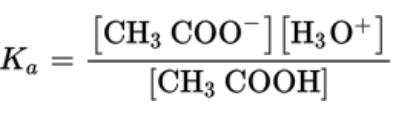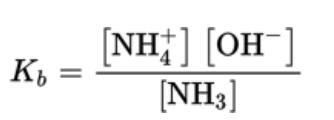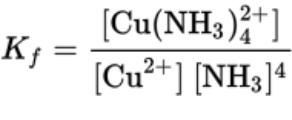Chapter 9: Chemical Equilibrium
- Dynamic equilibrium is when chemicals are reacting on a molecular scale but do not change in concentration
- When a reaction begins, the reactants decrease and the products increase. When equilibrium is reached, the reaction does not stop occurring, but the concentrations of products and reactants remain the same.
- Chemical equilibrium concepts are used to describe the compounds
The Equilibrium Expression
- The equilibrium concentrations are dependent on the initial concentration of the reactants. Different initial concentrations cause different equilibrium concentrations
- Equilibrium expression is always followed no matter the initial concentration.
- When you multiply the product concentrations and divide that value by all of the reactant concentrations multiplied together, you will obtain the equilibrium constant, K.
- Equilibrium constant depends on the specific reaction and temperature when equilibrium is reached
- K is the equilibrium constant symbol and can be specified with different subscripts
- Kc is equilibrium constant when concentration is in Molarity
- Kp is when partial pressure of gasses represents reactant and product
- Ksp is solubility product
- Ka is acid ionization constant
- Kb is base ionization constant
- If a reaction is written as aA + bB ⇄ pP + nN, then the equilibrium expression could be
Manipulating the Equilibrium Expression
- Equilibrium constant is written from the balance reaction
- To reverse direction of reaction, the reactant and products can be flipped.
- Coefficients can be multiplied or divided by constant factors
- Equations can be added or subtracted
Determining the Value of the Equilibrium Constant
To find K, simply measure the concentrations of products and reactants and use equilibrium expression
For O2 + 2SO2 ⇄ 2SO3 where the equilibrium concentration are [O2] = 2x10^-8 M, [SO2] = 3.4x10^-9 M, and [SO3] = .971 M
Using the Equilibrium Expression
Extent of Reaction and Thermodynamically Favorable Reactions
- If the equilibrium constant is very large, more products are present and favored at equilibrium.
- Very large is seen as greater than 10^10
- If a lot of product is formed, the reaction has gone nearly to completion
- If the constant is very small, more reactants are present and favored at equilibrium.
- Very small is seen as less than 10^-10
- If very little product has occurred, then virtually no reaction has occurred
- If K = 1, the equilibrium mixture has equal products and reactants
- Thermodynamically favorable reaction (TFP) has products form with very little assistance
- TFP is seen as constant greater than 1
- A reaction is seen as a thermodynamically unfavorable reaction, not TFP, when the equilibrium constant is less than 1.
The Reaction Quotient and Predicting the Direction of a Reaction
- Reaction quotient, Q, is obtained by using the equilibrium expression and using the values of product and reactant at any point in time, not just at equilibrium.
- This is different from K because K can only be obtained at equilibrium.
- Principles of Q
- If Q does not change with time, the reaction is in a state of equilibrium. Q = K
- Q = K is the reaction is at equilibrium
- Q < K if the reaction will move forward (produce more products) to reach equilibrium
- Q > K if the reaction will move in the reverse (produce more reactants) to reach equilibrium
Equilibrium Calculations
The Equilibrium Table
- Equilibrium table is used to organize given information in an problem to find the missing values
- Comprised of five lines: the balance reaction, initial concentrations, how much the initials change, the equilibrium values, and the answer
- For the reaction NO2 + SO2 ⇄ NO + SO3
| Reaction | NO2 | + | SO2 | ⇄ | NO | + | SO3 |
|---|---|---|---|---|---|---|---|
| Inital [ ] | |||||||
| Change | |||||||
| Eqlbrm | |||||||
| Answer |
- The change line will be represented by x and the coefficient of x correlates to the coefficient of the line it is in. In this table, x will have a coefficient of 1. However, if NO2 was 2 NO2, the x would be 2x.
- If a 4 L flask was comprised of 1 mole of each compound, then it would look like this (1 mol / 4 L = .250 M)
| Reaction | NO2 | + | SO2 | ⇄ | NO | + | SO3 |
|---|---|---|---|---|---|---|---|
| Inital [ ] | .250M | .250M | .250M | .250M | |||
| Change | -x | -x | +x | +x | |||
| Eqlbrm | .250 -x | .250 -x | .250+x | .250 +x | |||
| Answer |
Calculations of Equilibrium Constants
- Using the previous table, if it is then given that the equilibrium concentration of NO2 is .261M.
- If .250 - x = .261M, then x = -.011M
| Reaction | NO2 | + | SO2 | ⇄ | NO | + | SO3 |
|---|---|---|---|---|---|---|---|
| Inital [ ] | .250M | .250M | .250M | .250M | |||
| Change | -x | -x | +x | +x | |||
| Eqlbrm | .250 -x | .250 -x | .250+x | .250 +x | |||
| Answer | .261M | .261M | .239M | .239M |
Plugging the answer values to the equilibrium expression:
Determination of Equilibrium Concentrations by Direct Analysis
At equilibrium, the chemical reaction obeys the equilibrium expression and the expression can be used to calculate the concentrations
For the reaction H2 + I2 ⇄ 2 HI, where K = 49, [I2] = .2M, [HI] = .05M, [H2] can be calculated:
Determination of Equilibrium Concentrations from Initial Concentrations and Stoichiometric Relationships
- If given initial concentrations and equilibrium constant, equilibrium concentrations can be determined for all compounds
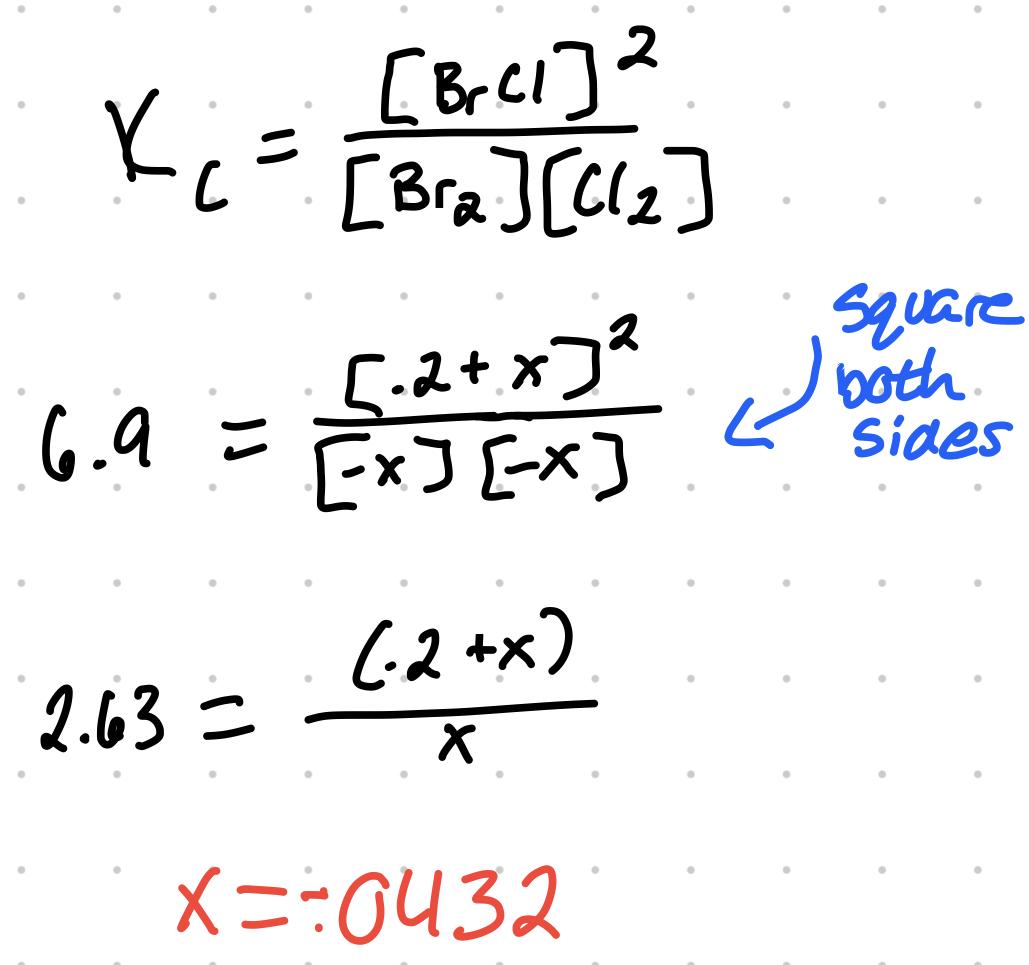
- For Br2 + Cl2 ⇄ 2BrCl, K = 6.9. If .1 mol BrCl is in a 500mL flask, find [Br2], [Cl2], and [BrCl].
- .1 mol BrCl / .5L = .2M
| Rxn | Br2 | + | Cl2 | ⇄ | 2BrCl |
|---|---|---|---|---|---|
| Initial [ ] | 0M | 0M | .2M | ||
| Change | -x | -x | +2x | ||
| Eqlbrm | -x | -x | .2M - 2x | ||
| Answer |
- Given the x value found, [Br2] and [Cl2] = .0432 and [BrCl] = .114M
Kp, An Equilibrium Constant for Gas-Phase Reaction
For the reaction H2 + I2 ⇄ 2HI, the Kp expression is
For the reaction 2S + 3O2 ⇄ 2SO3 where the partial pressure are S = .0035atm, O2 = .0021atm, and SO3 = .005atm, what’s the Kp?
Relationship Between Kp and Kc
Kp = Kc(RT)^(Δng)
- R = .0821
- Δng = change in moles of gas (products - reactants)
For the reaction 2NO (g) + O2 (g) ⇄ 2 NO2 (g) where the Kc is 5.6x10^12 at 290K, what’s the Kp?
Units of Equilibrium Constants
- There are no units because they are seen as dimensionless quantities
Special Equilibrium Constants
Solubility Product
- Solubility product can be used to find how much of an salt dissolves in water
- For the reaction Fe(OH)3 (s) ⇄ Fe (aq) + 3OH (aq), Ksp = [Fe][OH]
- Fe(OH)3 does not appear because it is solid.
- The column for Fe(OH)3 will not be used because it has no concentration
- If the Ksp is 1.6x10^-39, the concentrations can be found
| + |
|---|
- Using this X value, [Fe] = 8.8x10^-11 and [OH] = 2.6x10^-10 M
Weak-Acid and Weak-Base Equilibria
- Weak acids and weak bases ionize only slightly in water
- For the reaction CH3COOH (aq) + H2O ⇄ CH3COO (aq) + H3O (aq)
- For the reaction NH3 (aq) + H2O ⇄ NH4 (aq) + OH \n
- For weak acids, the equilibrium constant is called the acid ionization constant and given the symbol Ka
- Weak bases have the base ionization constant, Kb
Formation Constants
- Metal ions can react with anions and molecules to form complexes
- For the reaction Cu (aq) + 4NH3 (aq) ⇄ Cu(NH3)4 (aq)
- For the reaction Cu (aq) + 4NH3 (aq) ⇄ Cu(NH3)4 (aq)
- Kf, the formation constant, is used to represent the complex
- When the reaction is reversed, it is the dissociation constant, Kd and are inversely proportional to each other
Le Chatelier’s Principle
- If a reaction is in equilibrium and it is disturbed, a stressor is added, the reaction will react again to return to equilibrium.
Effects of Concentration
- If the reactant is increased, more product will become favored/produced to restore equilibrium
- If reactant decreases, the opposite occurs: more reactant is produced/favored
- If products are increased, more reactants are favored/produced
- If products are decreased, the opposite occurs
Effects on Pressure
- If more pressure is added to a reaction, the reaction will shift to produce more of the side with less moles of gas
- If the reactants have 2 mols of gas and products have 3, the reaction will produce more reactants
- If pressure is decreased, the reaction will shift to the side with more mols of gas
- If the reactants have 2 mol and the products have 3, the reaction will create more products
- If there are equal amounts of gas on each side of the reaction, no shifts will occur
Effects of Temperature
- Changing temperature is the only stressor that can change the value of the equilibrium constant
- If a reaction is exothermic, where reactants ⇄ products + heat
- If the temperature is increased more reactants will be produced, and the K will decrease
- If the temperature is decreased, more products will be formed and K will increase
- If a reaction is endothermic, where reactants + heat ⇄ products
- If the temperature is increased, more products will be made and K will increase
- If the temperature is decreased, more reactants will form and K will decrease