Somatosensation II: Pain
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
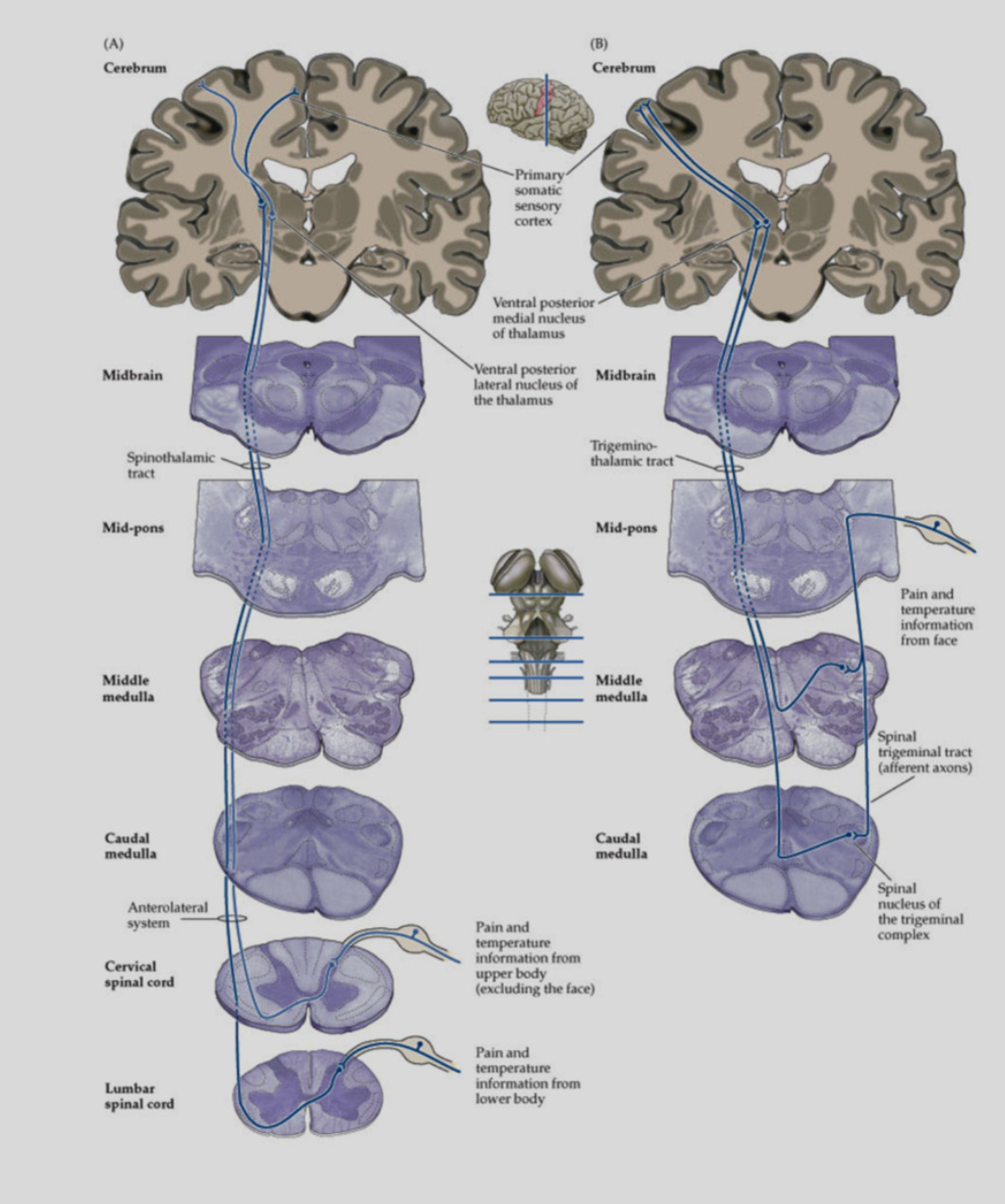
What are the two major central somatosensory pathways?
1. Dorsal Column–Medial Lemniscal (DCML) pathway – conveys fine touch, vibration, proprioception.
2. Spinothalamic Tract (STT; anterolateral system) – conveys pain, temperature, crude touch.
Second-order neurons from both systems decussate and project to the thalamus:
• VPL: sensory input from the body
• VPM: sensory input from the face
Decussation means crossing the midline. DCML crosses in the medulla, whereas STT crosses immediately in the spinal cord, explaining different patterns of sensory loss after lesions.
How do DCML and STT differ in lesion presentation?
Because the pathways cross at different levels, a unilateral spinal lesion often produces:
• Loss of fine touch/proprioception (DCML) on the same side as the lesion (crosses later).
• Loss of pain/temperature (STT) on the opposite side starting 1–2 segments below (crosses early).
What is pain?
According to the dual-aspect model Pain includes two components:
1. Sensory-discriminative: location, intensity, duration, and quality of the stimulus.
2. Affective–motivational: unpleasantness, emotional/behavioural impact, changes in arousal and mood.
These two components can be dissociated clinically—for example, lesions in the postcentral gyrus can remove sensory pain perception but leave emotional unpleasantness intact.
Nociceptors
Specialized sensory neurons that detect noxious mechanical, thermal, or chemical stimuli.
They mainly include A-delta fibres and C-fibres.
Differences between A-delta and C-fibres
A -delta fibres: thinly myelinated, fast conduction, responsible for sharp, well-localised “first pain.”
C-fibres: unmyelinated, slow conduction, responsible for dull, aching, diffuse “second pain.”
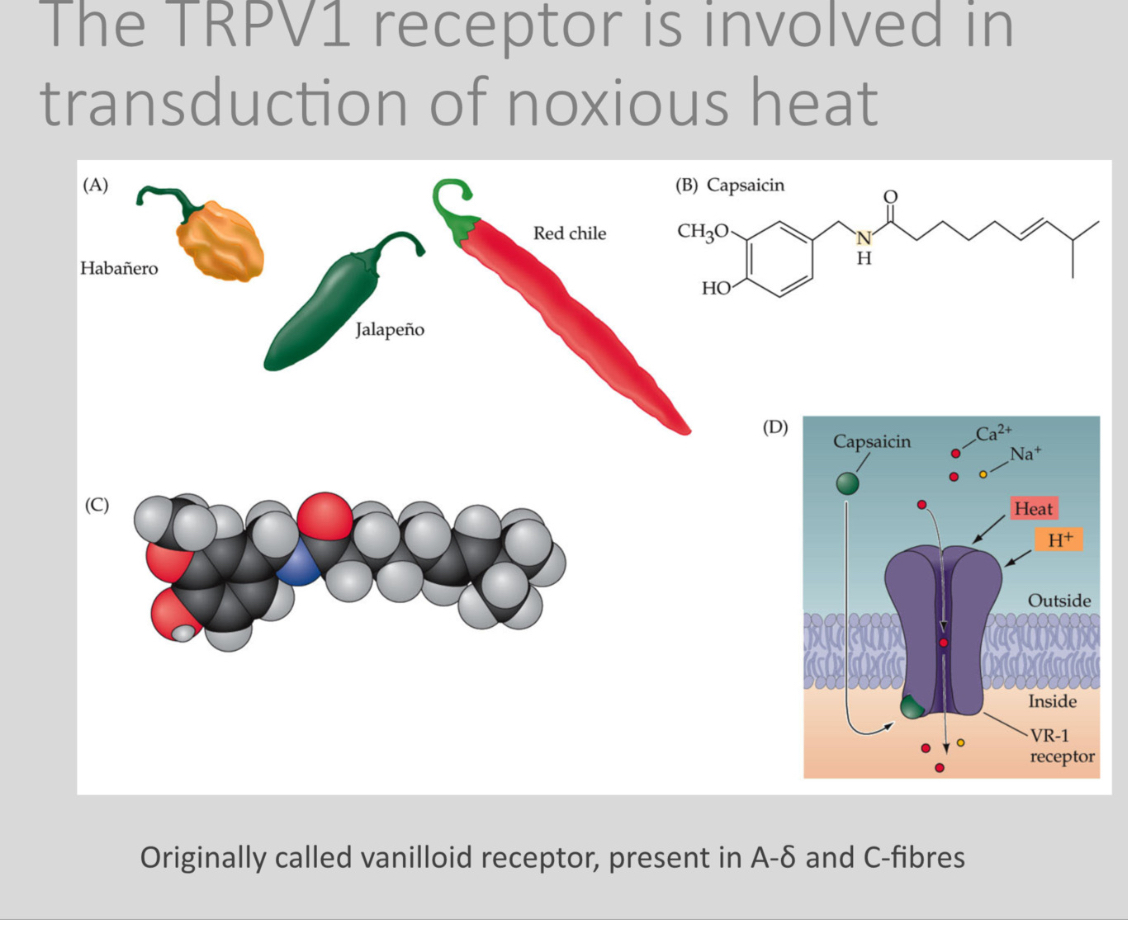
What is TRPV1 and how does it transduce heat pain?
TRPV1 is a heat-sensitive ion channel (originally called the vanilloid receptor) expressed in A-delta and C-fibres.
Capsaicin is found in chilli peppers
It opens in response to noxious heat, capsaicin, and low pH, allowing cations to enter and depolarize the nociceptor.
TRPV1 is why chilli feels “hot.” Capsaicin chronically activates and then desensitizes the channel, which is why capsaicin creams reduce chronic pain.
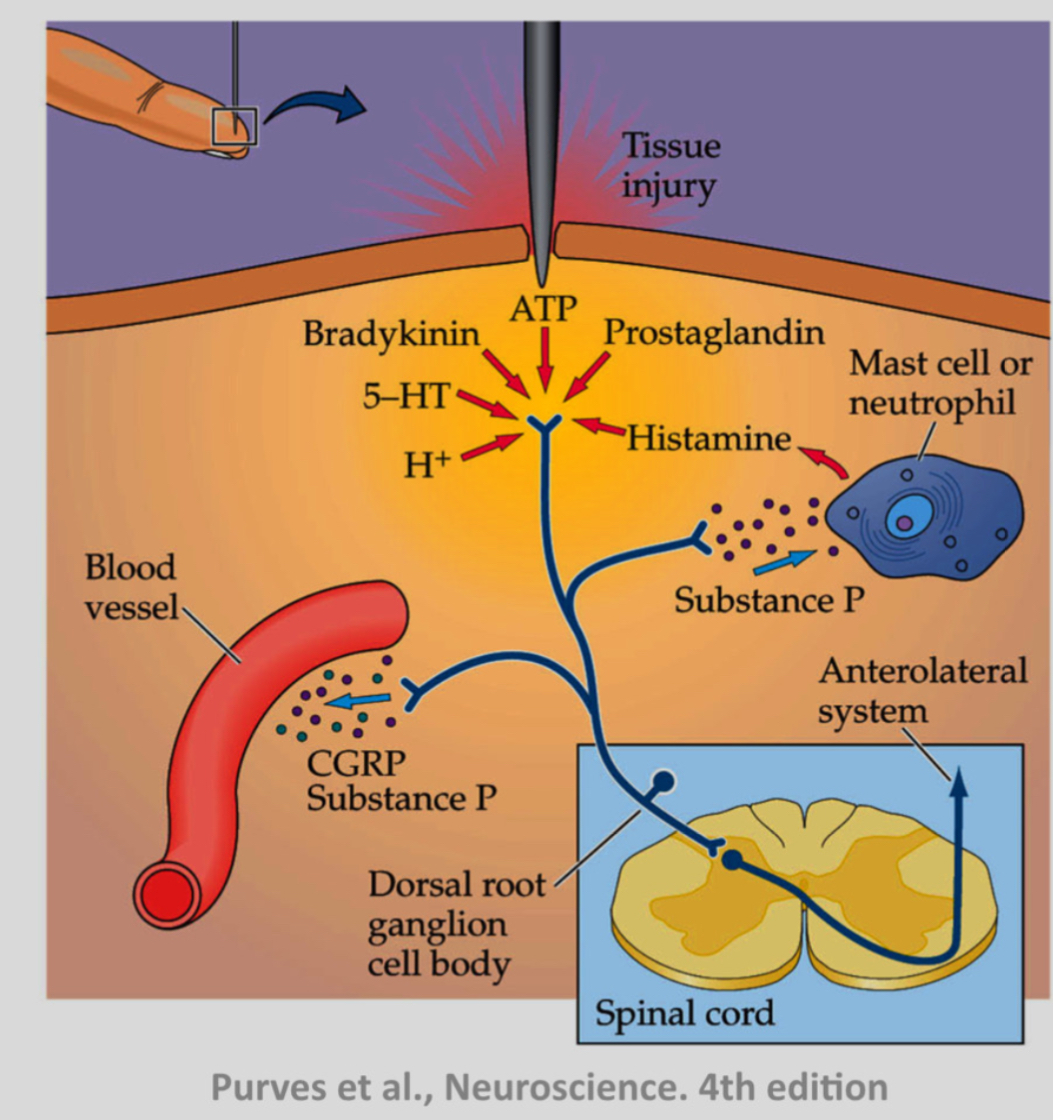
What maintains nociceptor activity after injury?
The inflammatory soup: cytokines, prostaglandins, bradykinin, ATP, and other mediators maintain depolarisation and sensitization of C-fibre terminals.
This leads to:
• Hyperalgesia: increased pain to normally painful stimuli
• Allodynia: pain from normally non-painful stimuli
Inflammation reduces the threshold of nociceptors, so they fire more easily. This is why injured skin becomes more sensitive.
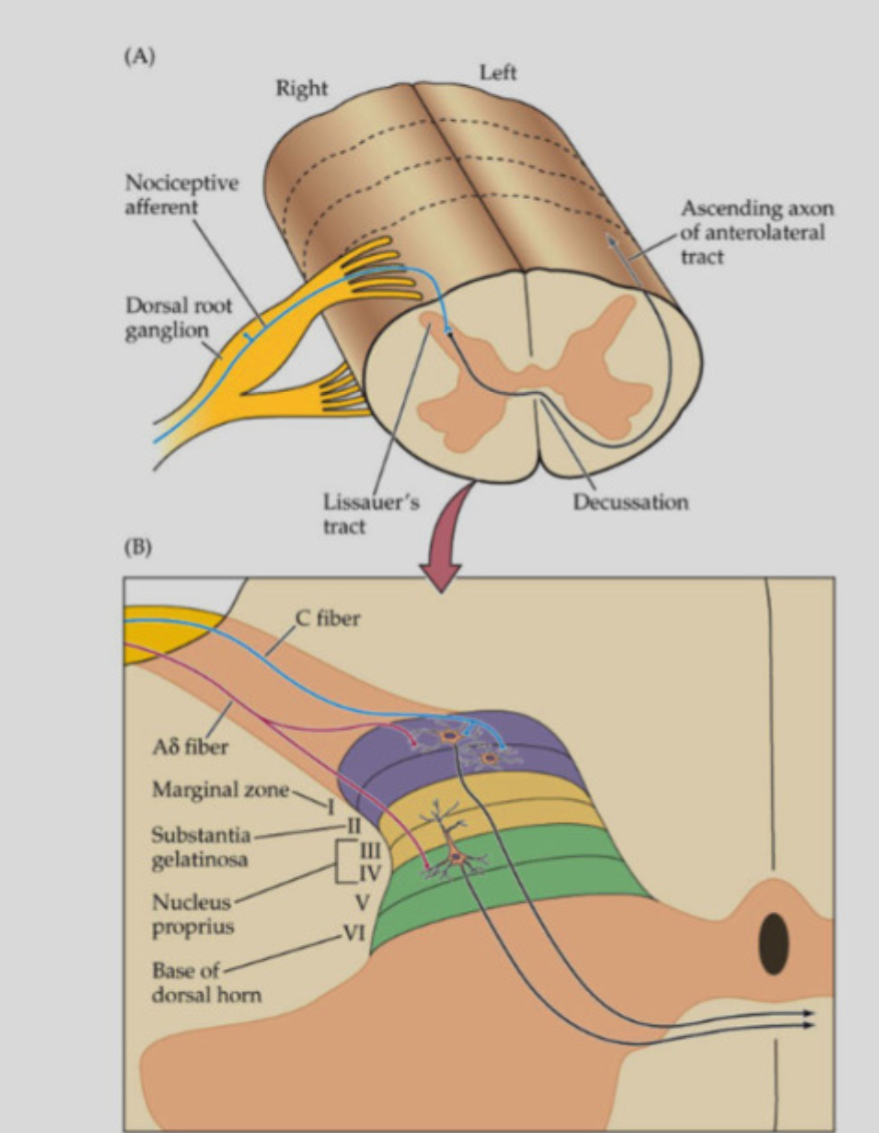
Features of the spinothalamic tract (STT)
• Originates from dorsal horn interneurons receiving A-delta and C-fibre input.
• Axons cross in the spinal cord and ascend in the anterolateral white matter.
• Some STT neurons are multimodal, receiving both nociceptive and non-nociceptive input.
• Some receive visceral afferents, contributing to referred pain.
What is referred pain?
Pain felt at a site distant from the actual source because visceral and somatic afferents converge onto the same dorsal horn neurons.
Example: myocardial ischemia causing left arm pain.
The brain interprets activity in these shared neurons as coming from the skin because somatic pain is more common.
Where does the STT project in the cortex?
The spinothalamic tract (STT) ultimately projects to the primary somatosensory cortex (S1).
Pain/temperature information ascends in the STT
It relays in the thalamus:
VPL for body
VPM for face
From the thalamus, third-order neurons project to S1 (postcentral gyrus)
Why S1 is involved
S1 provides:
Localization of pain (where it is)
Intensity discrimination (how strong)
This is why damage to S1 impairs pain localisation
Although STT reaches S1:
Stimulating S1 produces touch-like sensations, not pain
This means:
S1 is not sufficient to generate the painful feeling itself
Parallel processing:
STT and DCML:
Both project via the thalamus to S1
Remain parallel pathways
Do not converge on the same thalamic neurons
Other cortical targets of STT (pain experience)
Pain is distributed across multiple areas:
Insula → unpleasantness
Anterior cingulate cortex → emotional/motivational aspect
Prefrontal cortex → cognitive evaluation
These areas create the full pain experience
What are the two central systems for pain?
Pain is not processed by one single pathway
The brain splits pain into two parallel systems:
One answers “where is it and how strong?”
The other answers “how bad does this feel and how much do I care?”
These are called the lateral pain system and the medial pain system.
Lateral pain system (sensory–discriminative)
What it answers
Where is the pain?
How intense is it?
What type of stimulus caused it?
Pathway
Peripheral nociceptors → spinothalamic tract
Thalamus: VPL / VPM (specific sensory nuclei)
Cortex: Primary and secondary somatosensory cortex (S1, S2)
What you get
Precise localisation (e.g. “my left index finger”)
Sharpness and intensity
Spatial detail
Key point
This system makes pain informative, not emotional
Medial pain system (affective–motivational)
What it answers
How unpleasant is this?
How distressed am I?
Does this pain motivate avoidance, fear, or behaviour change?
Pathway
Peripheral nociceptors → spinothalamic tract (same entry)
Thalamus: midline / intralaminar nuclei (non-specific)
Cortex and limbic regions:
Anterior cingulate cortex (ACC)
Insular cortex
Amygdala, hypothalamus, brainstem nuclei
What you get
Emotional suffering
Urge to escape or protect
Autonomic responses (heart rate, sweating)
Key point
This system makes pain feel bad
Why both systems exist
Example
You touch a hot pan:
Lateral system: “Heat on my right hand, very strong”
Medial system: “This is awful, get away NOW”
You need both to survive.
Why stimulating S1 doesn’t cause pain
S1 belongs to the lateral system
It localises pain but does not generate suffering
Painfulness comes mainly from medial system areas (ACC, insula)
Why STT projects to many places
STT feeds both systems
Same spinal pathway → different thalamic nuclei → different cortical targets
How do we know the two components can dissociate?
A clinical case (Ploner et al., 1999) showed pain affect without pain sensation following a postcentral lesion.
Patients may recognise a stimulus as unpleasant but cannot localise or describe the pain.
This demonstrates that the emotional experience of pain and the sensory experience are processed by different circuits.
What modulates pain descendingly?
Brainstem systems release endogenous opioids (endorphins, enkephalins) that inhibit pain transmission in the dorsal horn.
Opiates mimic these effects.
Pain: Acute and Chronic
Acute pain:
• Triggered by nociceptor activation due to tissue damage
• Resolves with healing
• Mechanisms well understood
• Treatments largely effective (NSAIDs, opioids)
Chronic pain:
• Persists > 3 months or after healing
• May arise from nerve damage (neuropathic) or CNS dysfunction
• Often treatment-resistant
• Involves sensitisation, neuroplasticity, and cortical reorganization
Pain: Alternative distinction
• Nociceptor-driven pain: arises from ongoing peripheral tissue damage.
• Centrally maintained pain: occurs without peripheral injury; includes phantom limb pain and central pain syndromes (e.g., post-stroke).
Why is chronic pain difficult to treat?
Because mechanisms shift from peripheral nociceptor activity to central sensitisation, cortical changes, and altered thalamic processing.
Drug treatments target peripheral processes, so central ones persist.
Treatments for pain
• Acute pain: NSAIDs, opioids.
• Chronic pain: low-dose antidepressants (amitriptyline, duloxetine).
• Experimental: neurosurgery, deep brain stimulation.
What is anterior cingulotomy?
A surgical lesion that disconnects the anterior cingulate cortex, used for severe intractable pain.
It reduces the affective unpleasantness of pain while leaving sensory perception intact.
This procedure demonstrates the importance of the medial pain system in regulating the emotional component of pain.