PATH 310 Final
0.0(0)
Card Sorting
1/168
Earn XP
Description and Tags
Last updated 11:43 PM on 12/1/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
169 Terms
1
New cards
Symptoms of decreased hemoglobin (RBC's)
patients will present with fatigue as there is less oxygen delivery to the tissues and heart
2
New cards
symptoms of decreased platelets
patients will present with increased bleeding (unexplained or easy bruising and nosebleeds), since they play an important role in blood hemostasis
3
New cards
symptoms of decreased WBC's
patients will be more prone to infections due to a decrease in immune cells such as granulocytes, monocytes, and lymphocytes
4
New cards
symptoms of increased blasts
- patients may present with leukostasis, with high white blood cell counts causing increased blood viscosity and poor circulation
- Blasts may also infiltrate tissues leading to tissue damage and potential organ failure
- Blasts may also infiltrate tissues leading to tissue damage and potential organ failure
5
New cards
requirement of acute leukemia diagnosis
1) Clinical presentation
2) Morphology
3) Immunophenotyping
4) Cytogenetics
5) Molecular studies
2) Morphology
3) Immunophenotyping
4) Cytogenetics
5) Molecular studies
6
New cards
morphology in acute leukemia
1) blasts-tend to have a high nuclear cytoplasmic ratio (large nucleus with little cytoplasm)
- Chromatin of nucleus is open and light purple in colour
-Sometimes visible nucleoli
2) auer rods- needle-like cytoplasmic inclusions that are ONLY SEEN IN ACUTE MYELOID LEUKEMIA CELLS
○ If you don’t see these you cannot be sure if the patient has AML or ALL
- Chromatin of nucleus is open and light purple in colour
-Sometimes visible nucleoli
2) auer rods- needle-like cytoplasmic inclusions that are ONLY SEEN IN ACUTE MYELOID LEUKEMIA CELLS
○ If you don’t see these you cannot be sure if the patient has AML or ALL
7
New cards
aspiration of bone marrow
uses a syringe to remove liquid from the bone marrow cavity
○ Fluid is then used to make slide to view under a microscope and utilized in ancillary tests such as cytometry, cytogenetic-FISH analysis etc.
○ Fluid is then used to make slide to view under a microscope and utilized in ancillary tests such as cytometry, cytogenetic-FISH analysis etc.
8
New cards
bone marrow biopsy
uses a large-bore needle to remove a core of bone marrow, which is then examined under a microscope in thin sections to reveal the bone marrow architecture
○ Sample normally taken from the posterior iliac crest
~Ideal since as you age, the areas with active bone marrow decrease and are replaced by fat, but most of the axial skeleton bone marrow remains ACTIVE
○ Tissue examined can bed used for morphological and immunohistochemistry analysis
○ Sample normally taken from the posterior iliac crest
~Ideal since as you age, the areas with active bone marrow decrease and are replaced by fat, but most of the axial skeleton bone marrow remains ACTIVE
○ Tissue examined can bed used for morphological and immunohistochemistry analysis
9
New cards
Analyzing Bone Marrow Aspirate Histology for AML Diagnosis (normal vs cancer)
-Normal Smear: can see development of all hematopoietic cell lines, including WBC's, RBC's and platelets
-Acute Leukemia: shows increased number of blasts (need to represent at least 20% of the marrow cellularity)
○ Proliferation of blasts suppress the production of normal marrow maturation, leading to reduction in normal WBC''s, RBC's and platelets in peripheral blood
-Acute Leukemia: shows increased number of blasts (need to represent at least 20% of the marrow cellularity)
○ Proliferation of blasts suppress the production of normal marrow maturation, leading to reduction in normal WBC''s, RBC's and platelets in peripheral blood
10
New cards
Immunophenotyping in Acute Leukemia-Flow Cytometry
- Technique needed to diagnose and properly classify acute leukemia, due to morphological similarities between cases
- Uses laser-technology to cells incubated with fluorescently-tagged antibodies
- Cells are individually passed under a laser and will fluoresce if the cell has the antigen of interest
○ Useful to identify the proteins expressed on leukemia cells to determine their lineage and potentially guide treatment
- Alternative method: perform immunohistochemistry on bone marrow biopsy tissue sections
○ Antibodies attached to a colorimetric endpoint detection system
○Positive result = brown
- Uses laser-technology to cells incubated with fluorescently-tagged antibodies
- Cells are individually passed under a laser and will fluoresce if the cell has the antigen of interest
○ Useful to identify the proteins expressed on leukemia cells to determine their lineage and potentially guide treatment
- Alternative method: perform immunohistochemistry on bone marrow biopsy tissue sections
○ Antibodies attached to a colorimetric endpoint detection system
○Positive result = brown
11
New cards
Two-Hit of Acute Leukemia Formation
- Hit #1: requires a mutation that will block differentiation to prevent the immature progenitor cell from differentiating further
○ Often involves loss of function mutations occurring in transcription factors needed for differentiation
○Stems from chromosomal translocation events
- Hit #2: an advantage to enhance proliferation occurs
○ Gain of function mutation in enzymes involved in signalling pathways
○Pathways transmit signals to the nucleus and tell the cell to divide
○ Often involves loss of function mutations occurring in transcription factors needed for differentiation
○Stems from chromosomal translocation events
- Hit #2: an advantage to enhance proliferation occurs
○ Gain of function mutation in enzymes involved in signalling pathways
○Pathways transmit signals to the nucleus and tell the cell to divide
12
New cards
three recurring mutation associated with AML
1) T(8:21)(q22;q22)-
~T(8:21) indicates that a portion of chromosome 8 is translocated onto chromosome 21
□ Referred to as core-binding factor (CBF) rearrangement
~(q22;q22) indicates the neighbourhood involved in the translocation
□ Short arms = "p" arms
□ Long arms = "q" arms
2) Inv(16)- chromosomal material on chromosome 16 is INVERTED (material all stays within the same chromosome but flips around)
□Hard to find
3) T(15;17)- a portion of chromosome 17 is translocated onto chromosome 15, forming the der chromosome 15
~T(8:21) indicates that a portion of chromosome 8 is translocated onto chromosome 21
□ Referred to as core-binding factor (CBF) rearrangement
~(q22;q22) indicates the neighbourhood involved in the translocation
□ Short arms = "p" arms
□ Long arms = "q" arms
2) Inv(16)- chromosomal material on chromosome 16 is INVERTED (material all stays within the same chromosome but flips around)
□Hard to find
3) T(15;17)- a portion of chromosome 17 is translocated onto chromosome 15, forming the der chromosome 15
13
New cards
how do translocations cause AML
- Translocation impair cellular differentiation as they impact cellular machinery that controls maturation
- Normal Progenitor Cell: when stem cells are called upon to produce more mature cells, the core binding factors lead to transcription of target genes and the activation of maturation programs
- T(8;21): CBFa (RUNX1) is fused with another protein ETO (RUNX1T1) which impairs the function of CBFa and CBFb
○ This cellular machinery can no longer activate the target gene transcription and the cells remain locked in the immature phase
- Inv(16): CBFb is fused with another protein SMMHC (MYH11), which also impairs this cellular machinery's function resulting in maturation arrest
- Normal Progenitor Cell: when stem cells are called upon to produce more mature cells, the core binding factors lead to transcription of target genes and the activation of maturation programs
- T(8;21): CBFa (RUNX1) is fused with another protein ETO (RUNX1T1) which impairs the function of CBFa and CBFb
○ This cellular machinery can no longer activate the target gene transcription and the cells remain locked in the immature phase
- Inv(16): CBFb is fused with another protein SMMHC (MYH11), which also impairs this cellular machinery's function resulting in maturation arrest
14
New cards
Next Gen Seq for AML Mutation Detection
- Now routinely used in all new leukemia cases
- Allows for millions of individual dna strands to be sequenced in parallel, while incorporating fluorescent DNA bases (G, C, T and A)
○ These bases terminate the sequencing reaction and fluoresce, which can be captured by a camera after each PCR cycle
- Bioinformatics programs align the short sequences to a reference human genome and any mismatch between the two sequences, above the background error rate inherent with NGS, shows the presence of a mutation in the genome
- PCR also used as it is more cost effective and can obtain the results faster
- Allows for millions of individual dna strands to be sequenced in parallel, while incorporating fluorescent DNA bases (G, C, T and A)
○ These bases terminate the sequencing reaction and fluoresce, which can be captured by a camera after each PCR cycle
- Bioinformatics programs align the short sequences to a reference human genome and any mismatch between the two sequences, above the background error rate inherent with NGS, shows the presence of a mutation in the genome
- PCR also used as it is more cost effective and can obtain the results faster
15
New cards
Analyzing Bone Marrow Biopsy Histology for AML Diagnosis (normal vs leukemia)
- Bone marrow biopsies can be procured at the same time as bone marrow aspirates
- These tests are often done after a full diagnostic panel for confirmation rather than for initial diagnosis
- Normal: has fat within the marrow; as you age the amount of fat within the marrow is inversely proportional to your age and increases as age increases
○ Eg. Someone who is 20 years old would have ~20% fat and ~80% cells
- Leukemia: cells take over bone marrow completely, so almost no fat is seen
- These tests are often done after a full diagnostic panel for confirmation rather than for initial diagnosis
- Normal: has fat within the marrow; as you age the amount of fat within the marrow is inversely proportional to your age and increases as age increases
○ Eg. Someone who is 20 years old would have ~20% fat and ~80% cells
- Leukemia: cells take over bone marrow completely, so almost no fat is seen
16
New cards
types of AML Treatment
- Type of treatment chosen is influenced by type of leukemia (AML or ALL), age of patient and intent of the treatment (curative or palliative)
1) Combination Chemotherapy: drugs target DNA synthesis and prevent proliferation of the leukemic cells; intent is to kill the mutated stem cell and allow normal stem cells to survive and repopulate in the marrow
○ Induction- given to achieve complete remission
○ Consolidation- given to prevent relapse
2) Supportive Care: involves transfusions, antibiotics, nutrition and psychosocial support
1) Combination Chemotherapy: drugs target DNA synthesis and prevent proliferation of the leukemic cells; intent is to kill the mutated stem cell and allow normal stem cells to survive and repopulate in the marrow
○ Induction- given to achieve complete remission
○ Consolidation- given to prevent relapse
2) Supportive Care: involves transfusions, antibiotics, nutrition and psychosocial support
17
New cards
the standard 3 + 7 AML treatment (daunorubicin and cytarabine)
- Used to treat t(8;21) and fusion of RUNX1-RUNXX1T1, since there currently are no specific targeted agents available for this fusion
○ Kills all rapidly dividing cancer cells and is not particularly targeted, leading to potential toxic side effects
1) Daunorubicin: first administered via IV for 3 days
○ Interacts with DNA by intercalation and inhibition of macromolecular biosynthesis
○ Also inhibits progression of the enzyme topoisomerase II, which relaxes supercoils in DNA for transcription
2) Cytarabine: administered via IV for 7 days, following the 3 day treatment
○ Similar enough to human deoxycytidine to be incorporated into human DNA, but different enough to kills cells
○ Kills all rapidly dividing cancer cells and is not particularly targeted, leading to potential toxic side effects
1) Daunorubicin: first administered via IV for 3 days
○ Interacts with DNA by intercalation and inhibition of macromolecular biosynthesis
○ Also inhibits progression of the enzyme topoisomerase II, which relaxes supercoils in DNA for transcription
2) Cytarabine: administered via IV for 7 days, following the 3 day treatment
○ Similar enough to human deoxycytidine to be incorporated into human DNA, but different enough to kills cells
18
New cards
All-trans retinoic acid (ATRA)
- The t(15;17) mutation involves the RARA gene (retinoic acid receptor alpha)
○ Retinoic acid = vitamin A
- If leukemia cells are provided with ATRA (a form of vitamin A), it will induce t(15;17) blast differentiation
- ATRA is a non-toxic targeted therapy
○ It specifically targets blast cells, causing them to mature into normally-functioning granulocytes
- Arsenic trioxide is also added to the regimen to enhance the differentiation and killing of leukemic cells
○ Retinoic acid = vitamin A
- If leukemia cells are provided with ATRA (a form of vitamin A), it will induce t(15;17) blast differentiation
- ATRA is a non-toxic targeted therapy
○ It specifically targets blast cells, causing them to mature into normally-functioning granulocytes
- Arsenic trioxide is also added to the regimen to enhance the differentiation and killing of leukemic cells
19
New cards
main goal of bone marrow transplantation
-to achieve graft-vs-leukemia effect
○ Ability of donor immune cells to eliminate host leukemic cells after prior chemo
○ Ability of donor immune cells to eliminate host leukemic cells after prior chemo
20
New cards
factors that must be considered when conducting a bone marrow transplant
○ Age- bone marrow transplants are a viable option for younger patients (
21
New cards
mutant IDH inhibitors
- IDH1 and 2 are enzymes that catalyze the oxidative decarboxylation of isocitrate to alpha-ketoglutarate in the citric acid cycle
- Has been found that people with a mutated IDH1 or IDH2 produce an "onco-metabolite" called 2HG, which accumulates in the body
○ This blocks HSC differentiation contributing to the development of AML
- Has been found that people with a mutated IDH1 or IDH2 produce an "onco-metabolite" called 2HG, which accumulates in the body
○ This blocks HSC differentiation contributing to the development of AML
22
New cards
papillomaviruses
- Small, non-enveloped, double stranded DNA (dsDNA) viruses that are species-specific and widespread amongst mammals
23
New cards
how is HPV transmitted
- Sexual contact or close personal contact (skin to skin) with an infected person
-It is an acquired infection
-It is an acquired infection
24
New cards
what is the significance/what does it mean if a patient has HPV antibodies
- If a patient has antibodies for HPV, it indicates that this person has been exposed to HPV at some point
○ Does not tell you if the infection never happened, occurred in the past, or is currently present
- Antibodies give you a good idea of the serological prevalence of STI's and can be used to rule out people with no viral exposure
- A negative PCR test indicates that there is no detectable viral DNA in that particular tissue site
○ Does not tell you if the infection never happened, occurred in the past, or is currently present
- Antibodies give you a good idea of the serological prevalence of STI's and can be used to rule out people with no viral exposure
- A negative PCR test indicates that there is no detectable viral DNA in that particular tissue site
25
New cards
Serologic Prevalence of HPV in the US
- Around 75% of sexually active individuals will contract an HPV infection at some point
○ Majority will occur without symptoms and resolve without any form of intervention
- 1% of population have an active HPV infection at any given time
- 60% of US population has had a previous infection of HPV
○ Majority will occur without symptoms and resolve without any form of intervention
- 1% of population have an active HPV infection at any given time
- 60% of US population has had a previous infection of HPV
26
New cards
risk factors for cervical cancer
-oral contraceptive
-multiparity
-cigarette smoking
-sexually active
-over 25 years old
-lack of hpv screening
-lack of condom use
-multiparity
-cigarette smoking
-sexually active
-over 25 years old
-lack of hpv screening
-lack of condom use
27
New cards
risk factors for HPV
-age
-sex
-lack of condom use
-age of first intercourse / # of partners
-circumcision
-over 25
-lack of HPV screening
- HPV is unique amongst STI's because it does not need to have transfer of mucosal secretions
- Infects keratinocytes and epithelial cells, so barrier methods do not always protect against transmission
- Second most important risk factor for contracting HPV is age
○ Partly because of the maturation of the cervical mucosa that happens in women as they enter puberty and exit into their twenties
- In women who are 15-25, the cervical mucosa layer extends lower into the genital tract compared to older women
-sex
-lack of condom use
-age of first intercourse / # of partners
-circumcision
-over 25
-lack of HPV screening
- HPV is unique amongst STI's because it does not need to have transfer of mucosal secretions
- Infects keratinocytes and epithelial cells, so barrier methods do not always protect against transmission
- Second most important risk factor for contracting HPV is age
○ Partly because of the maturation of the cervical mucosa that happens in women as they enter puberty and exit into their twenties
- In women who are 15-25, the cervical mucosa layer extends lower into the genital tract compared to older women
28
New cards
the natural cycle of HPV infection
29
New cards
where are hpv infections isolated in the female reproductive system
the cervix
30
New cards
what is the cervix and what does it connect
-Narrow part of the uterus, connecting the uterus to the vagina
31
New cards
three sections of the cervix
1) External os- the opening that leads into the vagina and is composed of squamous epithelium
2) Endocervical canal- the passageway from the vagina to the uterine cavity
~Separates the external and internal os
~Lined with glandular epithelium
3) Internal os- the opening into the uterus
32
New cards
the transformation zone of the cervix
-site of initial HPV infection
- The area of the cervix where the squamous epithelium of the external os meets the glandular epithelium of endocervical canal
- Area where HPV INFECTS SUSCEPTIBLE CELLS
- The area of the cervix where the squamous epithelium of the external os meets the glandular epithelium of endocervical canal
- Area where HPV INFECTS SUSCEPTIBLE CELLS
33
New cards
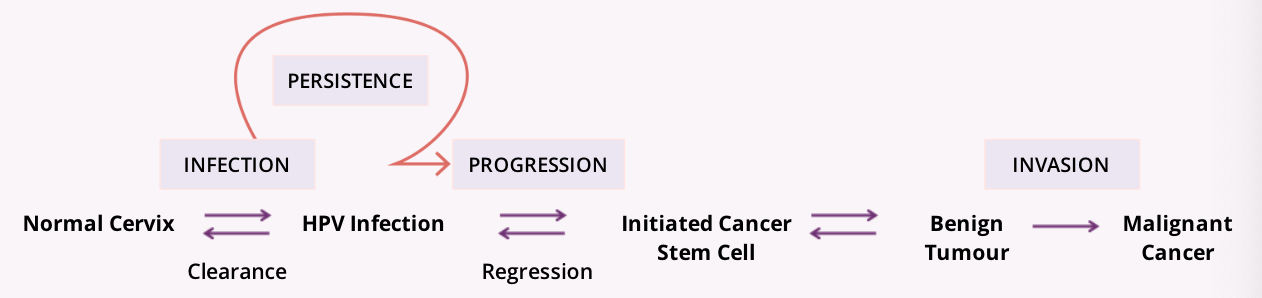
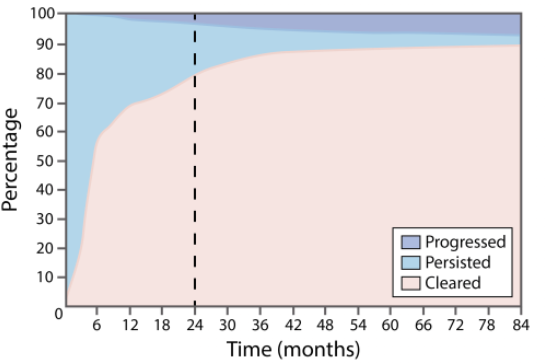
three possible outcomes after HPV infection
1) Clearance- occurs within 2 years of infection
○The immune system is able to recognize the virus and initiates an immune response, resulting in the development of HPV type-specific immunity
2) Persistence- in ~10% of cases, the infection does not clear on its own
○HPV tethers itself to the host DNA and continuously replicates at low levels, building up a reserve of the virus
○Immune system is never engaged and the virus persists
3) Progression- only a fraction of persistently infected cells will become initiated cervical cancer stem cells
○Initiated stem cells have undergone genetic transformation as a result of HPV infection, and are able, if unchecked, to generate a benign cervical tumour
□ As time goes on, there is an increasing proportion of people that are progressing to more significant clinical manifestations
○The immune system is able to recognize the virus and initiates an immune response, resulting in the development of HPV type-specific immunity
2) Persistence- in ~10% of cases, the infection does not clear on its own
○HPV tethers itself to the host DNA and continuously replicates at low levels, building up a reserve of the virus
○Immune system is never engaged and the virus persists
3) Progression- only a fraction of persistently infected cells will become initiated cervical cancer stem cells
○Initiated stem cells have undergone genetic transformation as a result of HPV infection, and are able, if unchecked, to generate a benign cervical tumour
□ As time goes on, there is an increasing proportion of people that are progressing to more significant clinical manifestations
34
New cards
mechanism of HPV infection
1) microabrasions
2) basal keratinocytes
3) either viral assembly or chromosomal integration
2) basal keratinocytes
3) either viral assembly or chromosomal integration
35
New cards
microabrasions
- Sexual intercourse causes microtears within the genital tract as a result of friction
-HPV uses these microtears within the mucosal lining as a portal of entry
-HPV uses these microtears within the mucosal lining as a portal of entry
36
New cards
basal keratinocytes
- HPV travels down and infects the basal keratinocytes (the layer as far away from the lumen as possible)
- From here, there are two equally probable ways the virus can continue the infection process (viral assembly or chromosomal integration)
- From here, there are two equally probable ways the virus can continue the infection process (viral assembly or chromosomal integration)
37
New cards
viral assembly
- As the infected keratinocytes differentiate, they move up towards the mucosa and bring the virus along with them
○ During this movement, the virus enters different stages of gene expression
- When in the suprabasal layer, the virus is in early gene expression
○ It is producing proteins that encode for viral DNA
- In the terminal keratinocyte layers, the virus is in late gene expression
○ It producing proteins that encode for structural proteins of the virus
- By the time the cells reach the lumen, the virus is assembled and infectious, and is released into surrounding tissues, spreading infection
- Does not lead to HPV-mediated cancer
○ During this movement, the virus enters different stages of gene expression
- When in the suprabasal layer, the virus is in early gene expression
○ It is producing proteins that encode for viral DNA
- In the terminal keratinocyte layers, the virus is in late gene expression
○ It producing proteins that encode for structural proteins of the virus
- By the time the cells reach the lumen, the virus is assembled and infectious, and is released into surrounding tissues, spreading infection
- Does not lead to HPV-mediated cancer
38
New cards
chromosomal integration
- Instead of assembling virus particles, the virus integrates its genome into the host DNA
- Integration of the HPV genome into the chromosome can result in uncontrollable oncoprotein production and host cell transformation
- Often leads to HPV-mediated cancer
- Integration of the HPV genome into the chromosome can result in uncontrollable oncoprotein production and host cell transformation
- Often leads to HPV-mediated cancer
39
New cards
mechanism of persistence
- If the virus is recognized by the immune system, an immune response is initiated and HPV type-specific immunity is developed
○ Results in clearance of the virus
- If the HPV infection leads to viral persistence via chromosomal integration the immune system is not engaged
○ The virus disrupts normal cell cycle control, promoting uncontrolled cell division and the accumulation of genetic damage
□ Damage can lead to cervical intraepithelial dysplasia (CIN)
~A premalignant transformation and abnormal growth of squamous cells on the surface of the cervix
~CIN is not cancer and is usually curable
○ Results in clearance of the virus
- If the HPV infection leads to viral persistence via chromosomal integration the immune system is not engaged
○ The virus disrupts normal cell cycle control, promoting uncontrolled cell division and the accumulation of genetic damage
□ Damage can lead to cervical intraepithelial dysplasia (CIN)
~A premalignant transformation and abnormal growth of squamous cells on the surface of the cervix
~CIN is not cancer and is usually curable
40
New cards
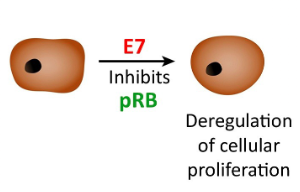
E7 oncoprotein
inhibits the tumour suppressor gene retinoblastoma (pRB) to stop cell cycle arrest
○ The primary transforming protein which facilitates the proliferation of deregulated cells
○ The primary transforming protein which facilitates the proliferation of deregulated cells
41
New cards
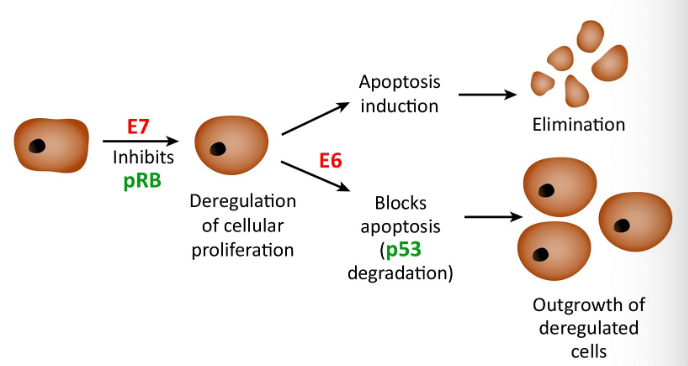
E6 oncoprotein
-interferes with the normal function of p53
○ P53- protein that decides whether a cell is stable enough to undergo cellular proliferation or should be marked for apoptosis
-Once E7 inhibits pRB, a cell would normally undergo cell apoptosis, however, E6 inhibits p53 to block this mechanism, allowing for further cell deregulation
○ P53- protein that decides whether a cell is stable enough to undergo cellular proliferation or should be marked for apoptosis
-Once E7 inhibits pRB, a cell would normally undergo cell apoptosis, however, E6 inhibits p53 to block this mechanism, allowing for further cell deregulation
42
New cards
actions of E6 and E7 allows for and leads to the development of?
the action of E6 and E7 protein allow for the uncontrolled replication and development of cervical lesions, which can lead to cervical cancers
43
New cards
how does the HPV genome integrate into host dna
- The HPV genome is integrated into the host DNA in a high proportion of individuals with high grade carcinoma
○ Integration may be an important biomarker for distinguishing HPV infection from benign tumours, but might not be necessary to cause invasion since not all women with invasive cancers have measurable integration
- DNA methylation, chromosomal instability and telomerase activation play an important role in cancer development
○ Integration may be an important biomarker for distinguishing HPV infection from benign tumours, but might not be necessary to cause invasion since not all women with invasive cancers have measurable integration
- DNA methylation, chromosomal instability and telomerase activation play an important role in cancer development
44
New cards
how does the HPV infection progress into cervical cancer
- HPV infects basal layer cells and replicates in the basal cells
- Virus expresses E6 and E7 gene, which interferes with normal cell function
○ Results in non-scheduled cell replication
~Accumulation of cells that disrupts structure of epithelial tissue
- Once cell is infected the viral capsid sheds and the HPV genome is shuttled into the nucleus
- Virus expresses E6 and E7 gene, which interferes with normal cell function
○ Results in non-scheduled cell replication
~Accumulation of cells that disrupts structure of epithelial tissue
- Once cell is infected the viral capsid sheds and the HPV genome is shuttled into the nucleus
45
New cards
HPV 16
- carcinogenic
- 40% of infections progress to benign tumours after 3-5 years of persistent infection
- 40% of infections progress to benign tumours after 3-5 years of persistent infection
46
New cards
steps of cervical cancer prevention
1) Screening- Pap smear
2) Triage of results- the bethesda screening system is used to triage the results of a pap smear
3) Colposcopy- used to get a closer look at any concerning results
4) Treatment
5) Post-treatment follow up- important to determine treatment effectiveness
~If successful, patient can return to a normal screening schedule
2) Triage of results- the bethesda screening system is used to triage the results of a pap smear
3) Colposcopy- used to get a closer look at any concerning results
4) Treatment
5) Post-treatment follow up- important to determine treatment effectiveness
~If successful, patient can return to a normal screening schedule
47
New cards
cervical cancer screening
- Test is very effective
- HPV infection and progression is linked to the histologic and cytologic appearance of the cervical tissue
○ Used to screen for evidence of high risk infections and premalignant tumours
-Histology: more invasive as it requires biopsy material
- To detect early stages of cervical disease, the less invasive, cytological (study of isolated cells) technique is very effective
- Classification for stages of cervical disease is nearly identical
- HPV infection and progression is linked to the histologic and cytologic appearance of the cervical tissue
○ Used to screen for evidence of high risk infections and premalignant tumours
-Histology: more invasive as it requires biopsy material
- To detect early stages of cervical disease, the less invasive, cytological (study of isolated cells) technique is very effective
- Classification for stages of cervical disease is nearly identical
48
New cards
pap smear procedure
- Quick and relatively cheap
- It is a diagnostic technique used to isolate and analyze the mucosal cells in the cervix
- Involves the use of a cervical brush to scrape surface mucosal cells off the cervix
○ Cells are smeared onto a slide or swirled in an alcohol solution and dispensed onto slides
- The results allow for cervical samples to be integrated into an algorithm to prevent progression to cancer
- It is a diagnostic technique used to isolate and analyze the mucosal cells in the cervix
- Involves the use of a cervical brush to scrape surface mucosal cells off the cervix
○ Cells are smeared onto a slide or swirled in an alcohol solution and dispensed onto slides
- The results allow for cervical samples to be integrated into an algorithm to prevent progression to cancer
49
New cards
bethesda screening system
- Used to triage the cells collected via a pap smear
- This system consists of standardized diagnostic categories corresponding to the different stages of cervical cancer
○ Normal
○ ASCUS
○ LSIL
○ HSIL
○ Carcinoma
- This system consists of standardized diagnostic categories corresponding to the different stages of cervical cancer
○ Normal
○ ASCUS
○ LSIL
○ HSIL
○ Carcinoma
50
New cards
normal squamous mucosa
- There is no evidence of any abnormal cells, lesions or malignancies
- The basal cells where proliferation occurs are maturing into healthy surface epithelium
- The basal cells where proliferation occurs are maturing into healthy surface epithelium
51
New cards
Atypical squamous cells of undetermined significance (ASCUS)
- It is an early stage of progression, where you can see change that are somewhat abnormal but not quite clearly a low grade squamous intraepithelial lesion (LSIL)
- No histological equivalent
- Cytology allows for a category of mild atypia (ASCUS) which is not quite LSIL, but will allow the system to avoid missing anyone
- Nuclei are only mildly enlarged
- Only a suggestion of perinuclear clearing
- No histological equivalent
- Cytology allows for a category of mild atypia (ASCUS) which is not quite LSIL, but will allow the system to avoid missing anyone
- Nuclei are only mildly enlarged
- Only a suggestion of perinuclear clearing
52
New cards
Low grade squamous intraepithelial lesion (LSIL)
- Cell nuclei are dark and enlarged with perinuclear clearing
○ Nuclei can sometimes be binucleated
- Proof that the individual has the beginning of an HPV infection
- Cells are infected but don’t represent pre-malignant tumours
○ Nuclei can sometimes be binucleated
- Proof that the individual has the beginning of an HPV infection
- Cells are infected but don’t represent pre-malignant tumours
53
New cards
High grade squamous intraepithelial lesion (HSIL)
- There is a loss of clearing with busy looking epithelium
○ Each cell within this lesion has more nucleus relative to the cytoplasm
- Indicates that the infection has been present for an extended period of time
- Cells are pre-malignant and will likely progress to a carcinoma
○ Each cell within this lesion has more nucleus relative to the cytoplasm
- Indicates that the infection has been present for an extended period of time
- Cells are pre-malignant and will likely progress to a carcinoma
54
New cards
carcinoma
- Viral DNA has integrated into the host cell genome and normal cellular regulation has been altered
- There is intense dysregulation of cell structure and the cells may not resemble the normal squamous cells at all
- There is intense dysregulation of cell structure and the cells may not resemble the normal squamous cells at all
55
New cards
how do you manage ASCUS or LSIL
-individual will be scheduled to repeat the pap smear in 6 months, as it will likely resolve itself
○ If it is still abnormal, they will be referred for a colposcopy
○ If it is still abnormal, they will be referred for a colposcopy
56
New cards
how would you manage someone with HSIL or carcinoma
-individual will be sent for a colposcopy
57
New cards
how often are healthy individuals screened for cervical cancer
-every three years
58
New cards
changes seen in LSIL to HSIL progression
- As the cell progresses to a HSIL cell, there are higher levels of E6 and E7 oncoproteins
- These proteins interrupt cellular processes, such as apoptosis and cell cycle arrest
- There is increased integration of the viral genome into host DNA
- These proteins interrupt cellular processes, such as apoptosis and cell cycle arrest
- There is increased integration of the viral genome into host DNA
59
New cards
what is a colposcopy and when is it performed
- Performed when a patient receives concerning Pap smear results
- A gynecologist uses a colposcope to magnify the cervix for better visualization of the tissue
○ Differs from a pap smear because magnification provides detail beyond capacity of the human eye
- The doctor can determine what the next step would be in cervical cancer prevention
- A gynecologist uses a colposcope to magnify the cervix for better visualization of the tissue
○ Differs from a pap smear because magnification provides detail beyond capacity of the human eye
- The doctor can determine what the next step would be in cervical cancer prevention
60
New cards
three available treatments after abnormal colposcopic biopsy results
- If there are any abnormal areas identified during the colposcopy, the gynecologist will biopsy the tissue
- The biopsy is sent to pathology, where it will be analysed to confirm the presence of HPV or cervical cancer
- The treatment method depends on biopsy results and age of patient
○ Cryotherapy
○ Loop electrosurgical excision procedure
○ Cold knife cone biopsy
- The biopsy is sent to pathology, where it will be analysed to confirm the presence of HPV or cervical cancer
- The treatment method depends on biopsy results and age of patient
○ Cryotherapy
○ Loop electrosurgical excision procedure
○ Cold knife cone biopsy
61
New cards
explain cryotherapy
- A surgical procedure used to freeze and destroy abnormal cervical tissue
- A speculum is inserted into the vagina and a cryoprobe with nitrogen gas is used to freeze the tissue
- In the following weeks, the dead cervical tissue is shed, similar to a period
- Post-treatment is followed up with a colposcopy and pap smear
○ If results are normal, patient return to routine screening
- A speculum is inserted into the vagina and a cryoprobe with nitrogen gas is used to freeze the tissue
- In the following weeks, the dead cervical tissue is shed, similar to a period
- Post-treatment is followed up with a colposcopy and pap smear
○ If results are normal, patient return to routine screening
62
New cards
loop electrosurgical excision procedure (LEEP)
- A loop of electrosurgical wire is passed through the cervical os to remove a donut-shaped piece of tissue
○ Tissue then sent to pathology
- Post-treatment is followed up with a colposcopy and pap smear
○ If results are normal, patient return to routine screening
○ Tissue then sent to pathology
- Post-treatment is followed up with a colposcopy and pap smear
○ If results are normal, patient return to routine screening
63
New cards
cold knife cone biopsy
- Similar to the LEEP procedure
- Instead of using a electrical loop, a scalpel is used to excise the abnormal tissue
- Post-treatment is followed up with a colposcopy and pap smear
○ If results are normal, patient return to routine screening
- Instead of using a electrical loop, a scalpel is used to excise the abnormal tissue
- Post-treatment is followed up with a colposcopy and pap smear
○ If results are normal, patient return to routine screening
64
New cards
how do HPV vaccines work
- Vaccines are made with recombinant technology in which protein form virus-like particles (VLPs) of the L1 protein
- They are non-infectious and lack live biological products
- Does not provide protection of already exposed; it has to be done before exposure
- They are non-infectious and lack live biological products
- Does not provide protection of already exposed; it has to be done before exposure
65
New cards
two available HPV vaccines
- Gardasil 4- protects against four types of HPV (6, 8, 26 and 18)
○ Quadrivalent vaccine
○ Ontario now uses Gardasil 9, after it was approved in 2015
- Cervarix- approved in 2010 for females between 9 and 45 but only targets 2 types of HPV (16 and 18)
○ Bivalent
○ Ontario chose to use Gardasil 9 over Cervarix
○ Quadrivalent vaccine
○ Ontario now uses Gardasil 9, after it was approved in 2015
- Cervarix- approved in 2010 for females between 9 and 45 but only targets 2 types of HPV (16 and 18)
○ Bivalent
○ Ontario chose to use Gardasil 9 over Cervarix
66
New cards
Why are HPV 16 and 18 present in all formulations of HPV? Why would you want to continue to add HPV genotypes to vaccine formulations?
-HPV types 16 and 18 are the most common types of HPV and the most common types attributed to a high risk of developing cervical cancer
○ By preventing initial infection with these HPV types, you can decrease the chance of future cervical cancer development in a population
-HPV types 6 and 8 most commonly cause genital warts
○ By integrating more HPV genotypes into one vaccine, you can decrease the prevalence of multiple HPV-related cancers and other clinical manifestations
○ By preventing initial infection with these HPV types, you can decrease the chance of future cervical cancer development in a population
-HPV types 6 and 8 most commonly cause genital warts
○ By integrating more HPV genotypes into one vaccine, you can decrease the prevalence of multiple HPV-related cancers and other clinical manifestations
67
New cards
when and why is the HPV vaccine administrated in females
- Vaccine is introduced in females due to their risk of cervical cancer as a result of infection
- Administered in grade 7 and 8 in the hopes that the females are not yet sexually active and/or exposed to the virus
○ HPV vaccines does not protect you if you already have the serotype
- Administered in grade 7 and 8 in the hopes that the females are not yet sexually active and/or exposed to the virus
○ HPV vaccines does not protect you if you already have the serotype
68
New cards
when and why is the HPV vaccine administered in males
- While males are not at risk for cervical cancer, they can still contract HPV and pass it along to their sexual partners, as well as develop genital warts and penile cancers
- Administration of the vaccine to males decreases the likelihood of them passing it along to females
- Should vaccinate in grade 7 and 8, like females
- Administration of the vaccine to males decreases the likelihood of them passing it along to females
- Should vaccinate in grade 7 and 8, like females
69
New cards
what are prion diseases and which species are affected
- A family of rare, progressive and fatal neurodegenerative disorders
- Affect both humans and animals
- Affect both humans and animals
70
New cards
what are prions and how do they lead to prion diseases
- Prion = proteinaceous infectious particle = protein-only infectious agents
- The prion gene (PRNP) is encoded on chromosome p20 within the human genome is produces a normal cellular prion protein (PrP)
- Function = unknown
- Has high levels of expression in neurons and is though to influence synaptogenesis or maintenance of synapses
- Can misfold resulting in protein deposition in the brain, causing progressive neurological diseases
- The prion gene (PRNP) is encoded on chromosome p20 within the human genome is produces a normal cellular prion protein (PrP)
- Function = unknown
- Has high levels of expression in neurons and is though to influence synaptogenesis or maintenance of synapses
- Can misfold resulting in protein deposition in the brain, causing progressive neurological diseases
71
New cards
PrPc (cellular) prion protein
- normally non-infectious
- Present in peripheral tissues, white blood cells and platelets
- Highly conserved among species
- Rich in a-helices, is protease-sensitive (susceptible to deterioration by proteases) and detergent soluble
- Present in peripheral tissues, white blood cells and platelets
- Highly conserved among species
- Rich in a-helices, is protease-sensitive (susceptible to deterioration by proteases) and detergent soluble
72
New cards
PrPSc (Scrapie) prion protein
the pathogenic prion protein is rich in beta-sheets, insoluble and protease-resistant (resistant to deterioration by proteases)
73
New cards
what are the three methods through which a conformational change within a prion protein can manifest?
1) infectious = PrPSc
2) mutation = PrPM
3) sporadic = PrPSc
Normal = PrPc
2) mutation = PrPM
3) sporadic = PrPSc
Normal = PrPc
74
New cards
infectious (PrPSc) conformational change of prion proteins
- if an individual is exposed to the pathogenetically folded prion protein, it will cause the proteins that don’t have that conformation to assume that conformation
~Causes a continuous cascade of this pathogenesis
~1% of prion diseases
~Causes a continuous cascade of this pathogenesis
~1% of prion diseases
75
New cards
mutation (PrPM) conformational change of prion proteins
- conformational changes can be genetic
~Change in the aa sequence that results in increased susceptibility to develop misfolded prion proteins
~15%
~Change in the aa sequence that results in increased susceptibility to develop misfolded prion proteins
~15%
76
New cards
sporadic (PrPSc) conformational change
- unknown what factors cause these conformational changes
~85%
~85%
77
New cards
what are the various pathologies associated with prion disease
- The increased presence of beta sheets found in misfolded prions results in pathological changes to the structure of brain tissue
1) Prion Deposition: deposition of misfolded prion protein in the brain
○ Deposits can lead to may other pathologies as they grow and spread
2) Synaptic and Dendrite Loss: accumulation of misfolded prions are suggested to play a role in synaptic damage and the loss of dendrites in an infected brain
○ Dendrite = where a neuron receives action potentials from surrounding neurons
3) Spongiform Encephalopathy: prion disease make the brain look like a sponge, as holes are produced in the brain tissues
○ Caused by formation of fibrils which results in amyloid plaque formation, affecting dendrite and neuronal function
4) Gliosis: caused by the deposition of these amyloid plaques in the brain
○ Occurs in response to injury
○ Refers to the reactive change and proliferation of glial cells, which are normally responsible for forming myelin, and providing support and protection for neurons
○ If gliosis persists = scarring within the brain
5) Neuronal Death: amyloid plaques lead to overall neuronal loss and can spread to other organs as well
1) Prion Deposition: deposition of misfolded prion protein in the brain
○ Deposits can lead to may other pathologies as they grow and spread
2) Synaptic and Dendrite Loss: accumulation of misfolded prions are suggested to play a role in synaptic damage and the loss of dendrites in an infected brain
○ Dendrite = where a neuron receives action potentials from surrounding neurons
3) Spongiform Encephalopathy: prion disease make the brain look like a sponge, as holes are produced in the brain tissues
○ Caused by formation of fibrils which results in amyloid plaque formation, affecting dendrite and neuronal function
4) Gliosis: caused by the deposition of these amyloid plaques in the brain
○ Occurs in response to injury
○ Refers to the reactive change and proliferation of glial cells, which are normally responsible for forming myelin, and providing support and protection for neurons
○ If gliosis persists = scarring within the brain
5) Neuronal Death: amyloid plaques lead to overall neuronal loss and can spread to other organs as well
78
New cards
what is Creutzfeldt-Jakob Disease (CJD)
- A neurodegenerative disorder caused by misfolded prion
- Represents ~85% of all human pion diseases
- Incidence rate = 1 in 1,000,000/year
- Represents ~85% of all human pion diseases
- Incidence rate = 1 in 1,000,000/year
79
New cards
what are the 4 types of CJD
○ Sporadic CJD- MOST COMMON; cause is unclear; incidence = 85-95% of cases
○ Variant CJD- most commonly caused by consuming contaminated meat from a cow with BSE (mad cow disease); incidence = unknown
○ Iatrogenic CJD- a condition that is caused by medical examination or treatment accidently; incidence =
○ Variant CJD- most commonly caused by consuming contaminated meat from a cow with BSE (mad cow disease); incidence = unknown
○ Iatrogenic CJD- a condition that is caused by medical examination or treatment accidently; incidence =
80
New cards
what are some clinical features of sporadic CJD
- Rapid, progressive dementia
- Short course of disease (~4-5 months from symptom onset until death)
- Cerebellar ataxia: the inability to coordinate balance, gait extremity and eye movements
- Myoclonic "muscle jerks"
- Muscle wasting
- Short course of disease (~4-5 months from symptom onset until death)
- Cerebellar ataxia: the inability to coordinate balance, gait extremity and eye movements
- Myoclonic "muscle jerks"
- Muscle wasting
81
New cards
what changes occur to the brain as a result of sporadic CJD
- spongiform degeneration is the most dramatic pathologic feature that can be seen in the brains of patients with sCJD
○ Vacuoles represent focal swellings of axonal and dendritic neuronal processes, associated with the loss of synaptic organelles and accumulation of abnormal membranes
~This affects grey and deep grey matter and are accompanied by reactive gliosis
○ Vacuoles represent focal swellings of axonal and dendritic neuronal processes, associated with the loss of synaptic organelles and accumulation of abnormal membranes
~This affects grey and deep grey matter and are accompanied by reactive gliosis
82
New cards
what would the brain of someone with sporadic CJD look like in an MRI
-patients show a hyperintense signal at the cortex and the caudate nucleus and putamen
○ Cortical rimming of the brain is indicated by a thick boundary on the periphery of the cerebral cortex
○ Cortical rimming of the brain is indicated by a thick boundary on the periphery of the cerebral cortex
83
New cards
what is the relationship between codon 129 and sporadic CJD
- There is a susceptibility focus in the primary structure of the prion protein at codon 129
- 71-89% of individuals with sCJD are homozygous for valine or methionine at codon 129, as opposed to 41-49% of the general population who are heterozygous
~Suggests that individuals who are homozygous for valine or methionine at prion codon 129 are more susceptible to develop sCJD
- 71-89% of individuals with sCJD are homozygous for valine or methionine at codon 129, as opposed to 41-49% of the general population who are heterozygous
~Suggests that individuals who are homozygous for valine or methionine at prion codon 129 are more susceptible to develop sCJD
84
New cards
how is variant CJD different than sporadic CJD
○ Has a longer course of disease (around 13-14 months)
○ Has EEGs without periodic discharges
○ There is no cortical rimming present with MRI scan
- It typically affects a younger population (17-42 years old) compared to sCJD (65-69 years)
- Genetic susceptibility is present with the vast majority of cases being homozygous for methionine at codon 129
○ Has EEGs without periodic discharges
○ There is no cortical rimming present with MRI scan
- It typically affects a younger population (17-42 years old) compared to sCJD (65-69 years)
- Genetic susceptibility is present with the vast majority of cases being homozygous for methionine at codon 129
85
New cards
how were prions spread in the food chain
- Animal feed made from beef and sheep meat and bone meal products
○ Fed to young cattle livestock
- Feed was infected with BSE, which the cattle then ate
○ Humans then ate the infected cattle
○ Fed to young cattle livestock
- Feed was infected with BSE, which the cattle then ate
○ Humans then ate the infected cattle
86
New cards
how does variant CJD reach the brain
- Prions survive various proteases and stomach acids
- Are absorbed by gut-related lymphoid tissue
- Make their way to brain via the sympathetic nervous system
- Are absorbed by gut-related lymphoid tissue
- Make their way to brain via the sympathetic nervous system
87
New cards
what are the clinical features of variant CJD
- Pulvinar Sign: in MRI results, a pulvinar sign is seen rather than cortical rimming
○ The bilateral hyperintensity of the pulvinar thalamic nuclei
- Protein deposits: it deposits significantly more prion protein within the brain tissue than sCJD
○ Helps explain the earlier clinical presentation of the disease
- Florid Plaque: consists of a dense core of prion protein fibrils and amyloid deposits that trap the neural processes
○ Plaque provokes an area of vacuolization around it
○ Distinct to vCJD subtypes
- Symptoms: begins to present with predominantly psychiatric features (eg. Apathy and depression), before moving into the neurological movement disorders
- Painful distal sensations are also very common
○ The bilateral hyperintensity of the pulvinar thalamic nuclei
- Protein deposits: it deposits significantly more prion protein within the brain tissue than sCJD
○ Helps explain the earlier clinical presentation of the disease
- Florid Plaque: consists of a dense core of prion protein fibrils and amyloid deposits that trap the neural processes
○ Plaque provokes an area of vacuolization around it
○ Distinct to vCJD subtypes
- Symptoms: begins to present with predominantly psychiatric features (eg. Apathy and depression), before moving into the neurological movement disorders
- Painful distal sensations are also very common
88
New cards
what are three surgeries that could transmit prion proteins in ICJD
1) Dural cadaveric flaps- in neurosurgical procedures, dural grafts originating from deceased individuals with CJD can cause CJD in the recipient
2) Pituitary extracts- if a pituitary donor had CJD it could be transmitted to the recipient who was treated with the derived hormone
~Procedure occurred before the ability to synthesize hormones used to treat endocrine disorders was developed
3) Blood transfusions- the process of transferring donated blood into a patients circulation intravenously
~Prions not generally recognized as blood-borne infectious agents
~Prion infects have been reported in 3 individuals who received blood infusion and then developed CJD
2) Pituitary extracts- if a pituitary donor had CJD it could be transmitted to the recipient who was treated with the derived hormone
~Procedure occurred before the ability to synthesize hormones used to treat endocrine disorders was developed
3) Blood transfusions- the process of transferring donated blood into a patients circulation intravenously
~Prions not generally recognized as blood-borne infectious agents
~Prion infects have been reported in 3 individuals who received blood infusion and then developed CJD
89
New cards
how do prion proteins get accidently transmitted during surgical or medical procedures (ICJD)?
- The pathogenic prion protein is very resistant to common denaturing and sterilizing agents
-Difficult to ensure that these instruments are completely cleaned after coming into contact with an infected patient
-Difficult to ensure that these instruments are completely cleaned after coming into contact with an infected patient
90
New cards
what is the inheritance pattern of familial/genetic CJD
- An autosomal dominant disease with high penetrance
- Usually occurs as a result of point mutations
- Usually occurs as a result of point mutations
91
New cards
how is familial CJD different than sporadic
-often presents in a younger population than sCJD
92
New cards
what are the two types of familial CJD
1) GSS
2) FFI
2) FFI
93
New cards
how do the 4 prion diseases present histologically?
- sCJD: typical spongiform degeneration of the grey matter and reactive gliosis
- vCJD: florid plaques consisting of dense core prion deposits surrounded by vacuoles (a halo)
- GSS: large prion protein amyloid plaques
- FFI: neuronal loss and gliosis occurs in the thalamus, inferior olives of the medulla oblongata and cerebellum
○ No vacuolar degeneration
- vCJD: florid plaques consisting of dense core prion deposits surrounded by vacuoles (a halo)
- GSS: large prion protein amyloid plaques
- FFI: neuronal loss and gliosis occurs in the thalamus, inferior olives of the medulla oblongata and cerebellum
○ No vacuolar degeneration
94
New cards
biochemical similarities between sCJD and iCJD
- always recorded the same migration patterns (Types 1-3) after proteinase K digestion
95
New cards
biochemical similarities between vCJD and BSE (mad cow disease)
-displayed a novel pattern (Type 4)
○ Biochemically BSE and vCJD are similar because they provide similar elution patterns after proteinase K digestion
~Supports the idea the vCJD is the result of BSE transmission to humans
○ Biochemically BSE and vCJD are similar because they provide similar elution patterns after proteinase K digestion
~Supports the idea the vCJD is the result of BSE transmission to humans
96
New cards
what is the Montreal cognitive assesment (MoCa)test and what does it test for
- Used to evaluate judgment, reasoning, memory, planning and problem-solving, in order to detect cognitive impairment
- Composed of 30 questions and takes between 12-14 mins
- Can receive a max score of 30
○ 26 or greater = normal cognitive health
○16 = Those with Alzheimer's
- Composed of 30 questions and takes between 12-14 mins
- Can receive a max score of 30
○ 26 or greater = normal cognitive health
○16 = Those with Alzheimer's
97
New cards
what is alzheimer's disease
- It is a progressive neurodegenerative disease
- The most common cause of dementia
○ Accounts for 50-75% of all cases
- Typical lifespan post-diagnosis = 4-8 years
○ In some rare cases, people live up to 20 years
- The most common cause of dementia
○ Accounts for 50-75% of all cases
- Typical lifespan post-diagnosis = 4-8 years
○ In some rare cases, people live up to 20 years
98
New cards
what are the common signs of alzheimer's
○ Frequently misplacing things
○ Getting lost (unable to retrace steps back home after going for walks)
○ Personality changes causing out of character behaviour
○ Difficulty having a conversation
○ Consistent poor judgment
○ Getting lost (unable to retrace steps back home after going for walks)
○ Personality changes causing out of character behaviour
○ Difficulty having a conversation
○ Consistent poor judgment
99
New cards
what is dementia
- A heterogenous group of symptoms affecting memory, thinking, social abilities and interferes with daily functioning
- A broader category used for cognitive impairment
- A broader category used for cognitive impairment
100
New cards
what are some physiological changes associated with dementia
○ Personality changes
○ Depression
○ Anxiety
○ Inappropriate behaviour
○ Agitation
○ Hallucinations
○ Memory loss
○ Difficulty communicating / finding words
○ Difficulty reasoning / problem solving / handing complex tasks
○ Depression
○ Anxiety
○ Inappropriate behaviour
○ Agitation
○ Hallucinations
○ Memory loss
○ Difficulty communicating / finding words
○ Difficulty reasoning / problem solving / handing complex tasks