Unit 2 Part 1
1/154
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
155 Terms
Cations
Alkalai = +1
Alkaline Earth = +2
Transition/Post Transition Metals
(ion charge)
Anions
End in ide
Periodic Table and Ion Formation
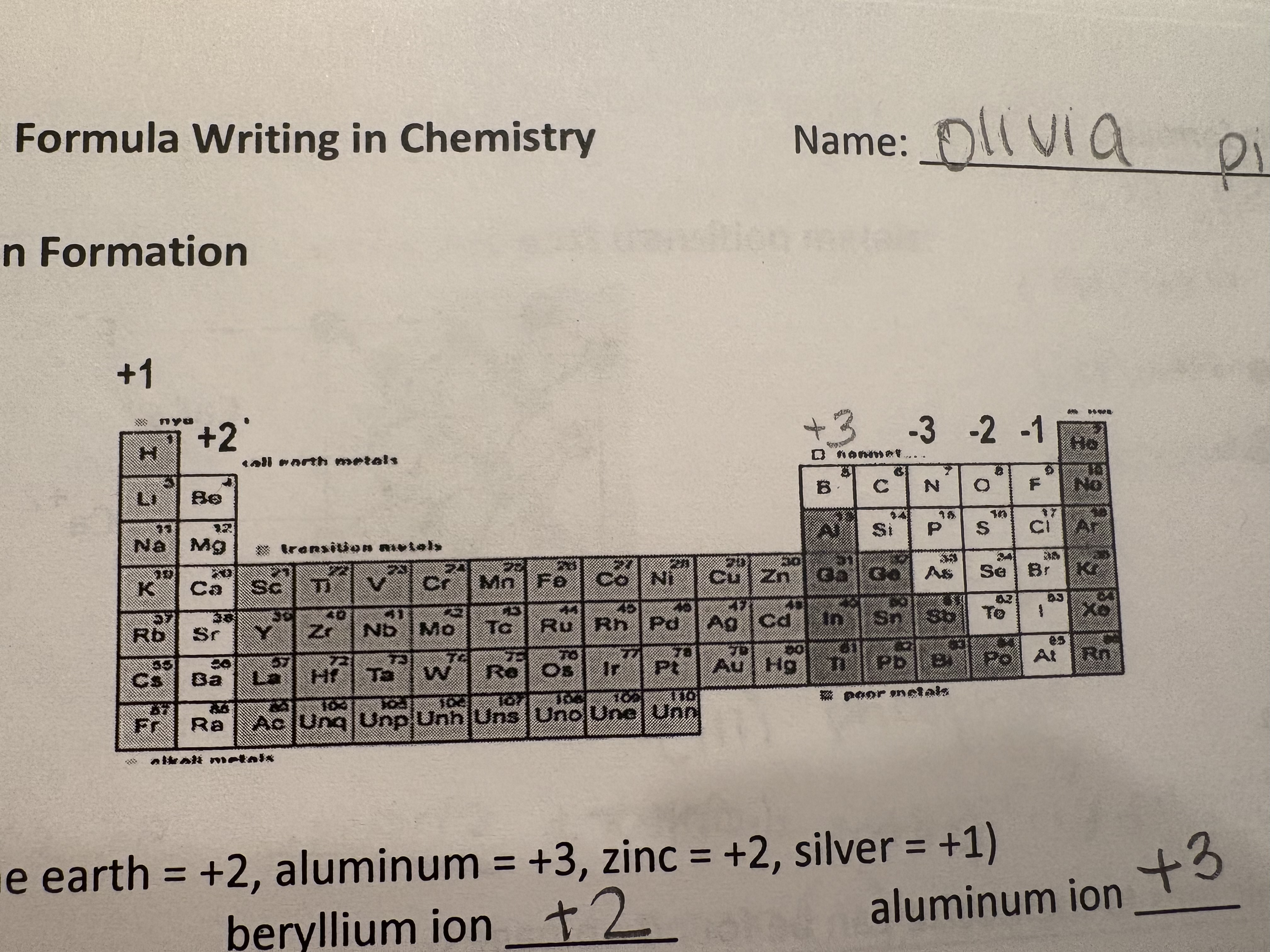
Binary Ionic Formation
Figure out charge
Figure out number that makes charges equal
Put how that number small
When writing names…
If it is a transition metal make sure to put roman numerals
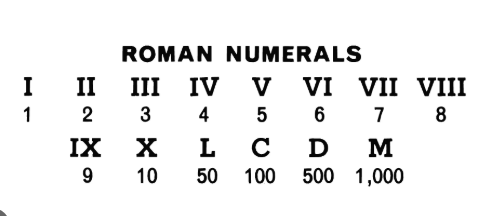
Roman Numerals
When writing formulas with polyatomic ions….
If the there is a charge - outside the ()
If there is no charge - no ()
Determining the oxidation number for transition/post transition metals: Steps
Put polyatomic Ions in ()
Write in charges for the anion
Multiply the anion charge by the subscript
Divide by the subscript for the cation
Determining the oxidation number for transition/post transition metals: Physical description

Naming Binary Molecular Compounds: 1
mono (Expect the first element, then it does not change)
Naming Binary Molecular Compounds: 2
di
Naming Binary Molecular Compounds: 3
tri
Naming Binary Molecular Compounds: 4
tetra
Naming Binary Molecular Compounds: 5
penta
Naming Binary Molecular Compounds: 6
hexa
Naming Binary Molecular Compounds: 7
hepta
Naming Binary Molecular Compounds: 8
octa
Naming Binary Molecular Compounds: 9
nona
Naming Binary Molecular Compounds: 10
deca
Ionic formula
metal and nonmetal or Ammonium and non-metal
Canceling Out
Molecular formula
2 Non-metal, only sharing electrons
Prefixes
Naming acids: Acids without oxygen
Hydro____ic acid
Naming acids: oxyacids stemming from ate ions
____ ic acid
Naming acid: oxyacids stemming from ite ions
____ous acid
If it begins with a metal
IONIC, uses charges, no prefixes
If it begins with a non-metal other than H
Molecular, use prefixes, no charges
If it begins with H
Use acid naming system
Rules for naming molecular compounds
First element: decide element nam
Second element: start with ide name
Use prefixes to show how many atoms each type there are
Don’t use mono on the first element
If you have “ao” or “oo” turn it into o
Acid
A compound in which 1 or more H ions are bonded to a negative ion. Name of acid is based on the name of the negative ion
NH+4
Ammonium
NO-2
Nitrite
Nitrite
NO-2
Ammonium
NH+4
NO-3
Nitrate
Nitrate
NO-3
OH-
hydroxide
hydroxide
OH-
SCN-
thiocyanate
thiocyanate
SCN-
cyanide
CN-
CN-
cyanide
permanganate
MnO-4
MnO-4
permanganate
hydrogen carbonate (bicarbonate)
HCO3-
HCO3-
hydrogen carbonate (bicarbonate)
hypochlorite
CIO-
CIO-
hypochlorite
CIO-2
chlorite
chlorite
CIO-2
CIO-3
chlorate
chlorate
CIO-3
CIO-4
perchlorate
perchlorate
CIO-4
C2H3O2-
acetate
acetate
C2H3O2-
CO32-
Carbonate
Carbonate
CO32-
sulfite
SO32-
SO32-
sulfite
SO42-
sulfate
sulfate
SO42-
O22-
peroxide
peroxide
O22-
CrO42-
chromate
chromate
CrO42-
Cr2O72-
dichromate
dichromate
Cr2O72-
PO43-
phosphate
phosphate
PO43-
PO33-
phosphite
phosphite
PO33-
Aluminum ion
Al+3
Sliver ion
Ag+1
Zinc Ion
Zn+2
Cadmium ion
Cd+2
Formula unit vs formula
Unit - Ionic compound only
Formula - Ionic or covalent
Draw with dot diagram
Dot diagram is dots in valence electrons (s + p)
Type 1 vs Type 2 metal
Type 1 - One charge
Type 2 - More than one charge (transition metals)
Diatomic elements
Hydrogen, Nitrogen, Fluoride, Oxygen, Iodide, Chlorine, bromine
Sulfur root and Phosphours
SulfurEND
PhosphEND - not acid
PhosphorEND - acid
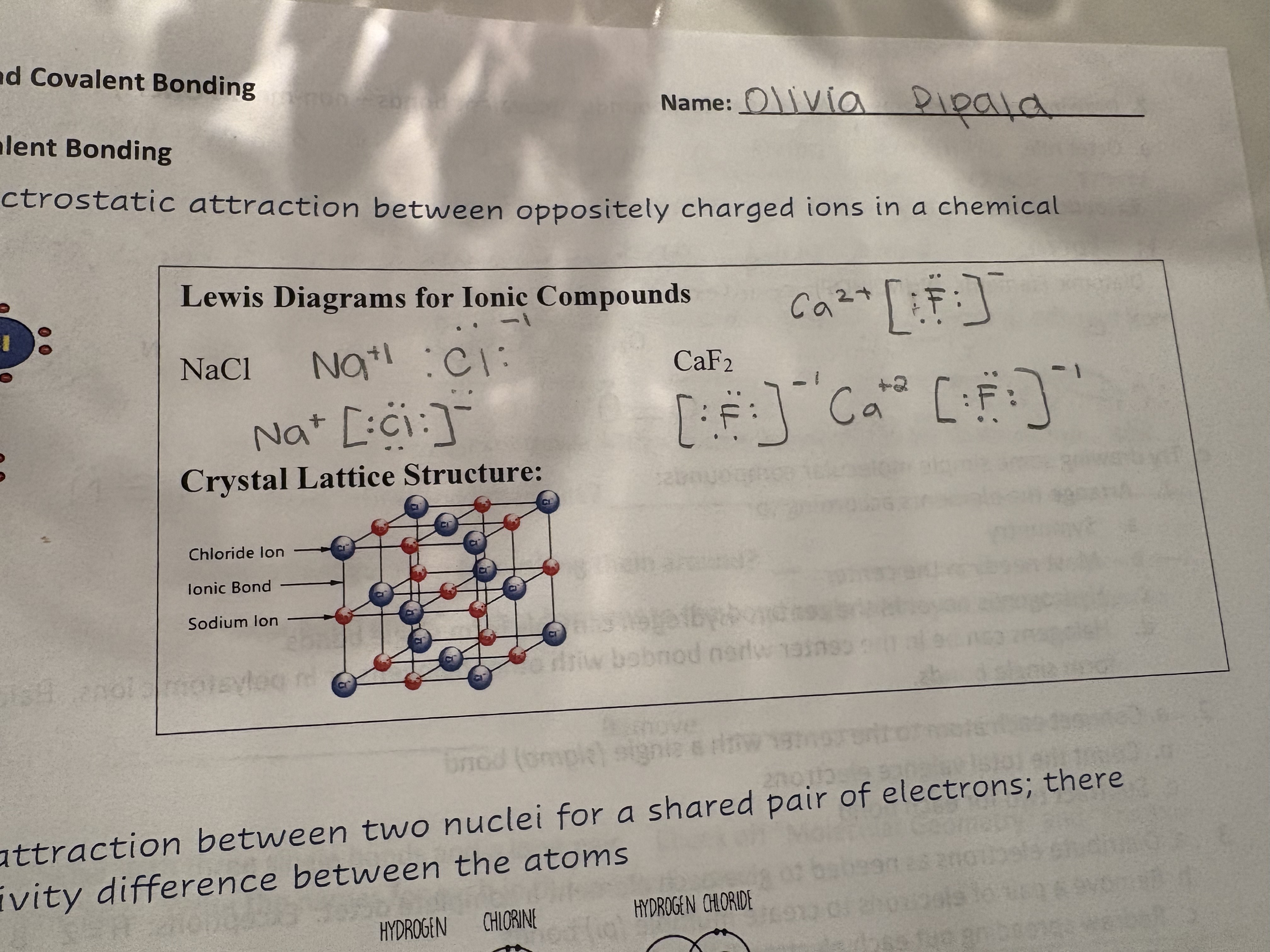
Ionic Bond
Electrostatic attraction between oppositely charged ions in a chemical compound
Lewis Diagram for Ionic Compound: Steps
Identify elements
Identify valence electrons
Draw valence electrons around each element
Show metal transferring electrons until it has 0
Show nonmetal with 8 electrons
Write with ion charged
Enclose anions with Brackets
Lewis Diagrams for Ionic Compound
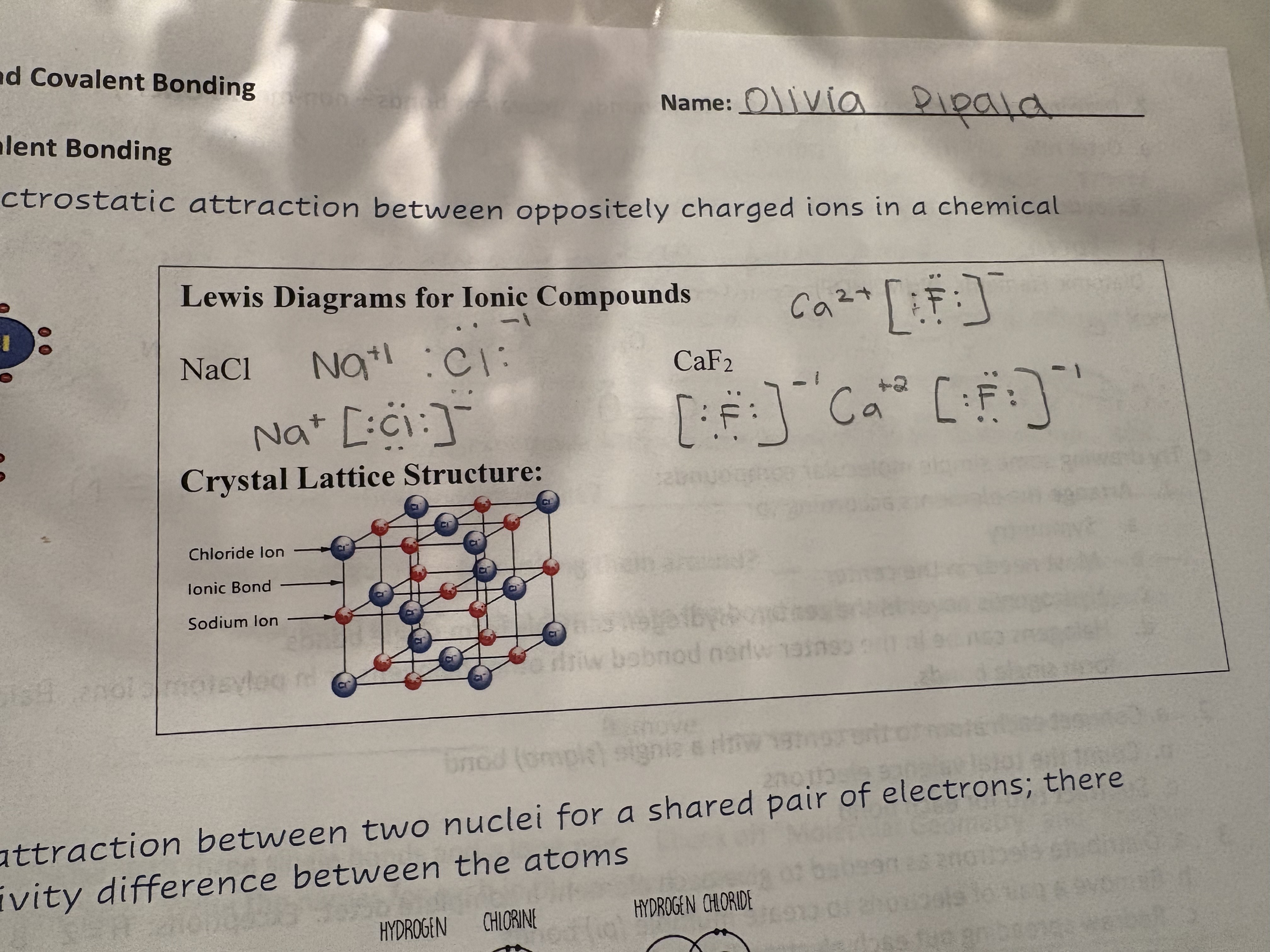
What does ionic bonds look like IRL
Crystal Lattice Structure
Crystal Lattice Structure
Covalent Bonds
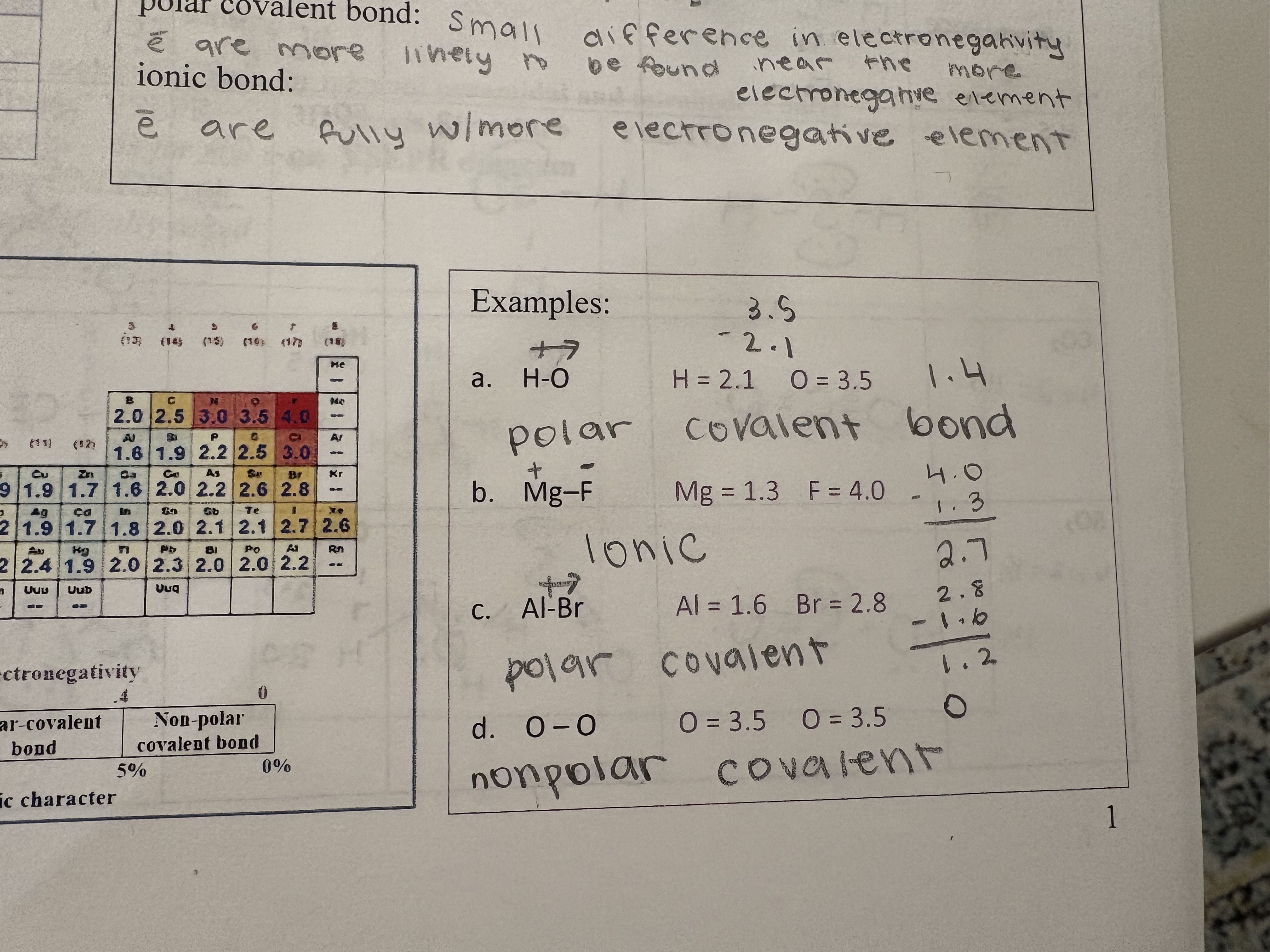
Attraction between 2 nuclei for a shared pair of electrons; there electronegativity differences between the atoms
Nonpolar covalent Bond
Very similar electronegativity
Electrons are shared equally
Nonpolar covalent bond/pure covalent; Electronegativity difference
< 0.4
Polar covalnt bond
Small differences in electronegativity
Electrons are more likely to be found near the more electronegative element
Polar covalent bond: Electronegativity difference
between 0.4 and 1.8
Ionic bond
Electrons are fully with more electronegative element
Ionic bond: electronegativity difference
>1.8
Steps to Nonpolar and Covalent Bonds
Subtract 2 elements
Figure out bonds
If there is an cation and anion: positive goes to negative arrow
Nonpolarand Polar Covalent bonds: Picture
Octet rule
Elements bond in order to have 8 electrons in their router shell: NONMETAL ONLY
1 bond line
2 electrons
Exceptions for Octet Rule
H forms 1 bond only and B can form 3 bonds (or 4)
How to draw simple molecular compounds
Count valence electrons
Find totals of valence electrons (multiply charge by valence electrons if needed)
Find the center element: one with the least valence electrons
Hydrogen is never in the middle
Start with connecting the elements with a line
Make sure both have 8 electrons
If the line does not cover all electrons, fill up non center elements sides
Add more lines or electrons on the center element to get all 8