PHYSICS IGCSE EDEXCEL ALL PAPER 1
1/91
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
92 Terms
speed
distance/time
acceleration
change in velocity/time taken
speed in a distance time graph
gradient
acceleration in a velocity time graph
gradient
distance in a velocity time graph
area
weight
mass x gravitational field strength
scalar
magnitude only
vector
magnitude and direction
friction
force that opposes motion, present if an objet is in motion
stopping distance
thinking distance + braking distance
faster speed increases both
larger mass increases braking distance
slower reaction time increases thinking distance
increased grip decreased braking distance
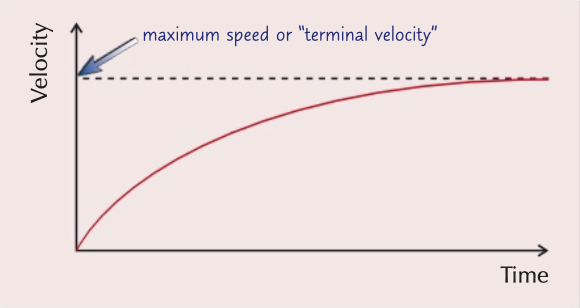
terminal velocity
weight is equal to drag meaning resultant force is zero, therefore acceleration is 0
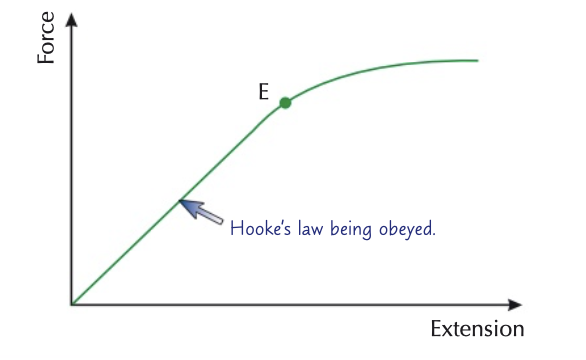
hooke’s law
extension of a helical spring is direction proportional to the applied force
there is an elastic limit where elastic behaviour turns into plastic behaviour
elastic behaviour: if the applied force is removed, the object will return back to its original shape
plastic behaviour: if the applied force is removed, the object will not return back to its original shape, therefore it is deformed
newton’s laws of motion
if resultant force on an object = 0, it will remain at its current velocity (incl. 0 – standing still)
inertia means no resultant force/ object will continue doing what it’s doing
zero acceleration
object accelerates in the direction of the resultant force
speed can remain constant but direction changes, meaning a changed velocity
if object A exerts a force on object B, then object B exerts an equal and opposite force on object A
requirements: 1) same type of force, 2) acts on two different objects
moments
moment = force x perpendicular distance from pivot
newton metres = newtons x metres
principle of moments: sum of clockwise moments equals sum of anticlockwise moments in order for a lever to be in equilibrium
in order for lever to be in equilibrium, f1x = f2y
insulation
to stop the flow of electricity
conduction
to allow the flow of electricity
double insulation
covering a metal object in plastic, therefore it acts as an insulator
earthing
carries excess current into the earth through a metal rod put into earth
circuit breakers
switch opens, therefore breaking the circuit if too much current is flowing through
benefit: reusable
mains electricity
230V
fuse
melts if current gets too high therefore breaking the circuit
drawback: one time use; after it melts it must be replaced
ammeter
measures current, must be placed in series
voltmeter
measures voltage, must be placed in parallel around the component under test
a.c. supply
current is constantly changing direction
d.c. supply
current keeps flowing in the same direction
voltage
current x resistance
metal filament lamp
as temperature increases, resistance increases
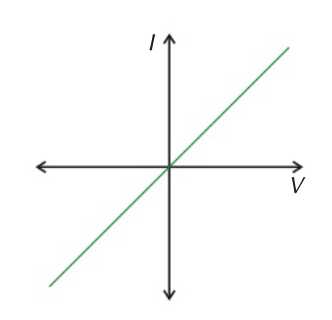
wire
current through a wire is proportional to voltage
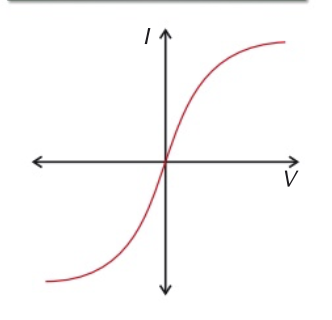
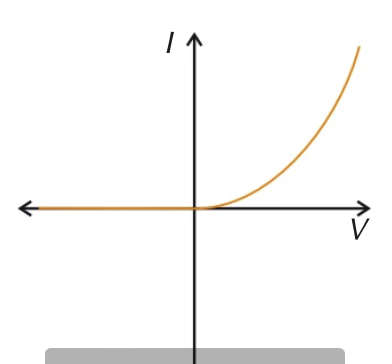
diode
current in a diode only flows through in one direction
LEDs
emit light when current flows through them in the forward direction
used in remote controls, digital clocks, traffic lights
don’t have a filament that can burn out
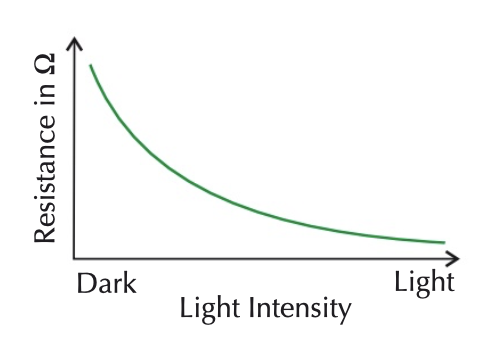
LDRs
changes resistance depending on how much light falls on it
in bright light, resistance decreases
in darkness, resistance increases
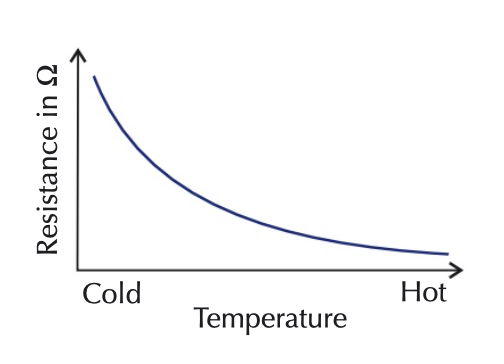
thermistors
temperature-dependent resistor
in hot conditions, resistance decreases
in cool conditions, resistance increases
series circuits
in series, voltage is split between components
E.g. power source is 6V, both lightbulbs have 3V (assuming they have the same resistance)
the current is the same everywhere in a series circuit
benefit: more simple (less wires required)
negative: components have a greater resistance
parallel circuits
in parallel, voltage is equal in components
E.g. power source is 6V, both lightbulbs have 6V (assuming they have the same resistance)
at a junction, current is conserved
current is split between branches in a parallel circuit
benefit: if one component breaks, the others will continue running
negative: while the bulbs may be brighter, the power source would drain faster
current
current = charge/time
definition: rate of flow of charge
current is conserved at a junction
voltage
voltage = energy/charge
definition: energy transferred per charge
1 volt = 1 joule/coulomb
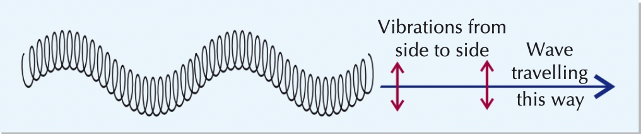
transverse waves
vibrations perpendicular to energy transfer
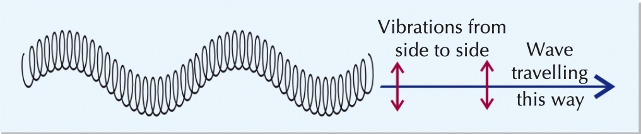
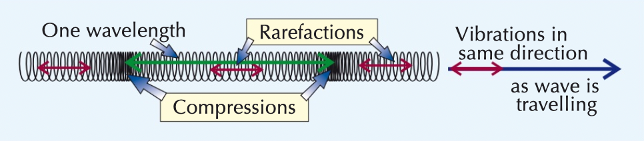
longitudinal waves
vibrations parallel to energy transfer
wave speed
λ x frequency
frequency
1/time
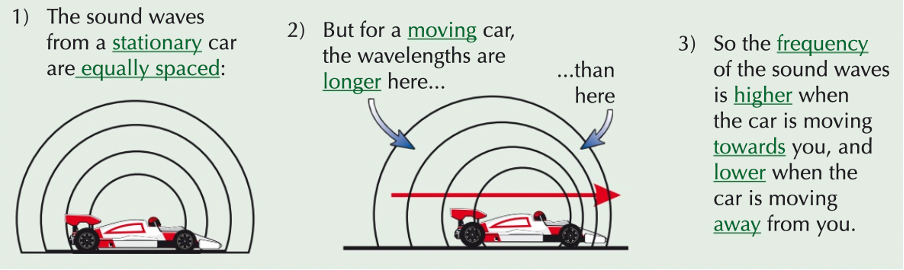
doppler effect
wavefronts are further apart, therefore meaning a longer wavelength
wavelength is inversely proportional to frequency
longer wavelength means a lower frequency, meaning a lower pitch
wave speed is constant
V = λf, if wavelength is longer so frequency must be lower to maintain the same wave speed
refraction
when a wave passes a boundary between two different density media, it changes speed and sometimes direction too
from more to less optically dense, wave goes away from the normal
from less to more optically dense, wave goes towards the normal
electromagnetic spectrum
radio waves → microwaves → infrared → visible light → ultraviolet → x-rays → gamma rays (increasing f, decreasing λ)
radio waves -> communication
microwaves -> cell phones, however heats internal tissues
infrared -> cooking food, night vision/thermal imaging, however can cause skin burns
visible light -> photography
ultraviolet -> testing fake bank notes, sterilisation, however may cause skin cancer by the mutation of skin cells
x-rays -> view internal structure of our bodies, however may cause cancer by the mutation of cells
gamma rays -> sterilise medical equipment, however may cause cancer by the mutation of cells
graphical method
take multiple incidences and refractions,
plot a graph of sin (i) against sin (r)
draw a straight line of best fit (should be directly proportional)
find gradient of line
to find n, gradient = diff in y/ diff in x
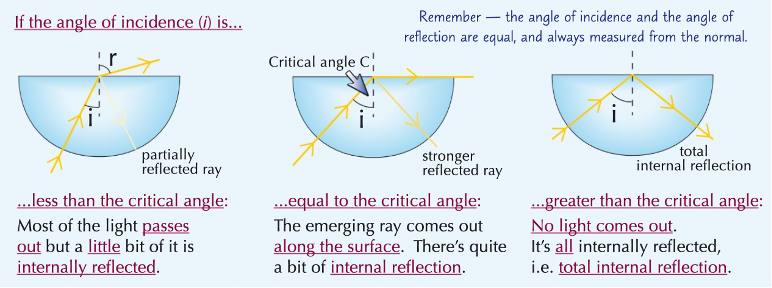
critical angle
if incidence is greater than C, all rays will be totally internally reflected
MUST take place at a boundary from a substance that is more optically dense to a substance that is less optically dense
total internal reflection
sound waves
humans can only hear sound waves from 20 – 20,000 Hz
sound waves are longitudinal waves
energy stores
kinetic, thermal, chemical, gravitational potential, elastic potential, electrostatic, magnetic, nuclear
principle of conservation of energy
energy can be stored, transferred between stores or dissipated - but it can never be created or destroyed
efficiency
useful/total x 100
sankey diagrams
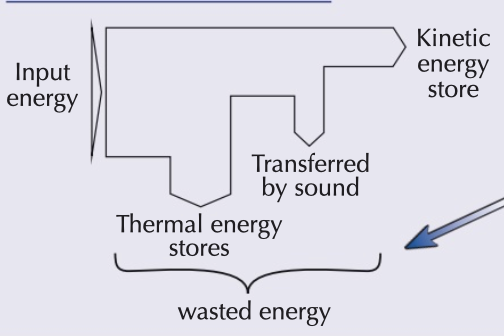
radiation energy transfer
thermal radiation is infrared radiation consisting of plenty of EM waves
an object that is hotter than its surroundings emits more radiation than it absorbs
an object that is cooler than its surroundings absorbs more radiation than it emits
conduction energy transfer
mainly in solids
vibrating particles transfer energy from their kinetic energy store to the kinetic energy store of neighbouring particles
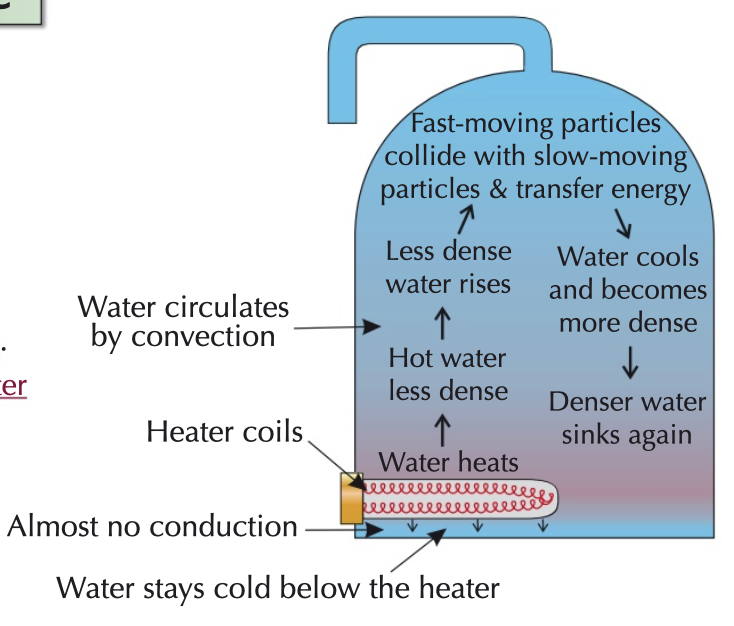
convection energy transfer
in liquids and gases
more energetic particles move from a hotter region to a cooler region, transferring energy as they do
convection currents are all about changes in density
colours in energy transfer
black → good absorber, bad reflector
white → good reflector, bad absorber
matte → good absorber
shiny → good reflector
work done
force x distance moved
kinetic energy store
KE = ½ x mass x speed²
density
mass/volume
pressure
force/area
absolute zero
-273 degrees celsius
particle collision theory
colliding gas particles create pressure
as gas particles move, they randomly collide into each other
they exert a force and their momentum and direction change
pressure created depends on speed and frequency
increasing temperature increases pressure
increasing volume decreases pressure
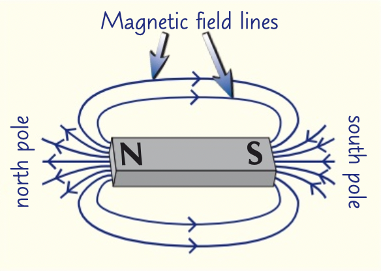
magnetic fields
region where magnetic materials experience a force
magnetic field lines show size and direction of magnetic fields, always from north to south
north to south
at least 3 lines
field lines closer at poles
magnetic materials
material that will turn into a magnet if it is brought into a magnetic field
iron
cobalt
nickel
magnetically hard
permanently magnetised, difficult to magnetise and demagnetise
e.g. steel
magnetically soft
temporarily magnetised, easy to magnetise and demagnetise
e.g. iron
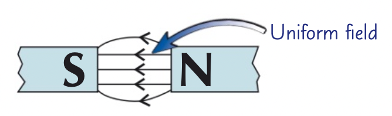
uniform field
evenly spaced
parallel
arrow from north to south
magnetic induction
when a magnetic material is brought into a magnetic field, it becomes a magnet (gets magnetised)
if brought to north pole, the side closest to the north pole will become the south pole
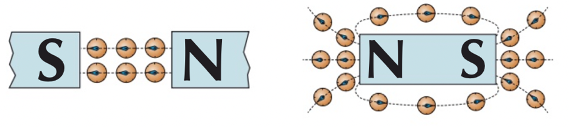
iron filings
place a white piece of paper over a bar magnet
sprinkle iron filings on top
gently tap the paper until magnetic field lines appear
compass
place multiple needle compasses around a bar magnet
needle of compass will align with the magnetic field
this will show the direction of the magnetic field
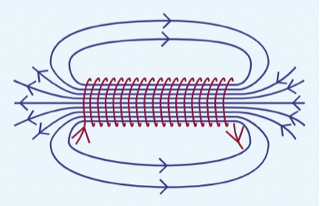
solenoid
when current flows through a current-carrying wire, it produces a magnetic field
magnetic field inside a current-carrying solenoid is uniform and strong
outside the bar, the field is one just like a magnet
ends of solenoid act as the north and south poles
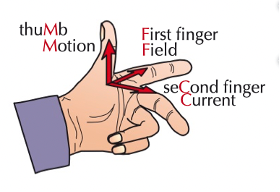
fleming’s left hand rule
current flowing through a wire produces a magnetic field
this magnetic field interacts with the magnetic field of the permanent magnet
this produces a force
motor effect
loudspeakers
current-carrying wire produces magnetic field
A.C. current, meaning a current that changes direction continuously
this magnetic field interacts with the magnetic field of a permanent magnet
this produces a force -> every time the direction of the current changes, the direction of the force changes as well
frequency of vibration correlates to the frequency of sound
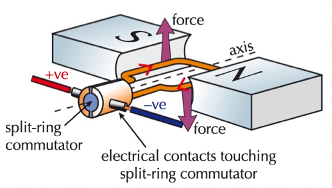
factors that speed up a D.C. electric motor
more current
more turns in the coil
stronger magnetic field
soft iron core in the coil
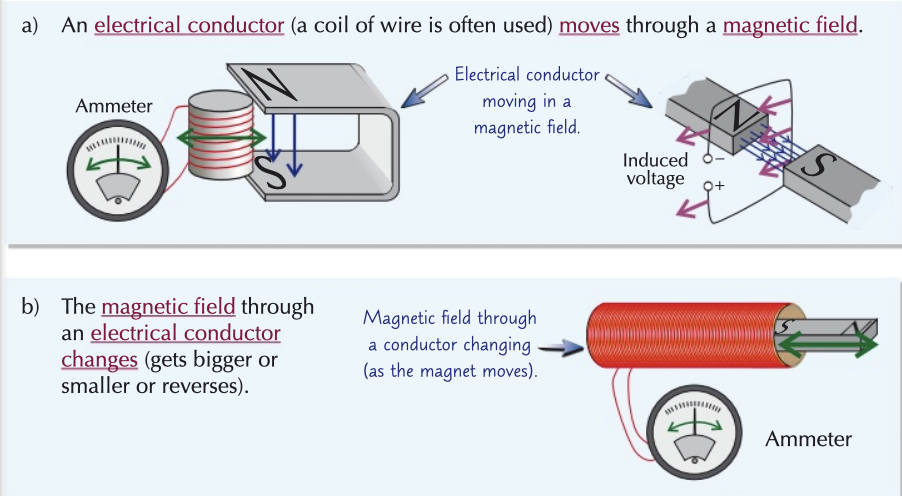
electromagnetic induction
the creation of a voltage in a wire which is experiencing a change in magnetic field
dynamo effect → using electromagnetic induction to generate electricity using energy from kinetic energy stores
to get a bigger voltage, increase
strength of magnet
number of turns on coil
speed of movement
structure of an atom
neutron number = mass - proton
isotopes: same proton number but different number of neutrons
proton
mass 1
charge +1
neutron
mass 1
charge 0
electron
mass 1/1000
charge -1
alpha radiation
helium nucleus
lowly penetrating
highly ionising
emitting an alpha particle causes proton number to decrease by 2, mass number decreases by 4
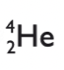
beta radiation
electron
moderately penetrating
moderately ionising
emitting a beta particle causes proton number to increase by 1, mass number stays the same
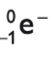
gamma radiation
electromagnetic wave
no mass, just energy
highly penetrating
lowly ionising
always happens after an alpha or beta decay
emitting gamma rays have no effect on the proton and mass number
neutron radiation
emitting a neutron causes proton number to stay the same, mass number decrease by 1
measuring radioactivity of a sample
measure background radiation using a GM detector (Bq)
measure Bq reading from a known distance (control) to radioactive source
subtract background radiation reading from total Bq reading
repeat 3 times and average concordant results, remove anomalies
half-life
time taken for half of the radioactive nuclei to decay
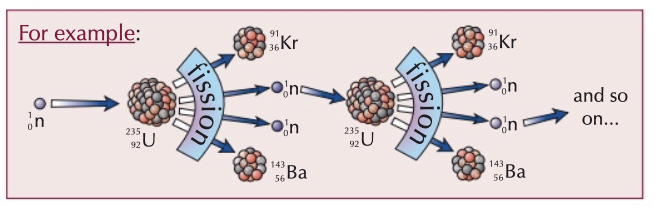
nuclear fission
splitting of a large parent nucleus into smaller daughter nuclei and neutrons which collide with other nuclei, causing a chain reaction
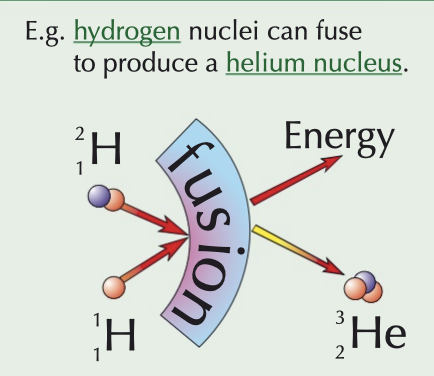
nuclear fusion
two small nuclei collide and fuse to create a larger, heavier nucleus
conditions → extremely high temperature (high kinetic energy) and pressure (to overcome electrostatic repulsion of like charges)
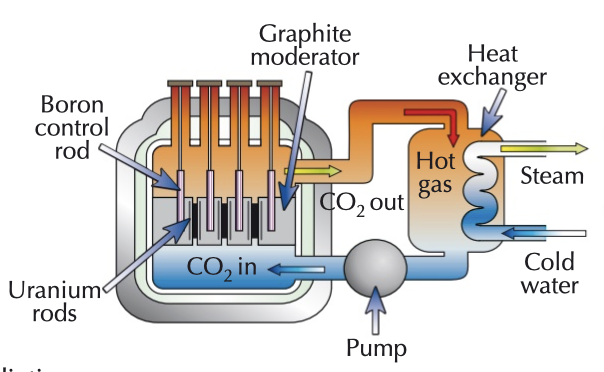
nuclear reactors
moderator (usually graphite or water) slows down neutrons
control rods (usually boron) limit rate of fission by absorbing excess neutrons
shielding (usually thick concrete) used to absorb ionising radiation
substance (usually CO2) pumped around to transfer energy to water in the heat exchanger
uses of nuclear radiation
medical tracers
radioactive source has to have a short half-life
sterilisation
of food and equipment
treating cancer
ionising radiation can kill or damage cells and tissues
industrial tracers and thickness gauges
risks of nuclear radiation
ionising radiation can damage cells and tissues
beta and gamma radiation can penetrate the skin and soft tissues
radiation collides with molecules in cells causing ionisation which damages or destroys molecules
irradiation
exposure to radiation
keeping sources in lead-lined boxes reduces risk of irradiation
contamination
radioactive particles getting onto objects
use gloves and tongs when handling sources
disposal
radioactive sources are difficult to dispose of
seal into glass blocks which are then sealed in metal canisters and buried underground
site must be geologically stable
universe
large collection of billions of galaxies
galaxy
large collection of stars
orbits
planets orbit the sun
comets orbit the sun
asteroids orbit the sun
moons orbit planets
elliptical orbit (elongated) → comets
circular orbit → planets
gravitational field strength affected by:
mass → higher mass higher g
distance → less distance higher g
orbital speed
2 x π x orbital radius/time period
stellar evolution
stars much bigger than the sun: nebula → protostar → main sequence star → red supergiant → supernova → neutron star or black hole
stars around the same size as the sun: nebula → protostar → main sequence star → red giant → white dwarf
star colour
(hottest) blue → white → yellow → orange → red (coolest)