Biochem - Metabolism Module
0.0(0)
0.0(0)
New
Card Sorting
1/162
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
163 Terms
1
New cards
What is the __**catabolic pathway**__?
**Break down** larger molecules into smaller substances
> Extract H/e- → Deliver it to the electron transport chain
Controlled by demand
> Extract H/e- → Deliver it to the electron transport chain
Controlled by demand
2
New cards
What is the __**anabolic pathway**__?
**Build** larger molecules from smaller substances
* Require ATP
* Require ATP
3
New cards
Dinitrophenol (DNP)
* An **uncoupler** (a molecule that **disrupts oxidative phosphorylation**)
* Prevent energy being stored as fat in the body (instead releasing as heat)
* Prevent energy being stored as fat in the body (instead releasing as heat)
4
New cards
ATP, ADP and AMP
Free energy required is produced by **ATP hydrolysis**, which makes them thermodynamically favourable.
* The rate of ATP synthesis = The rate of ATP use
* Cells can’t burn fuel without O2
* The rate of ATP synthesis = The rate of ATP use
* Cells can’t burn fuel without O2
5
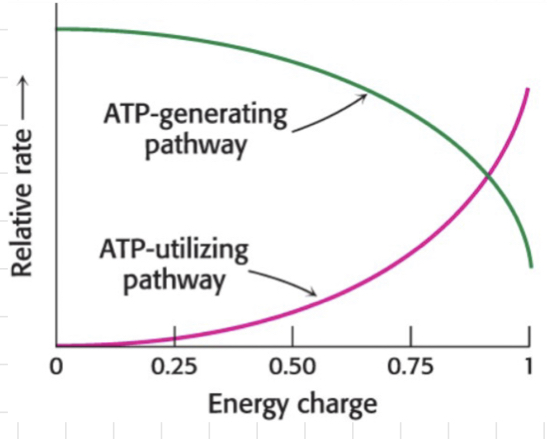
New cards
Energy demand
The rates of __**catabolic**__ **(ATP-demanding)** and __**anabolic**__ **(ATP-utilising) pathways** are regulated by the energy state within the cell.
* A small change in AMP can significantly affect the whole energy charge.
* A small change in AMP can significantly affect the whole energy charge.
6
New cards
What are ==__**kinases**__==__**,**__ @@__**phosphatases**__@@ **and** __**phosphorylases**____**?**__
* ==**Kinases**== **→** Catalyse a ==__**phosphorylation**__== reaction.
* @@**Phosphatases**@@ **→** Catalyse a @@__**dephosphorylation**__@@ reaction.
* **Phosphorylases** **→** Catalyse a __**phosphorolysis**__ reaction.
* @@**Phosphatases**@@ **→** Catalyse a @@__**dephosphorylation**__@@ reaction.
* **Phosphorylases** **→** Catalyse a __**phosphorolysis**__ reaction.
7
New cards
What are ==__**synthases**__== **and** @@__**synthetases**__@@?
* ==**Synthases**== **→** Catalyse ==__**condensation**__== reactions/ ==__**synthetic**__== process.
* ==**Without**== ATP
* ==Reversible==
* @@**Synthetases**@@ **→** Catalyse @@__**condensation**__@@ reactions.
* @@**With**@@ ATP
* @@Irreversible@@
* ==**Without**== ATP
* ==Reversible==
* @@**Synthetases**@@ **→** Catalyse @@__**condensation**__@@ reactions.
* @@**With**@@ ATP
* @@Irreversible@@
8
New cards
What is __**Coenzyme A**__?
* Carrier for acyl group
* Great for trapping metabolites within the cell
* Great for trapping metabolites within the cell
9
New cards
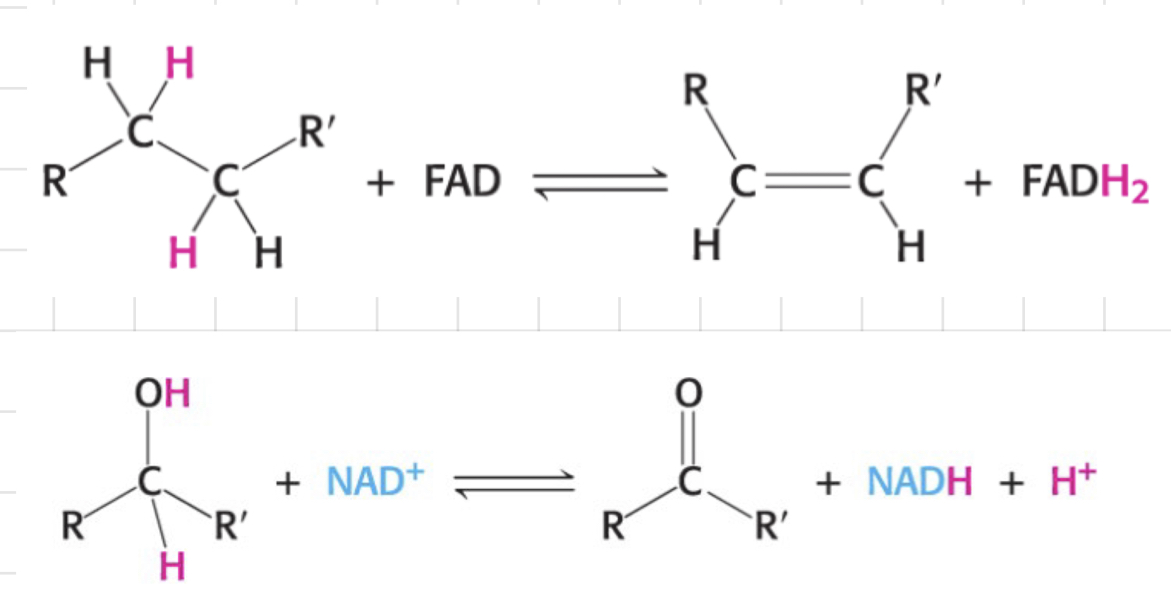
What are ==__**dehydrogenases**__==?
* Catalyse ==__**oxidation-reduction**__== reactions
* Transfer 2 H atoms from organic compounds to electron acceptors.
* Involved **NAD+** or **FAD** as cofactors.
* **NAD+** (Nicotinamide Adenine Dinucleotide)
* NAD+ is reduced to **NADH**
* Loves to oxidise **-CH2-COH-** to **-CH-CO-**
* **FAD (Flavin Adenine Dinucleotide)**
* Accept 2 H+ and 2 e-
* Become **FADH2**
* Loves to oxidise **-CH2-CH2-** to **-CH=CH-**
* Transfer 2 H atoms from organic compounds to electron acceptors.
* Involved **NAD+** or **FAD** as cofactors.
* **NAD+** (Nicotinamide Adenine Dinucleotide)
* NAD+ is reduced to **NADH**
* Loves to oxidise **-CH2-COH-** to **-CH-CO-**
* **FAD (Flavin Adenine Dinucleotide)**
* Accept 2 H+ and 2 e-
* Become **FADH2**
* Loves to oxidise **-CH2-CH2-** to **-CH=CH-**
10
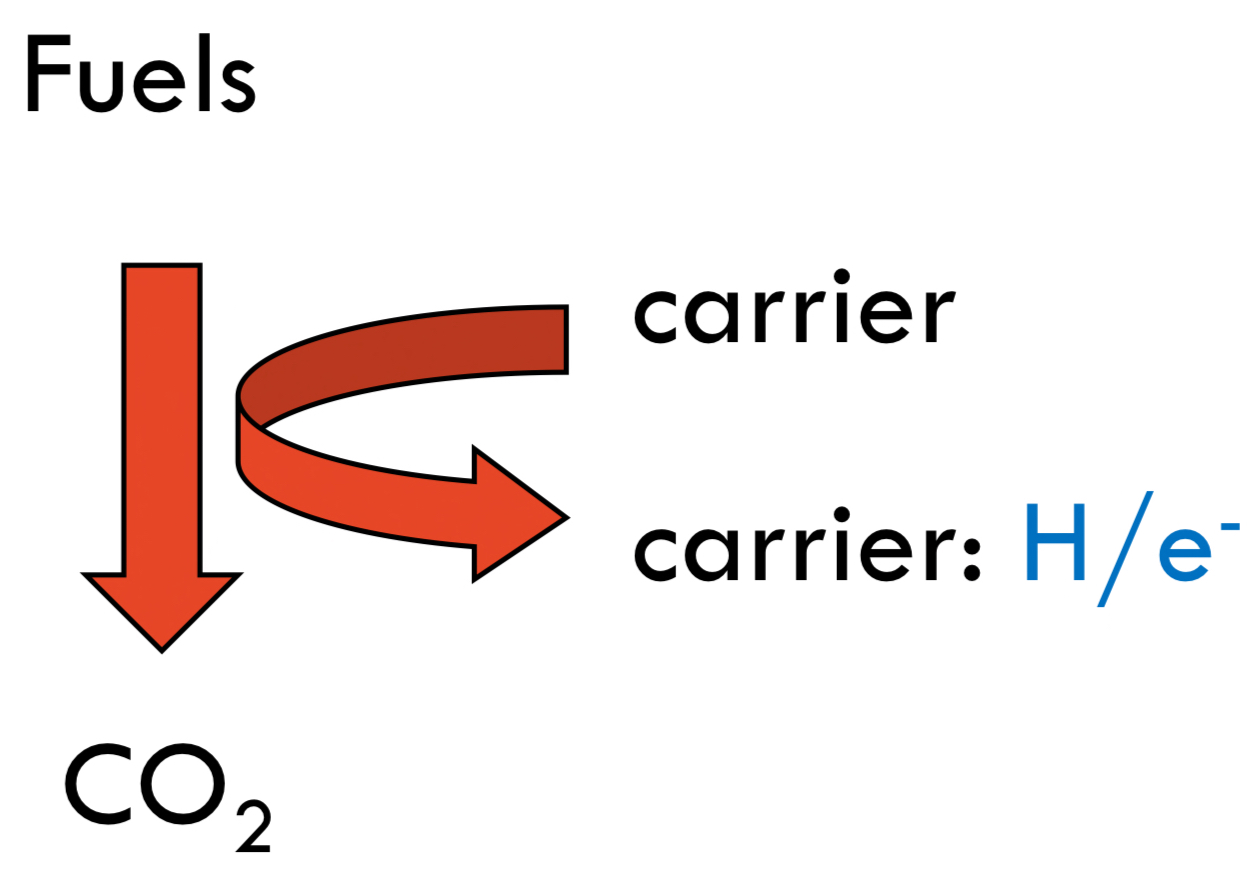
New cards
What is the role of hydrogen/electron carriers?
* H/e- carriers: **NAD+** or **FAD**
* They are also H/e-strippers.
* Both are limited supply (once they’re carrying H/e-, they can’t do any more stripping)
* They are also H/e-strippers.
* Both are limited supply (once they’re carrying H/e-, they can’t do any more stripping)
11
New cards
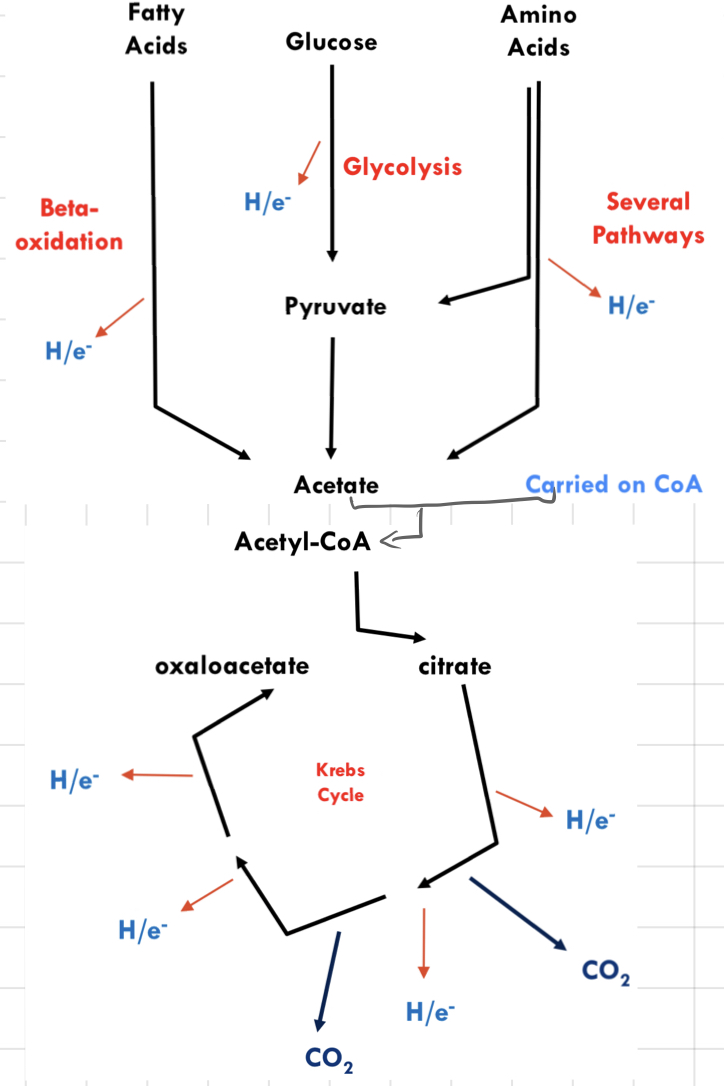
Strategy of **Fuel Oxidation**
__**Stage 1**__
* Rip H/e- out of fuels
* Fuels are broken up into **2-carbon** pieces (acetate)
__**Stage 2**__
* Rip H/e- out of acetate
* Compete for oxidation of C atoms to CO2
__**Stage 3**__
* Capturing the energy of H/e- as chemical/potential energy
* Reaction between H and O liberates lots of energy
* Formation of a **proton gradient**
* Limited by **oxidised NAD+** in resting muscle tissue
* Rip H/e- out of fuels
* Fuels are broken up into **2-carbon** pieces (acetate)
__**Stage 2**__
* Rip H/e- out of acetate
* Compete for oxidation of C atoms to CO2
__**Stage 3**__
* Capturing the energy of H/e- as chemical/potential energy
* Reaction between H and O liberates lots of energy
* Formation of a **proton gradient**
* Limited by **oxidised NAD+** in resting muscle tissue
12
New cards
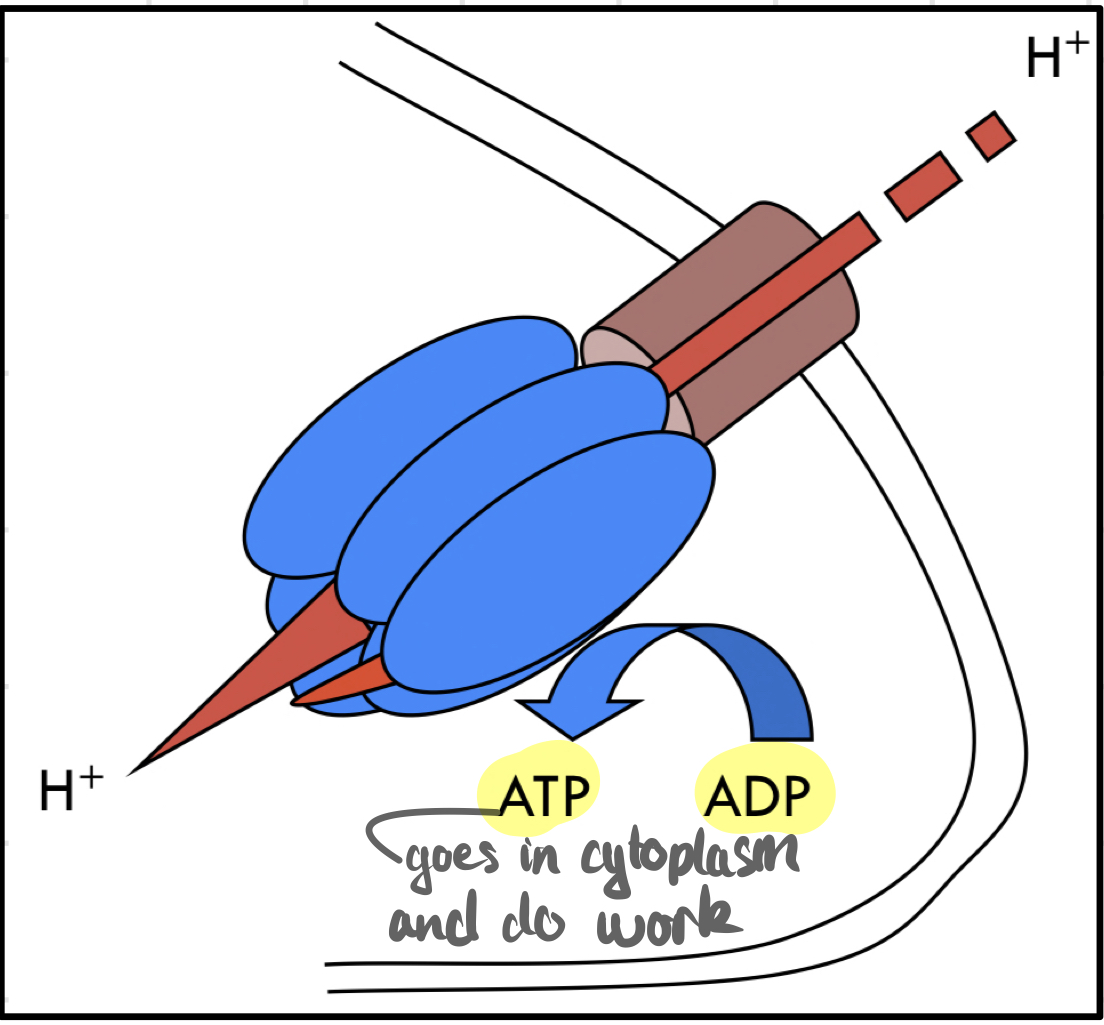
How do we make ATP with H+ gradient?
H+ flows under pressure through a channel in the inner mitochondrial membrane.
> They come in → Rotate another protein → Interact with subunits of **ATP synthase** → Generate ATP from ADP and phosphate
> They come in → Rotate another protein → Interact with subunits of **ATP synthase** → Generate ATP from ADP and phosphate
13
New cards
The 7 big concepts
1. H/e- carriers are in short supply
2. ADP is in short supply
* \[ATP\] = 5 mM.
* < 3 mM → Cells die
3. ATP is stable
4. Inner mitochondrial membrane is impermeable to H+
5. H+ only flow into the matrix if the ATP is being made
6. H+ pumps don’t work if the H+ gradient is very high
7. No H+ pumping, no H/e- movement down the ETC
14
New cards
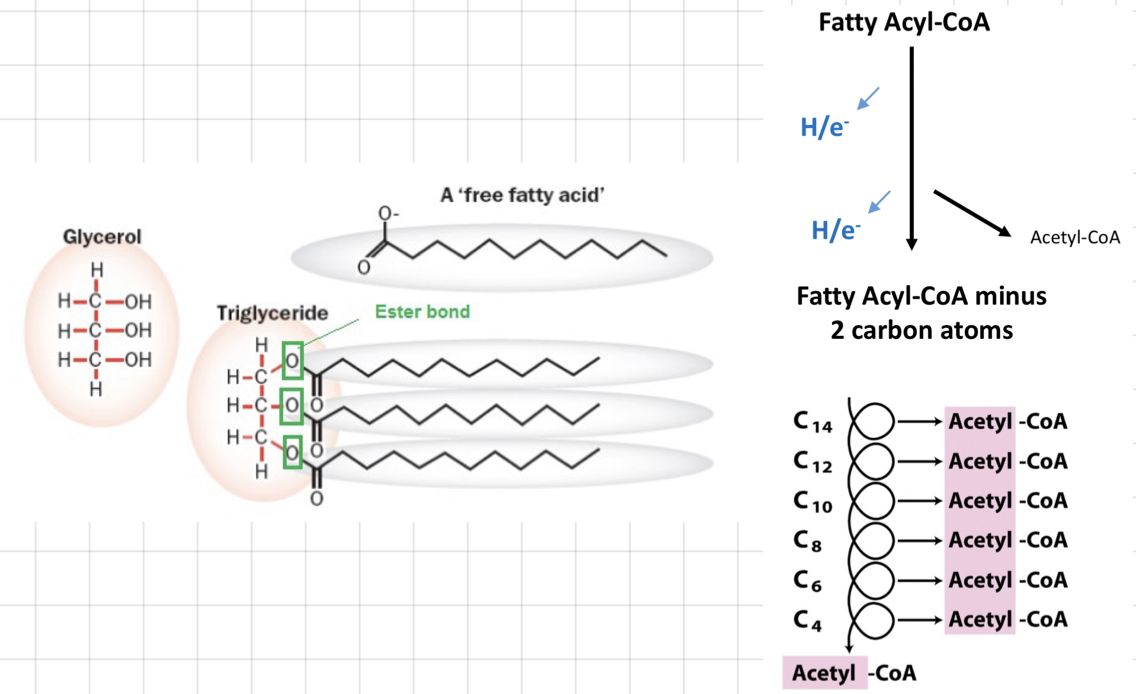
Fatty acids and ß-Oxidation
### __**Fatty Acids (FA)**__
* Nearly all C atoms are fully reduced
* Stored as **Triglyceride**
* Hydrophobic
* Very energy dense
* Huge store
* Can’t be used by brain
### __**ß-Oxidation**__
* **2 C atoms** are removed in the form of **acetyl-CoA** at the carbonyl terminal
* FAs trapped in the cytoplasm as **Fatty Acyl-CoA**
* Transported into mitochondria by H/e- carrier: **Carnitine**
> Help transport long-chain FAs into mitochondria to oxidise them to produce ATP.
* H/e- ripped out by **FAD** and **NAD+**
* FA part loses an acetate chunk
* Cycle repeats
* Nearly all C atoms are fully reduced
* Stored as **Triglyceride**
* Hydrophobic
* Very energy dense
* Huge store
* Can’t be used by brain
### __**ß-Oxidation**__
* **2 C atoms** are removed in the form of **acetyl-CoA** at the carbonyl terminal
* FAs trapped in the cytoplasm as **Fatty Acyl-CoA**
* Transported into mitochondria by H/e- carrier: **Carnitine**
> Help transport long-chain FAs into mitochondria to oxidise them to produce ATP.
* H/e- ripped out by **FAD** and **NAD+**
* FA part loses an acetate chunk
* Cycle repeats
15
New cards
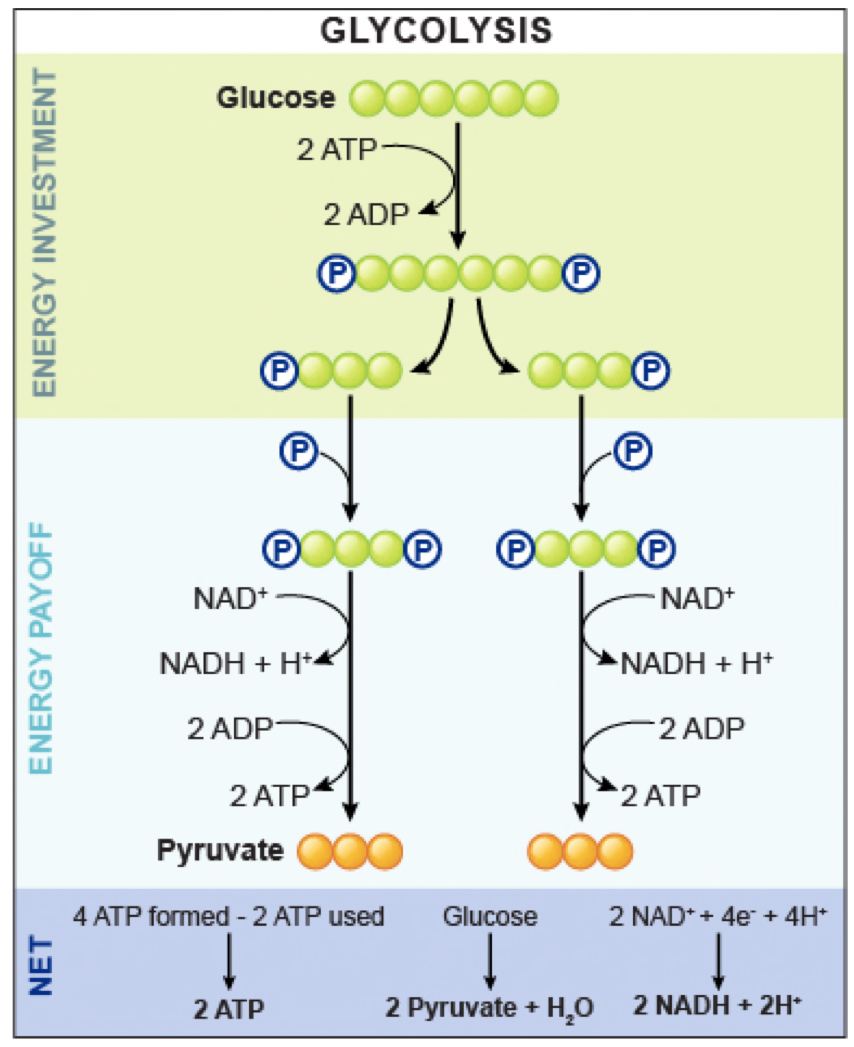
Glucose and Glucose Oxidation
### __**Glucose**__
* Reasonably reduced
* Stored as **Glycogen**
* Hydrophilic
* Low store (300g)
* Used by all tissues (esp. by the brain)
* Most readily available fuel (glucose transporters move to the cell surface)
### __**Glucose Oxidation/Glycolysis**__
* All tissues
* Wholly cytosolic
* No O2
* Very fast but inefficient
* Pyruvate must be transported into mitochondria for full oxidation.
* Reasonably reduced
* Stored as **Glycogen**
* Hydrophilic
* Low store (300g)
* Used by all tissues (esp. by the brain)
* Most readily available fuel (glucose transporters move to the cell surface)
### __**Glucose Oxidation/Glycolysis**__
* All tissues
* Wholly cytosolic
* No O2
* Very fast but inefficient
* Pyruvate must be transported into mitochondria for full oxidation.
16
New cards
Protein
* Channel into pyruvate, acetyl-CoA or Krebs cycle
* Need to dispose of amine groups
* Store 5-20 kg
* Last alternative fuel source
* Don’t store protein since all of it has its functions
* Making protein requires lots of energy
* Need to dispose of amine groups
* Store 5-20 kg
* Last alternative fuel source
* Don’t store protein since all of it has its functions
* Making protein requires lots of energy
17
New cards
Muscle contraction and ATP
* Use ATP
* Actin and Myosin interaction - Filaments sliding across each other
* The faster the contraction, the faster ATP use
* Use ATP even at rest
* Maintain ion gradients
* Sacroplasmic reticulum and CA2+
* Compared to resting muscle cells, actively contracting muscle tissue has a ***higher rate of NAD+/NADH turnover***
* Actin and Myosin interaction - Filaments sliding across each other
* The faster the contraction, the faster ATP use
* Use ATP even at rest
* Maintain ion gradients
* Sacroplasmic reticulum and CA2+
* Compared to resting muscle cells, actively contracting muscle tissue has a ***higher rate of NAD+/NADH turnover***
18
New cards
How many types of muscle?
### @@__**Type 1**__@@ __**- Red + Slow**__
* Contract @@slow@@
* @@Many@@ mitochondria
* Good blood supply
### ==__**Type 2b**__== __**- White + Fast**__
* Contract ==rapid==
* ==Few== mitochondria
* ==Poor== blood supply
* Packed full of contractile filaments
* Contract @@slow@@
* @@Many@@ mitochondria
* Good blood supply
### ==__**Type 2b**__== __**- White + Fast**__
* Contract ==rapid==
* ==Few== mitochondria
* ==Poor== blood supply
* Packed full of contractile filaments
19
New cards
Pathways of Fuel Oxidation
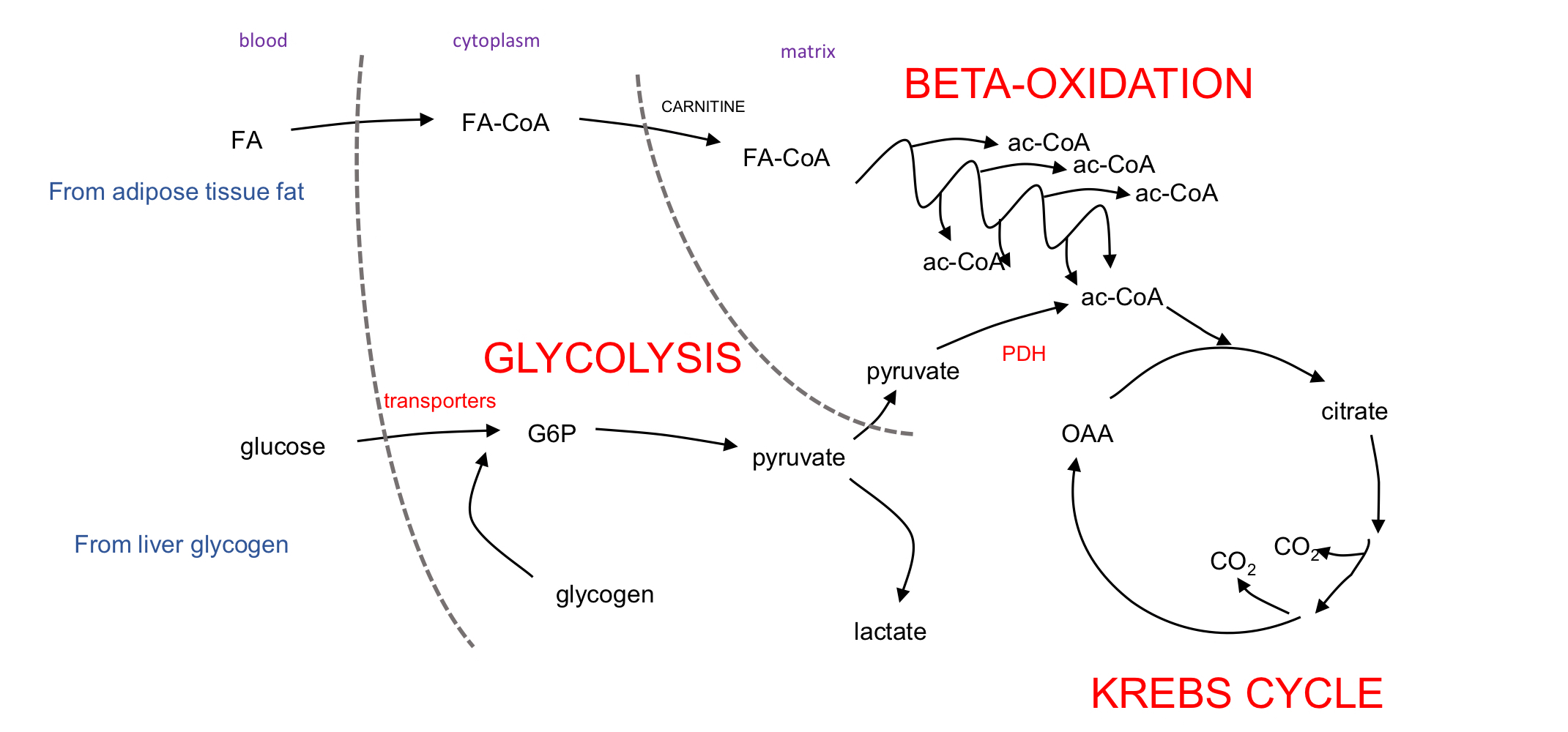
20
New cards
What happen once ATP is used?
* ↑ rate of ATP generation
* Once ATP is used → Greater availability of ADP
* ↑ ATP synthase
* ↑ ETC
* ↑ H/e- carriers/trippers
* ↑ Fuel oxidation
* Proton gradients diffuse faster, ie. H+ flow back into the matrix more quickly.
* ↓ Blood glucose
* Need to keep at 5 nM for brain
* Glucose homeostasis
* Once ATP is used → Greater availability of ADP
* ↑ ATP synthase
* ↑ ETC
* ↑ H/e- carriers/trippers
* ↑ Fuel oxidation
* Proton gradients diffuse faster, ie. H+ flow back into the matrix more quickly.
* ↓ Blood glucose
* Need to keep at 5 nM for brain
* Glucose homeostasis
21
New cards
What happen to energy in the body during __**gentle exercise**__?
* Glucose is used → Cannot be recycled directly
* After several minutes, __**fatty acids**__ take over
* Glucose stores (as glycogen) are **limited**
* Cannot convert FAs into glucose
* FAs substitute for glucose as a fuel
* FAs prevent glucose from being wastefully oxidised
* __**Glucose**__ still gets into the muscles until lactate is reached
* __**Lactate**__ (produced from pyruvate) goes to the liver for re-synthesis of glucose
* @@**Gluconeogenesis**@@
* Low insulin and High glucagon → Stimulate
* Glycogen breakdown in liver.
* Fat breakdown in white adipose tissue.
* After several minutes, __**fatty acids**__ take over
* Glucose stores (as glycogen) are **limited**
* Cannot convert FAs into glucose
* FAs substitute for glucose as a fuel
* FAs prevent glucose from being wastefully oxidised
* __**Glucose**__ still gets into the muscles until lactate is reached
* __**Lactate**__ (produced from pyruvate) goes to the liver for re-synthesis of glucose
* @@**Gluconeogenesis**@@
* Low insulin and High glucagon → Stimulate
* Glycogen breakdown in liver.
* Fat breakdown in white adipose tissue.
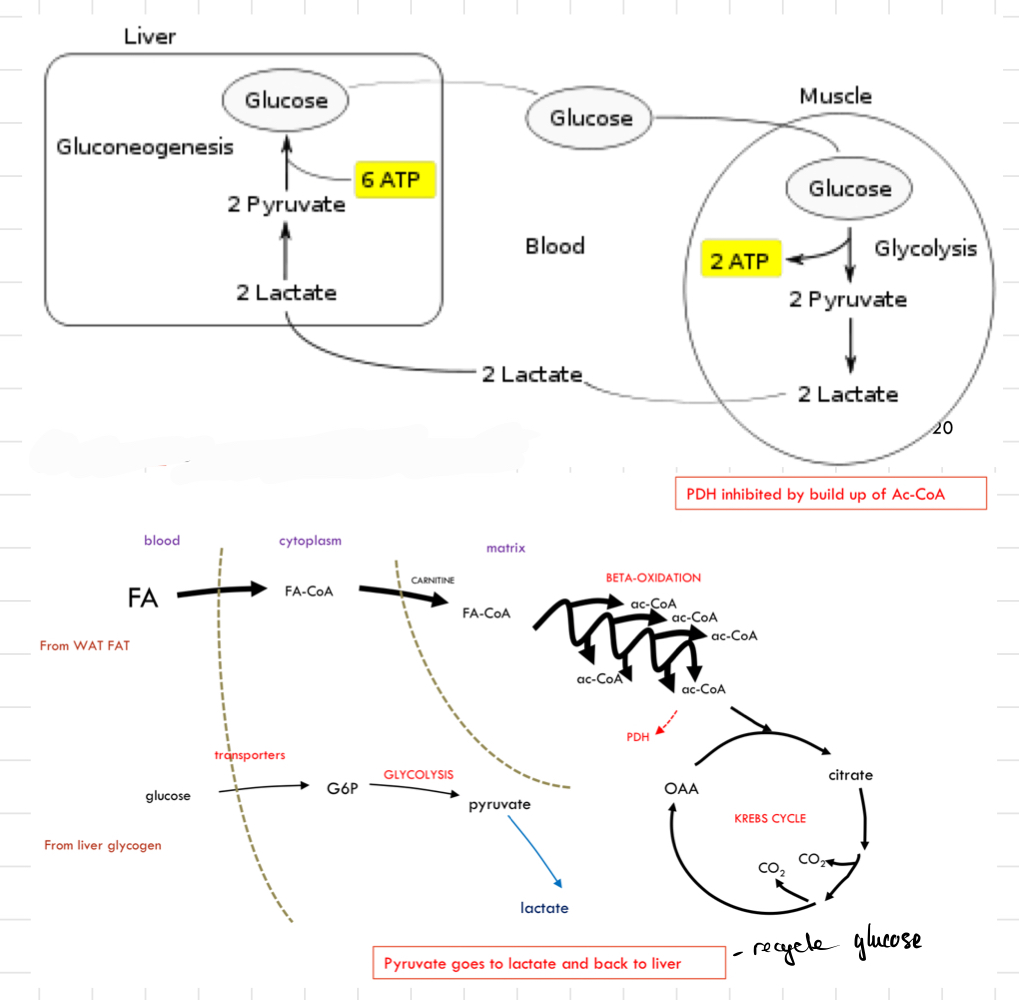
22
New cards
What happen to energy in the body during __**moderate exercise**__?
* ↑ Pace → ↑ Rate of FA utilisation
↳ Soon, FA oxidation enzymes are at their peak
* FA oxidation cannot maintain ATP production alone (inhibition on glucose oxidation is removed)
* __**Glucose oxidation**__ occurs
* Less glucose recycling
* A faster depletion of liver glycogen
* Mixture of FA oxidation and glucose oxidation
* Further ↑ pace by ↑ glucose oxidation
↳ FA oxidation going at full speed
↳ Soon, FA oxidation enzymes are at their peak
* FA oxidation cannot maintain ATP production alone (inhibition on glucose oxidation is removed)
* __**Glucose oxidation**__ occurs
* Less glucose recycling
* A faster depletion of liver glycogen
* Mixture of FA oxidation and glucose oxidation
* Further ↑ pace by ↑ glucose oxidation
↳ FA oxidation going at full speed
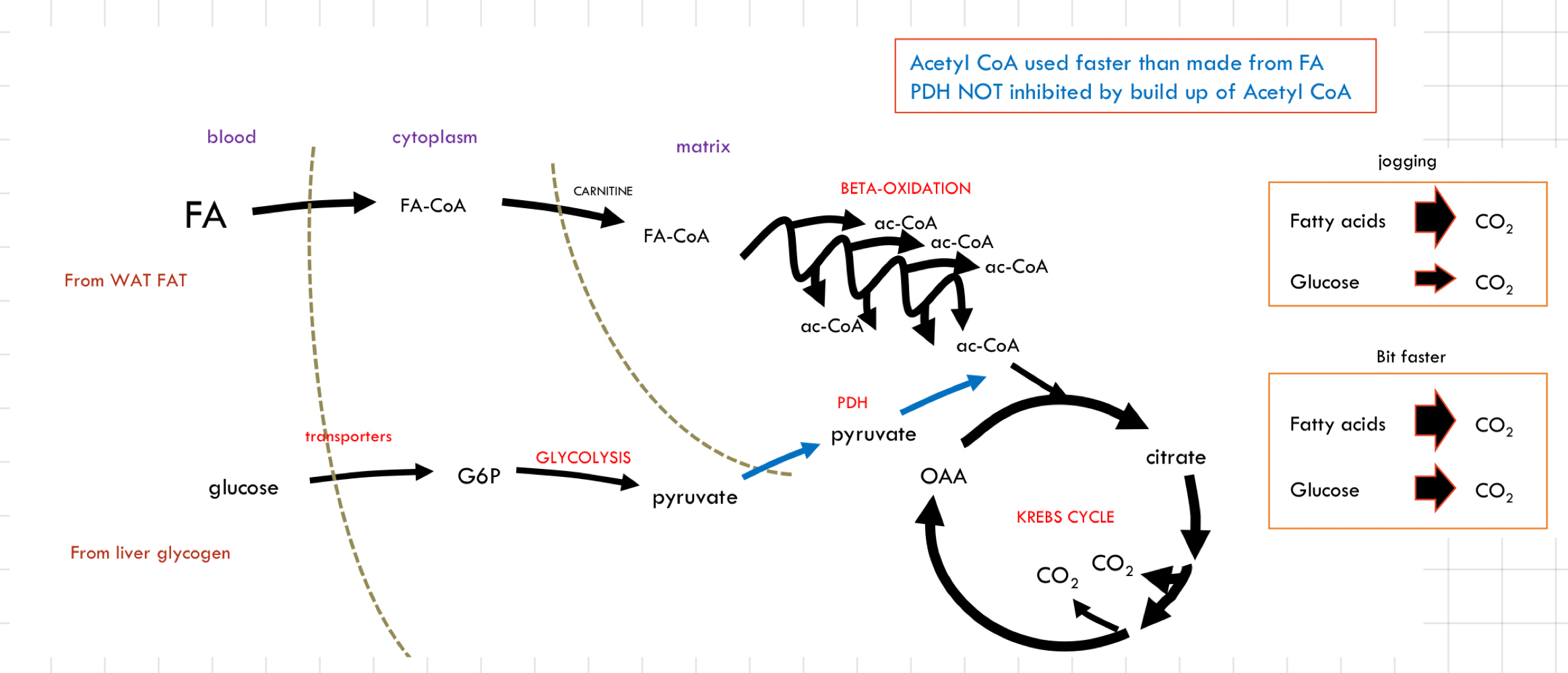
23
New cards
What happen to energy in the body during __**strenuous exercise**__?
* Muscle glycogen is now broken down
↳ **Endogenously stored**
* Limits on blood glucose oxidation
* The supply and transport of blood cannot keep up with the demand
* FAs are still going as fast as they can
↳ **Endogenously stored**
* Limits on blood glucose oxidation
* The supply and transport of blood cannot keep up with the demand
* FAs are still going as fast as they can
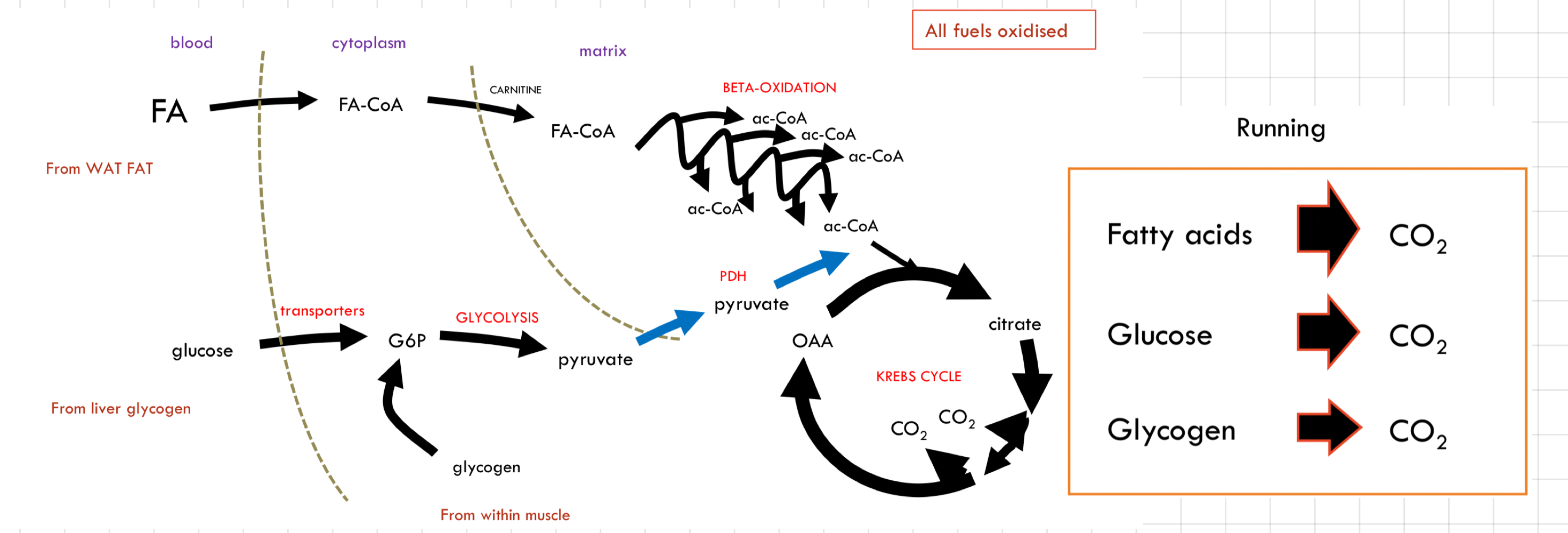
24
New cards
What happen to energy in the body during __**very strenuous exercise**__?
* ATP production cannot be met by oxidative phosphorylation
↳ Mitochondrial processes are too slow
* Extra glycolysis boost needed
* Glycolysis is very fast but inefficient
* ↑ Blood lactate levels
* Glucose must come from muscle glycogen
* Transport already at max
↳ Mitochondrial processes are too slow
* Extra glycolysis boost needed
* Glycolysis is very fast but inefficient
* ↑ Blood lactate levels
* Glucose must come from muscle glycogen
* Transport already at max
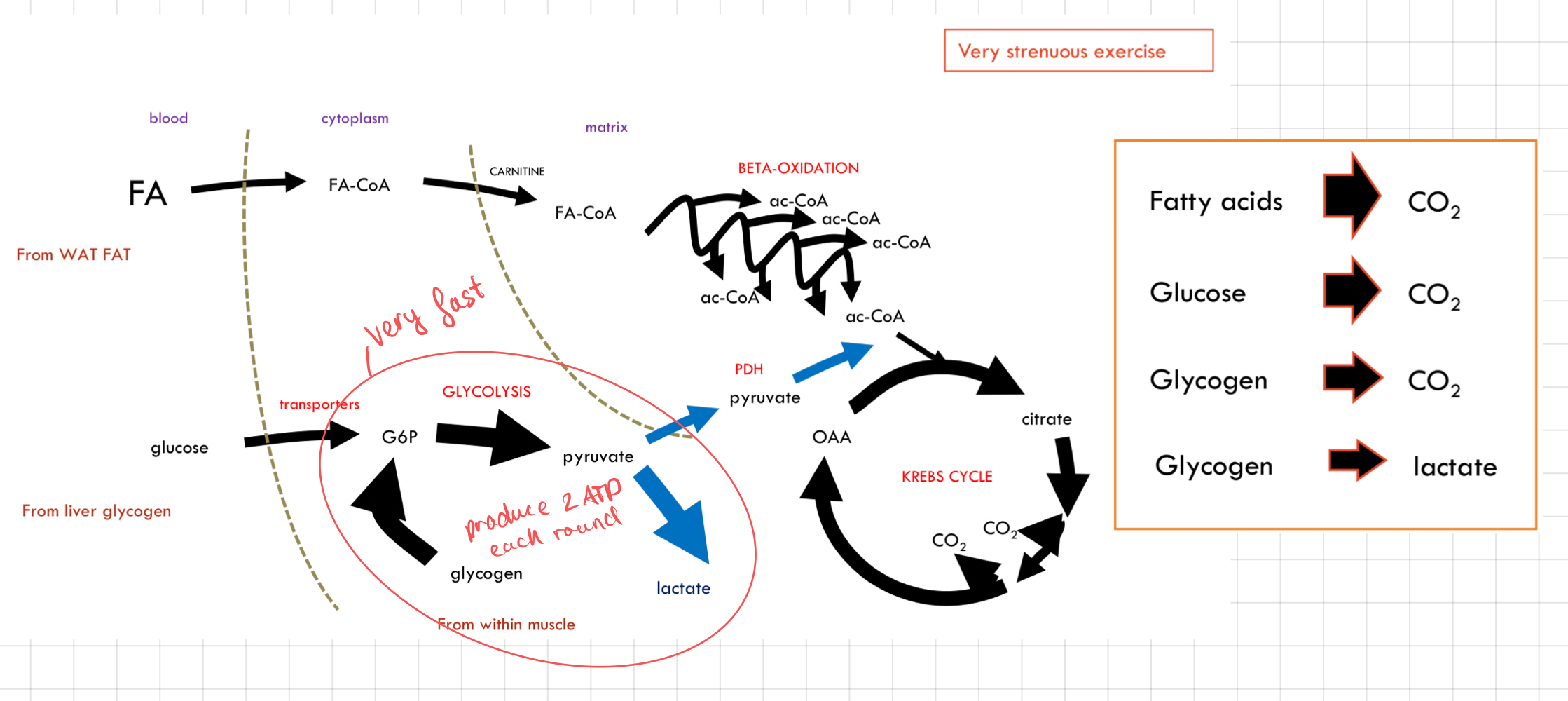
25
New cards
What happen to energy in the body during __**sprinting**__?
* Use Type 2b muscles → Very rapid ATP consumption.
* **Don’t use**
* FAS → Poor O2 supply, low mitochondria
* Blood glucose → Delay in transporter recruitment, poor fuel supply
* Glycolysis to lactate is very fast but creates a problem
↳ Lead to **lactic acidosis** due to lactate accumulation → Muscle fatigue and disrupt cellular processes
* Lots of lactate produce very quickly
* Poor blood supply takes away
* ATP regeneration is so inefficient
* Only 2 ATPs per glucose
* Regeneration of H/e- carrier (NAD+)
* **Don’t use**
* FAS → Poor O2 supply, low mitochondria
* Blood glucose → Delay in transporter recruitment, poor fuel supply
* Glycolysis to lactate is very fast but creates a problem
↳ Lead to **lactic acidosis** due to lactate accumulation → Muscle fatigue and disrupt cellular processes
* Lots of lactate produce very quickly
* Poor blood supply takes away
* ATP regeneration is so inefficient
* Only 2 ATPs per glucose
* Regeneration of H/e- carrier (NAD+)
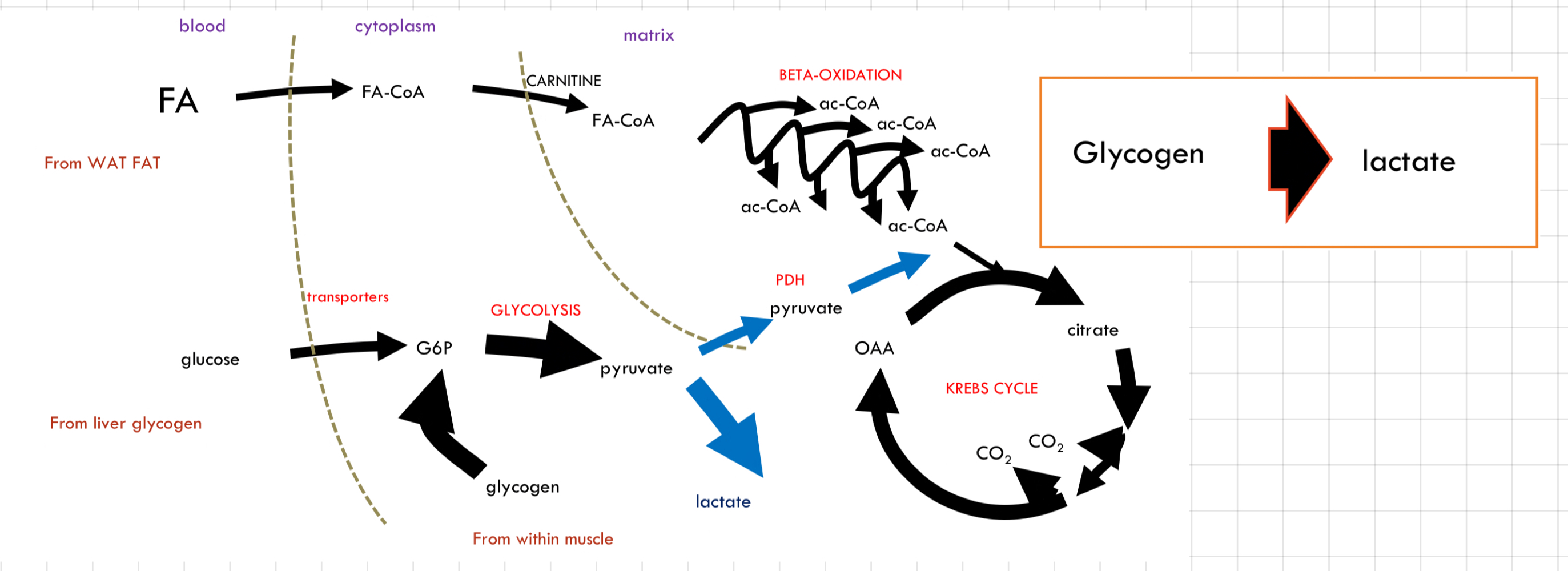
26
New cards
Why glycogen is important?
* ATP can only be produced by FA oxidation when glycogen is depleted
* Power output is lower when using only FAs
* Cannot sprint if there’s no glycogen
* Glucagon quickly provides glucose for energy production.
* Power output is lower when using only FAs
* Cannot sprint if there’s no glycogen
* Glucagon quickly provides glucose for energy production.
27
New cards
Creatine Phosphate (CP)
* An instant store of ATP (< 5 sec supply 15 nM)
* Creatine supplements ↑ CP levels
* Creatine increases the availability of creatine in the muscles, allowing higher levels of phosphocreatine.
### Creatine Phosphate + ADP → ATP + Creatine
* Creatine supplements ↑ CP levels
* Creatine increases the availability of creatine in the muscles, allowing higher levels of phosphocreatine.
### Creatine Phosphate + ADP → ATP + Creatine
28
New cards
Fatty Acid Oxidation/ß-Oxidation
Occurs in the ß-carbon atom
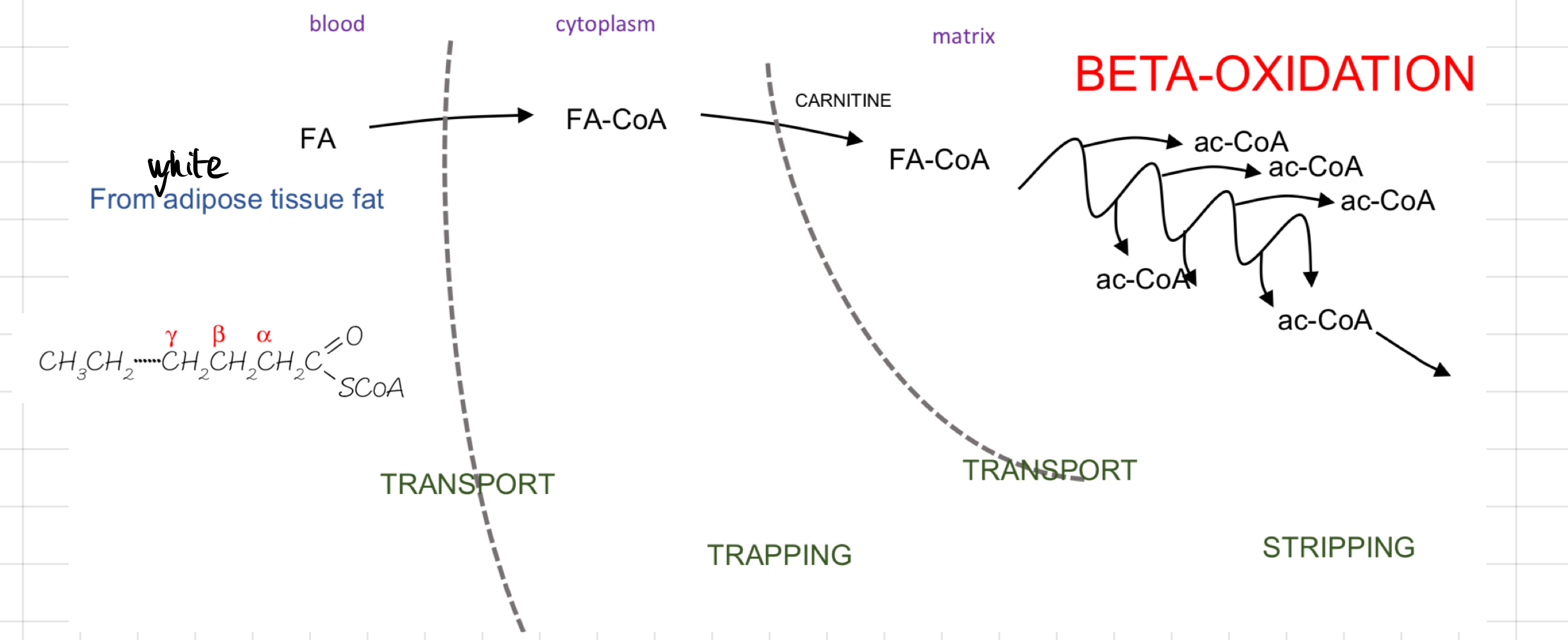
29
New cards
Transport of Fatty Acid
* Transported through the bloodstream bound to a protein called @@**albumin (ab)**@@.
* The cells produce ATP after taking up fatty acids and undergoing beta-oxidation.
* The cells produce ATP after taking up fatty acids and undergoing beta-oxidation.
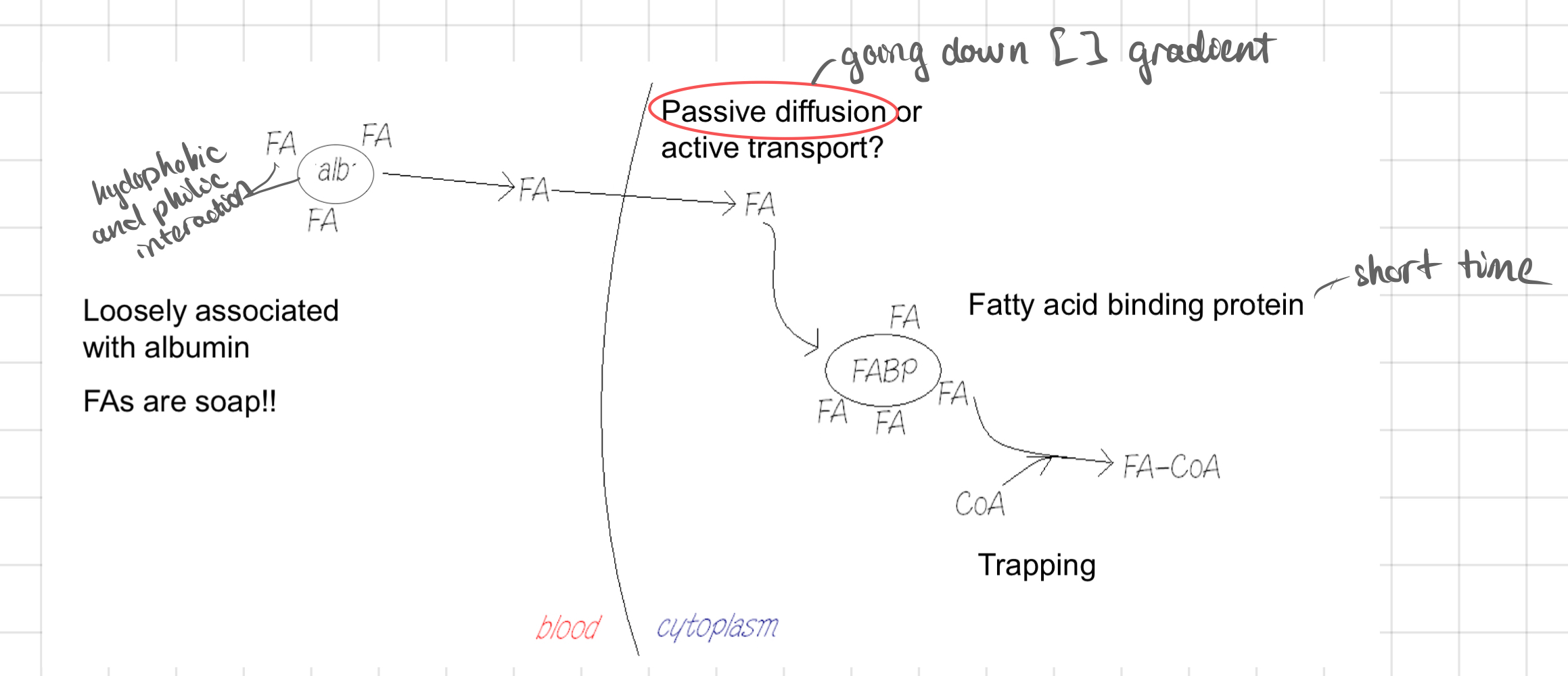
30
New cards
Transport of Fatty Acid: Mitochondria
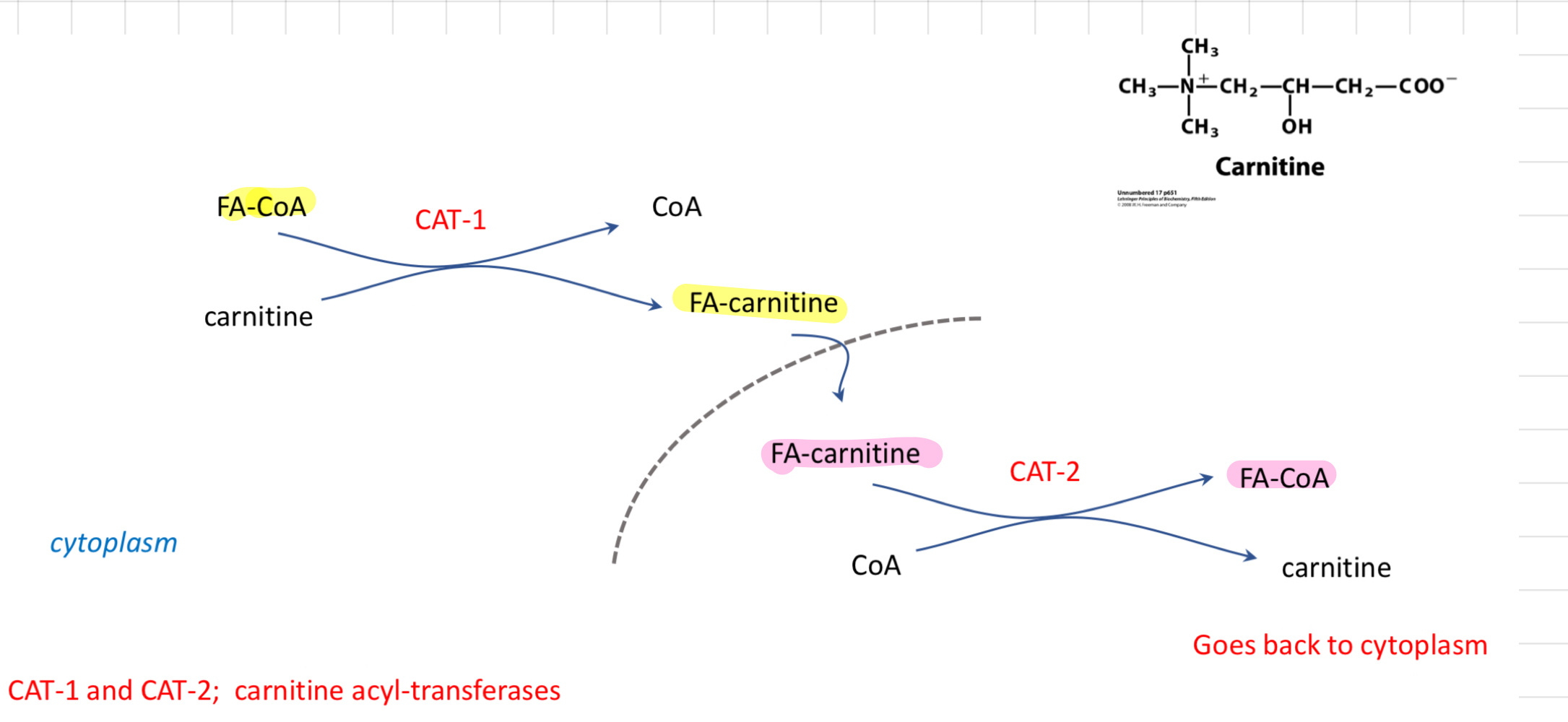
31
New cards
Trapping of Fatty Acid
* FA trapped by attachment to CoA
↳ The CoA will always be attached from now on
➙ Activates FA
* Requires lots of energy
* ATP is __**not**__ converted into ADP, but **AMP**
* **Pyrophosphate** is hydrolysed, pulling reaction over
* **Coenzyme A**
* Carrier of acyl group
* Great for trapping metabolites within the cell
↳ The CoA will always be attached from now on
➙ Activates FA
* Requires lots of energy
* ATP is __**not**__ converted into ADP, but **AMP**
* **Pyrophosphate** is hydrolysed, pulling reaction over
* **Coenzyme A**
* Carrier of acyl group
* Great for trapping metabolites within the cell
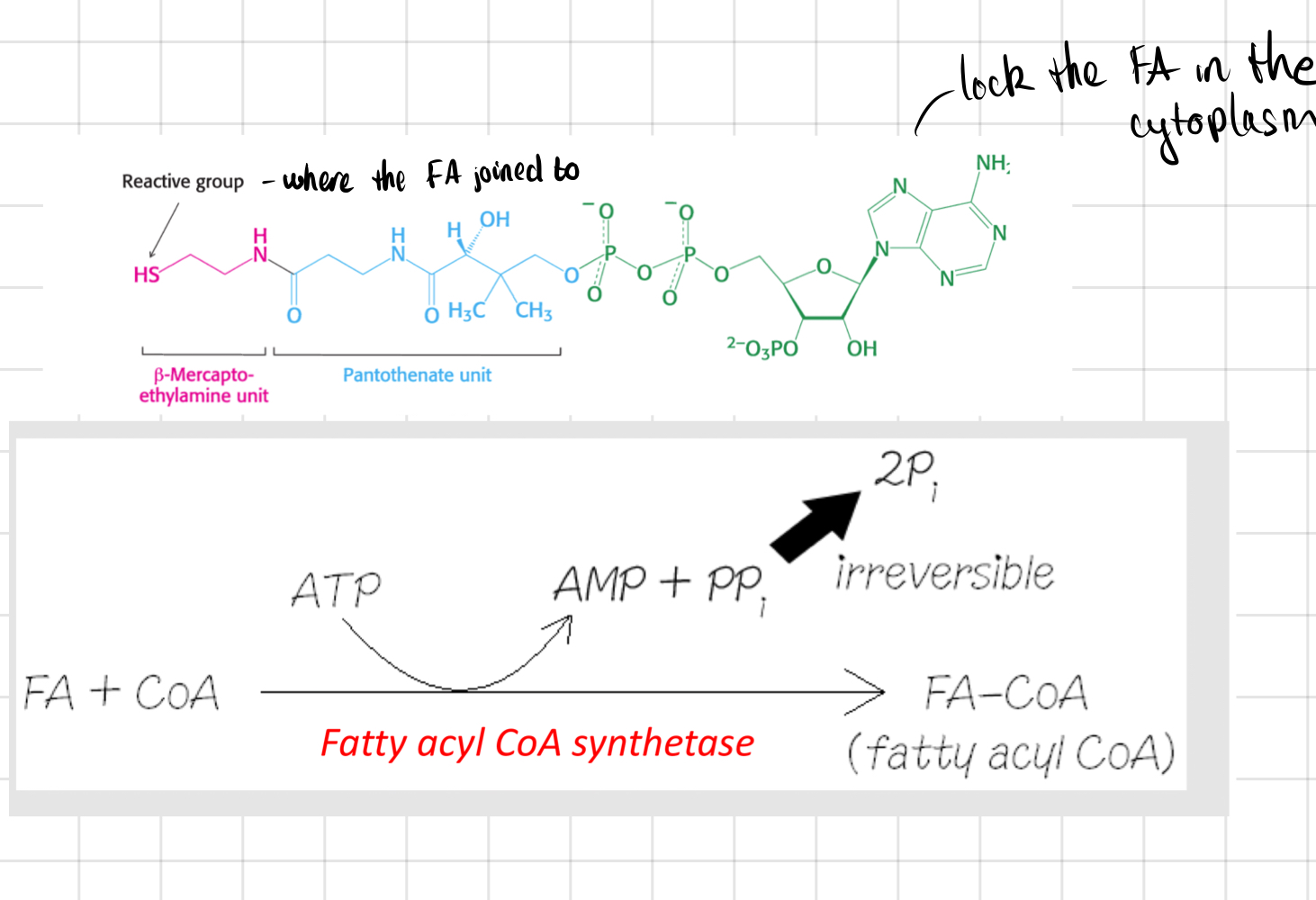
32
New cards
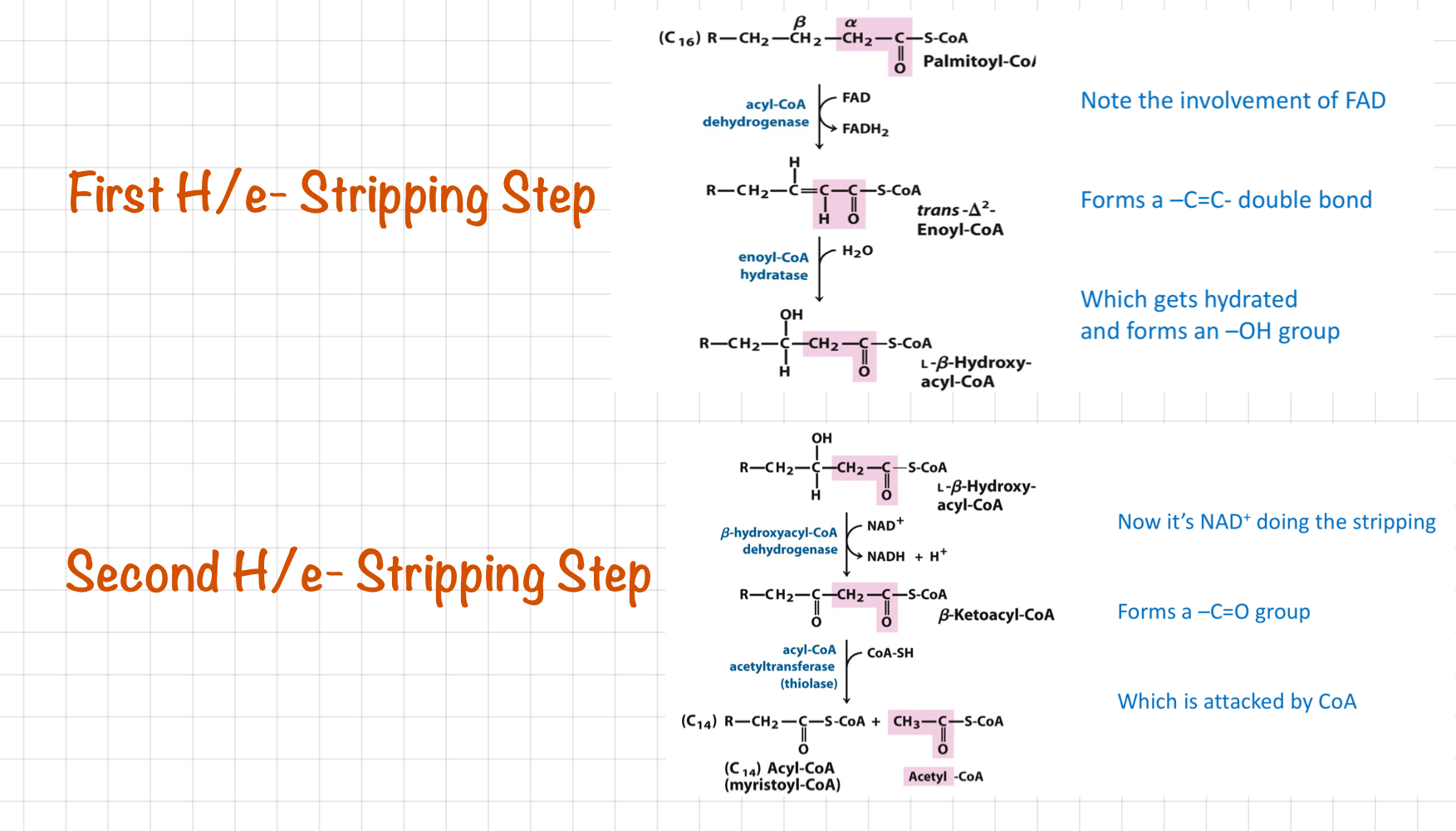
First and Second Stripping Steps
33
New cards
ß-Oxidation
* FAs trapped in the cytoplasm as __**Fatty Acyl-CoA**__
* Transported into mitochondria - Carrier: **Carnitine**
* H/e- ripped out by FAD and NAD+
* FA part loses an **acetate chunk**
* Cycle repeats
* Each round of ß-oxidation gives
* 1 acetyl CoA
* 1 NADH
* 1 FADH2
* Transported into mitochondria - Carrier: **Carnitine**
* H/e- ripped out by FAD and NAD+
* FA part loses an **acetate chunk**
* Cycle repeats
* Each round of ß-oxidation gives
* 1 acetyl CoA
* 1 NADH
* 1 FADH2
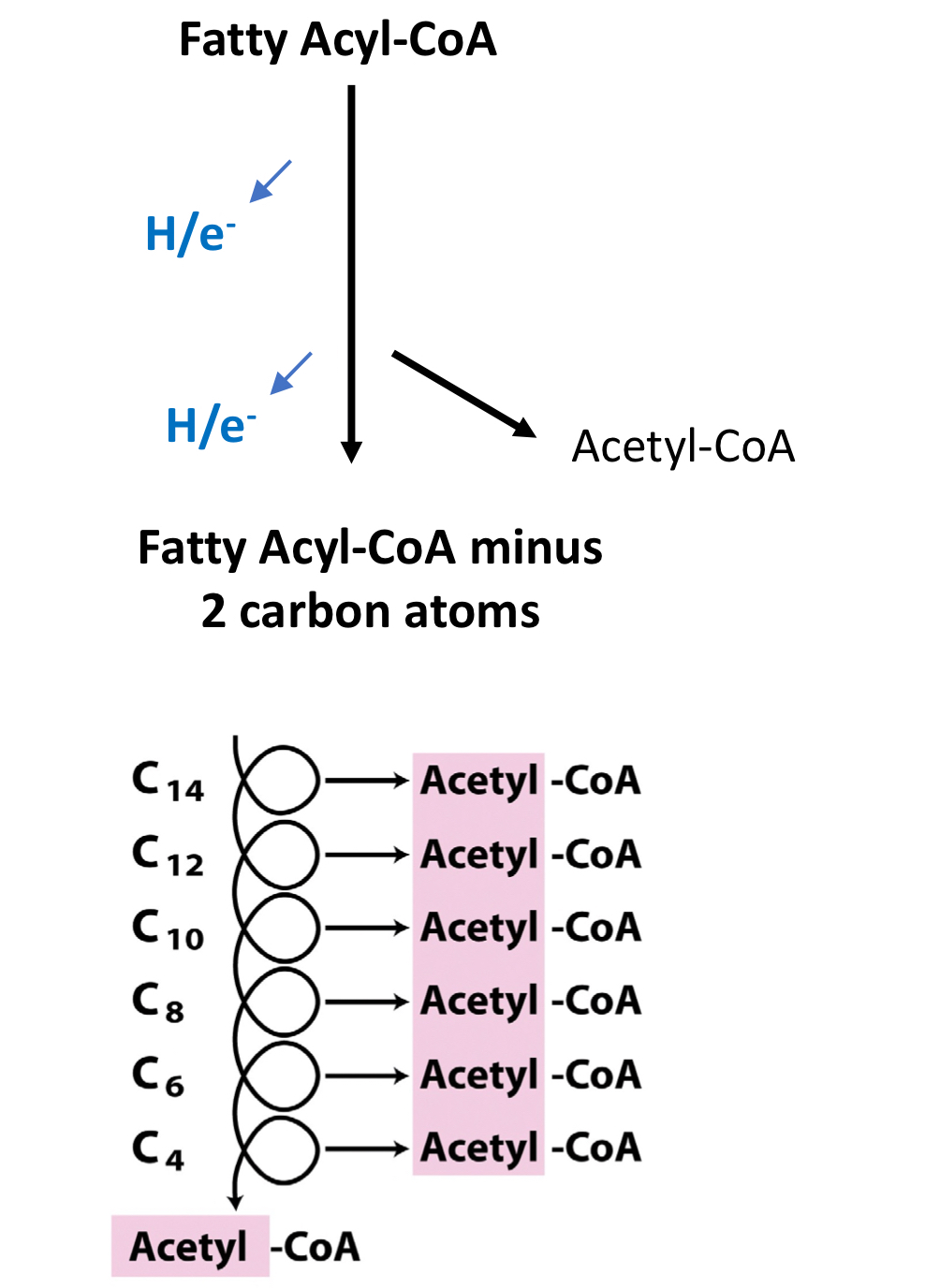
34
New cards
Glycolysis
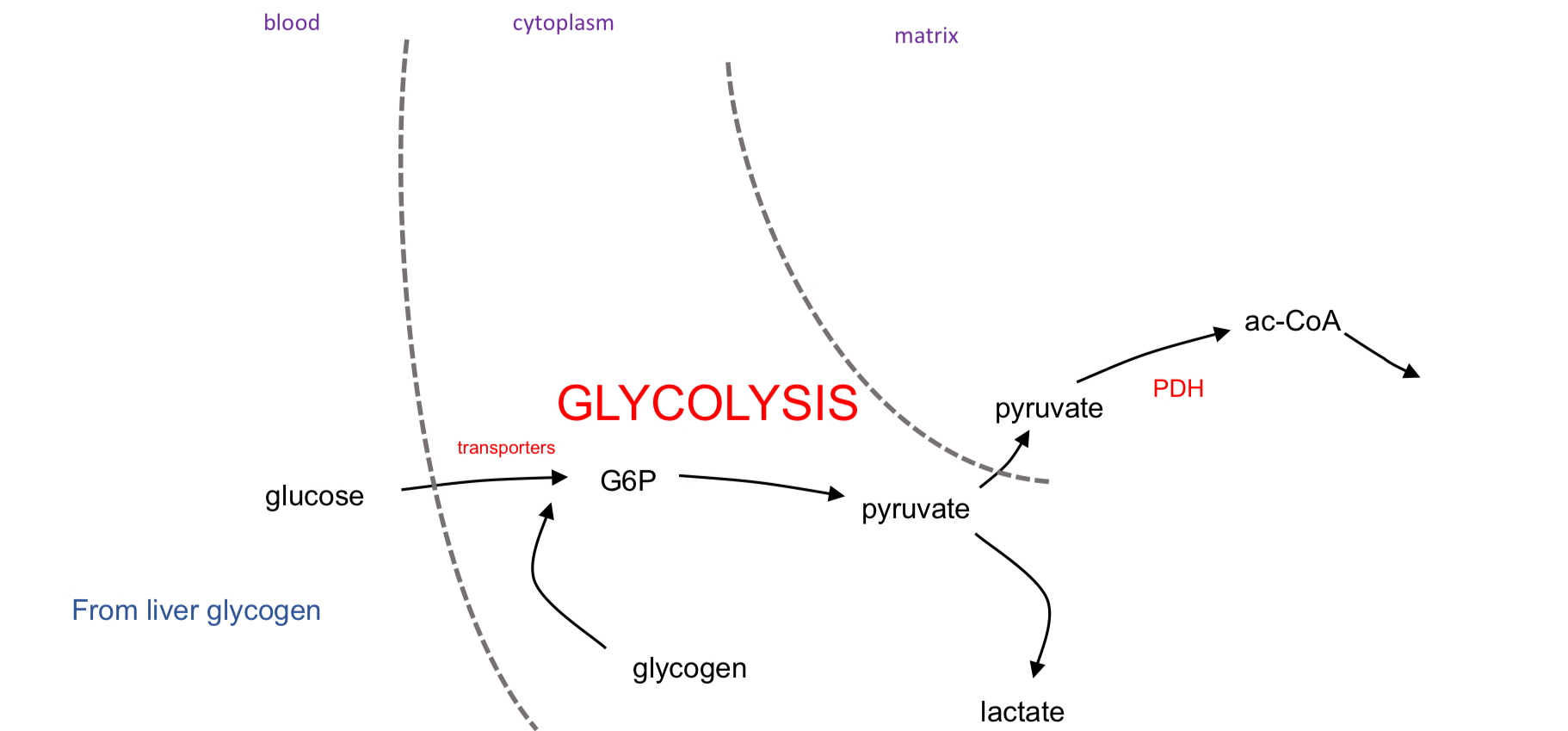
35
New cards
Glucose Uptake
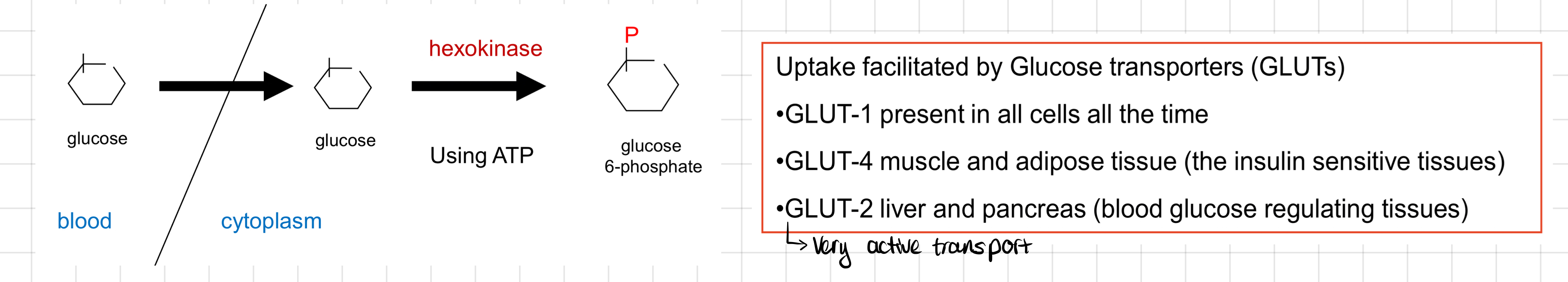
36
New cards
Early Glycolysis or **Investment Phase**
* Requires two ATP molecules to prepare glucose for further breakdown.
* It involves phosphorylation and rearrangement steps to convert glucose into **fructose-1,6-bisphosphate**.
* It involves phosphorylation and rearrangement steps to convert glucose into **fructose-1,6-bisphosphate**.
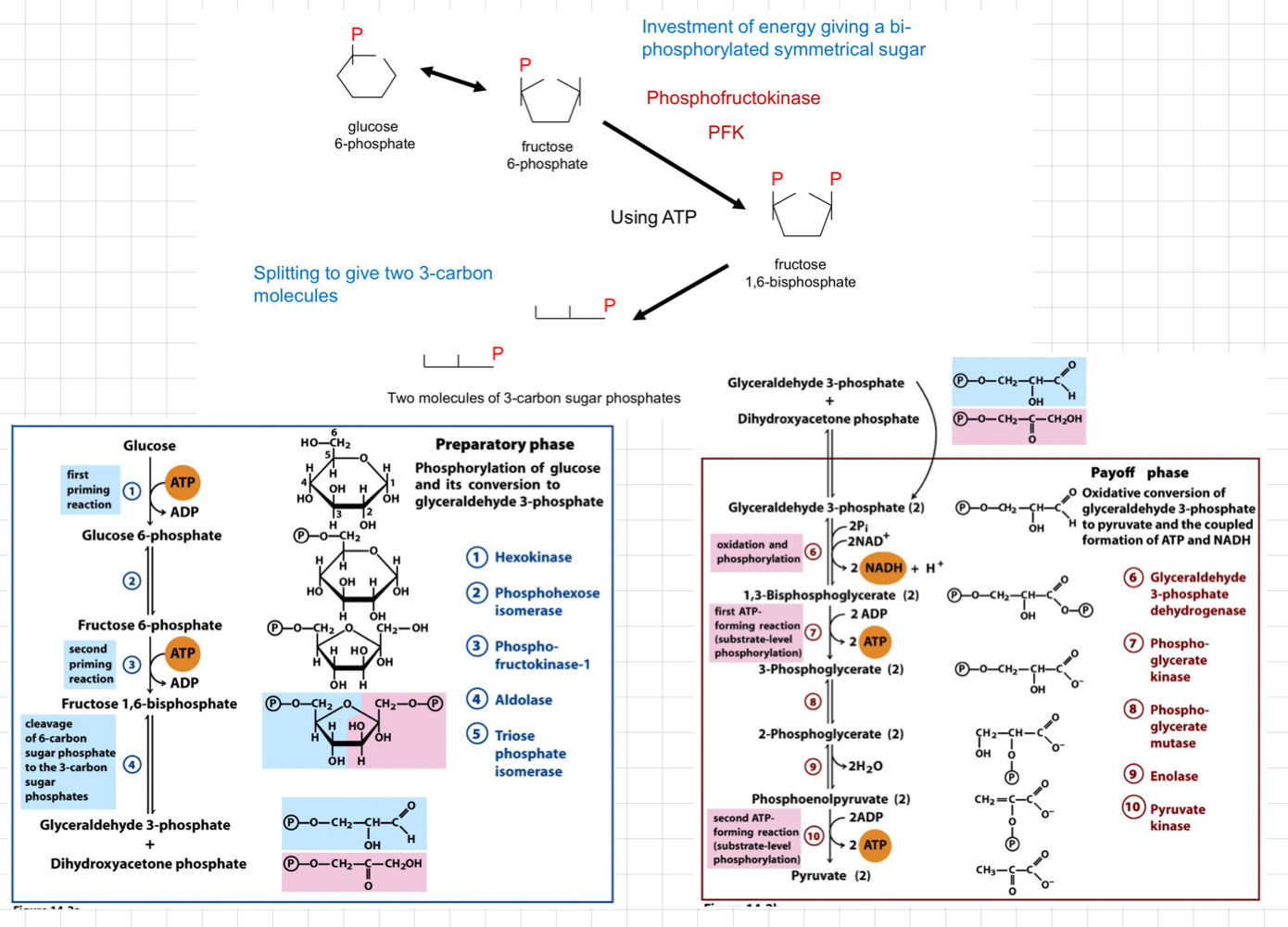
37
New cards
Second Glycolysis or **Return Phase**
* Conversion of fructose-1,6-bisphosphate into 2 molecules of **glyceraldehyde-3-phosphate (G3P)**.
* Pyruvate is produced through an enzymatic reaction that converts G3P molecules into ATP and NADH.
* Pyruvate is produced through an enzymatic reaction that converts G3P molecules into ATP and NADH.
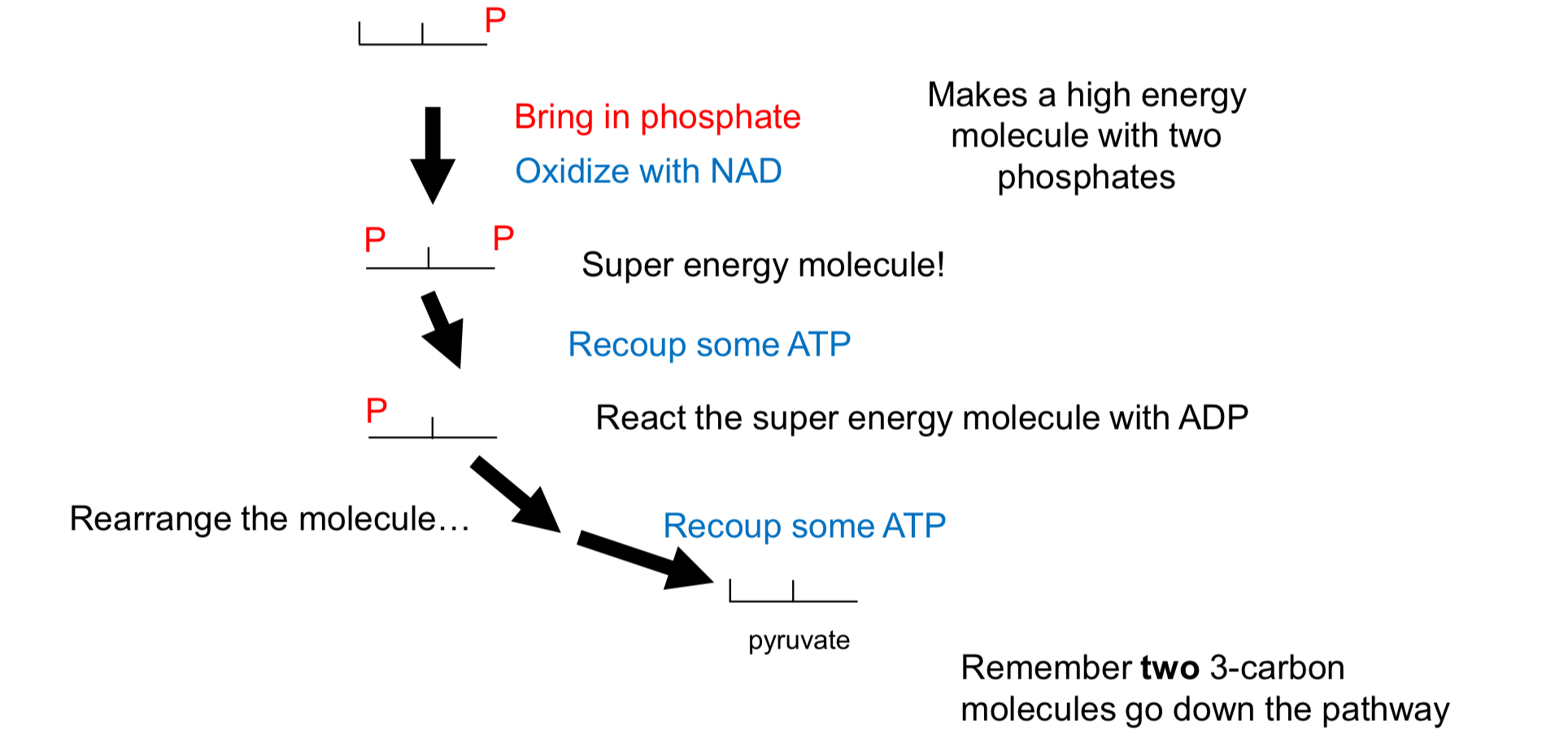
38
New cards
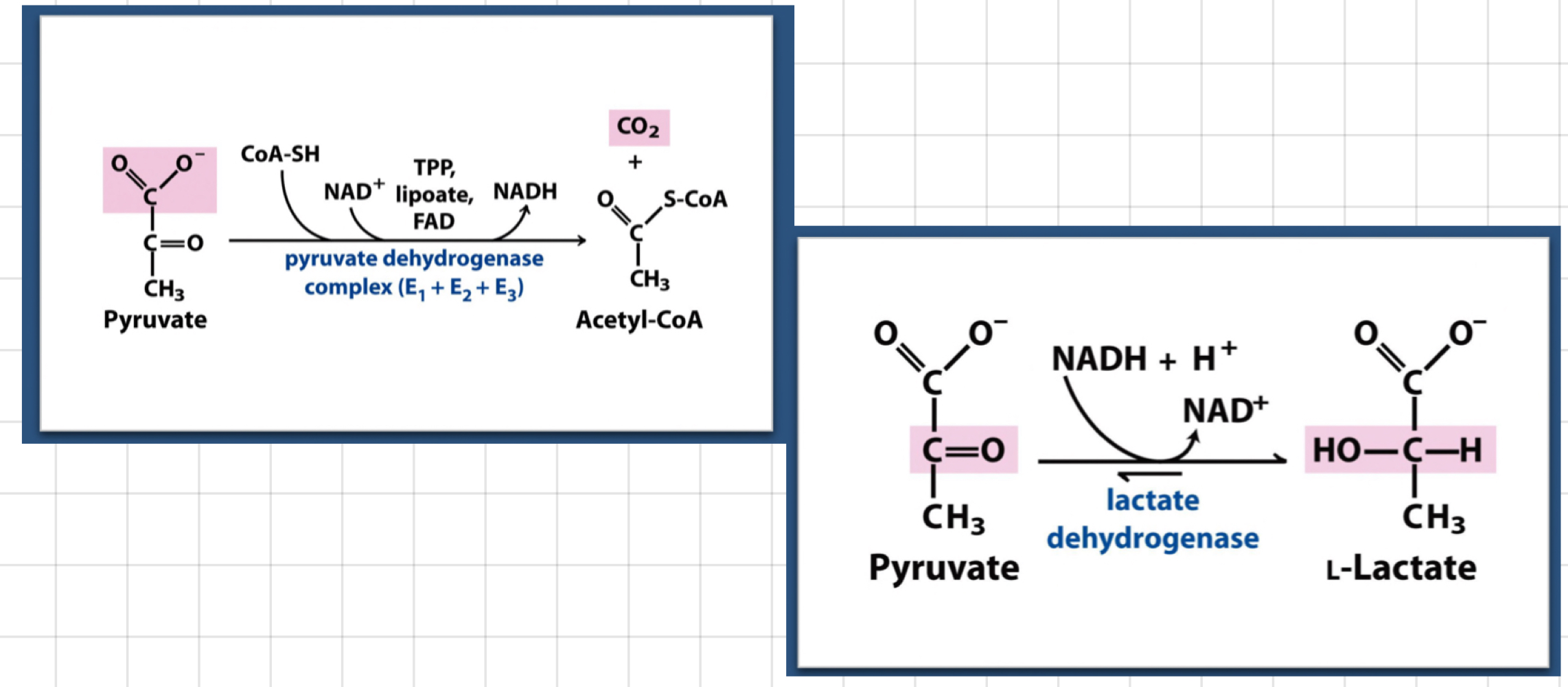
What happen completing glycolysis?
* 2 ATP, 2 pyruvate and 2 NADH (need to generate NAD+)
* Fate of pyruvate (Aerobic and Anaerobic)
* Get more ATP from full oxidation of pyruvate
* Need to transport into mitochondria
* Oxidise with **pyruvate dehydrogenase (PDH)**
* Reoxidise NADH quickly → Important
* Maintain the supply of NAD+
* Lactate production
* Alcohol production (in yeast)
* Keep everything cytosolic
* Fate of pyruvate (Aerobic and Anaerobic)
* Get more ATP from full oxidation of pyruvate
* Need to transport into mitochondria
* Oxidise with **pyruvate dehydrogenase (PDH)**
* Reoxidise NADH quickly → Important
* Maintain the supply of NAD+
* Lactate production
* Alcohol production (in yeast)
* Keep everything cytosolic
39
New cards
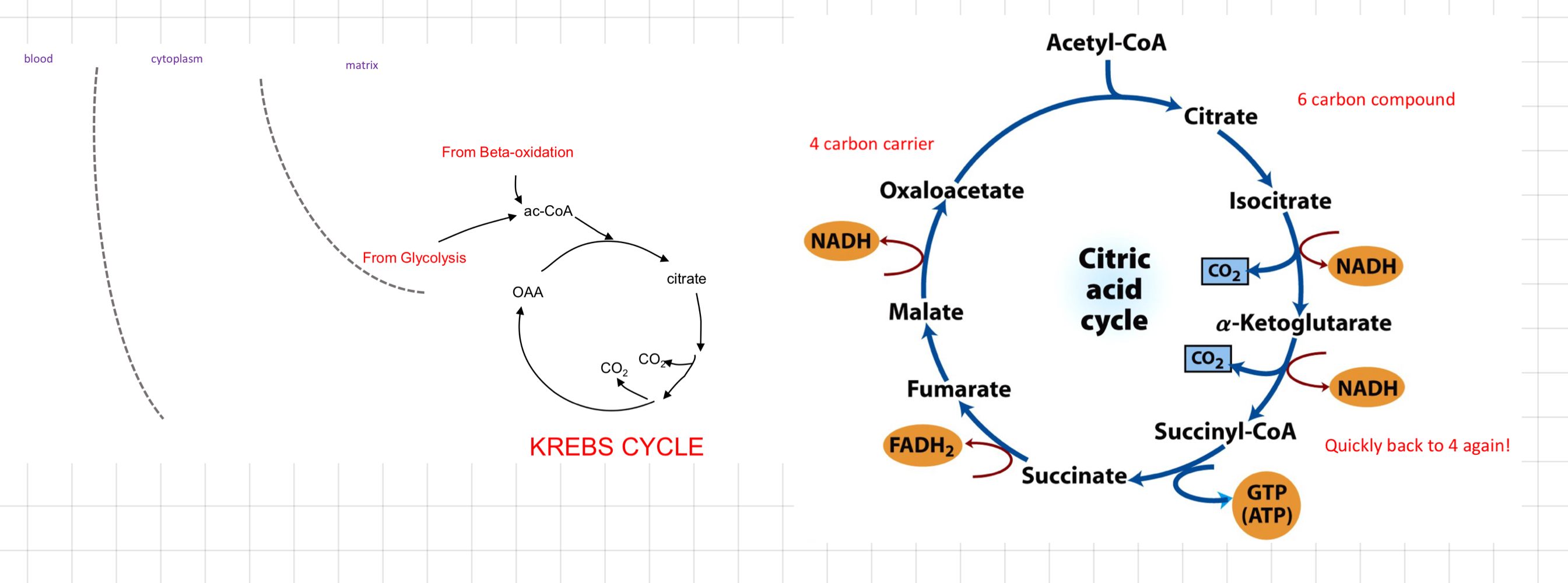
The Krebs Cycle
* Substrate: Acetyl CoA
↳ From FA oxidation and/or glucose oxidation
* Everything is in mitochondria
* Strategy
* Completely oxidise acetate carbons to CO2
* Produce lots of NADH, FADH2 and even ATP (not directly)
* Performing the reactions on a carrier molecule
* Regenerate the carrier
↳ From FA oxidation and/or glucose oxidation
* Everything is in mitochondria
* Strategy
* Completely oxidise acetate carbons to CO2
* Produce lots of NADH, FADH2 and even ATP (not directly)
* Performing the reactions on a carrier molecule
* Regenerate the carrier
40
New cards
What are the important features of Krebs Cycle?
* 2C atoms come in and 2C atoms release
* Generate:
* 3 NADH, 1 reduced FAD + 1 GTP
> 1 ADH → 2.5 ATP in oxidative phosphorylation
>
> 1 FADH2 → 1.5 ATP
>
> With GTP, ≈ 10 ATP per acetyl CoA
* ATP is __**not directly**__ generated
* **Oxaloacetate** is not net consume in the cycle (acts as carrier)
* Generate:
* 3 NADH, 1 reduced FAD + 1 GTP
> 1 ADH → 2.5 ATP in oxidative phosphorylation
>
> 1 FADH2 → 1.5 ATP
>
> With GTP, ≈ 10 ATP per acetyl CoA
* ATP is __**not directly**__ generated
* **Oxaloacetate** is not net consume in the cycle (acts as carrier)
41
New cards
Regulation pathways of the Krebs Cycle
* Mainly by availability of cofactors
* NAD+, FAD, ADP (more of these → Faster they go)
* Inhibited by a high ‘energy charge’ – ATP : ADP ratio
* NAD+, FAD, ADP (more of these → Faster they go)
* Inhibited by a high ‘energy charge’ – ATP : ADP ratio
42
New cards
What happens if there is no proton gradient?
* Will burn all of stored fuel
* No driving force for ATP synthesis
* No back-pressure to stop H+ pumping
* No restriction, no H/e- movement down the transport chain to O2
* Instant regeneration of NAD from NADH
* Massive fuel oxidation rate
* Massive O2 consumption
* No ATP production → Low ATP synthesis and cell death (
* No driving force for ATP synthesis
* No back-pressure to stop H+ pumping
* No restriction, no H/e- movement down the transport chain to O2
* Instant regeneration of NAD from NADH
* Massive fuel oxidation rate
* Massive O2 consumption
* No ATP production → Low ATP synthesis and cell death (
43
New cards
What does __**uncoupling**__ mean?
ATP synthesis and electron transport chain are disrupted.
44
New cards
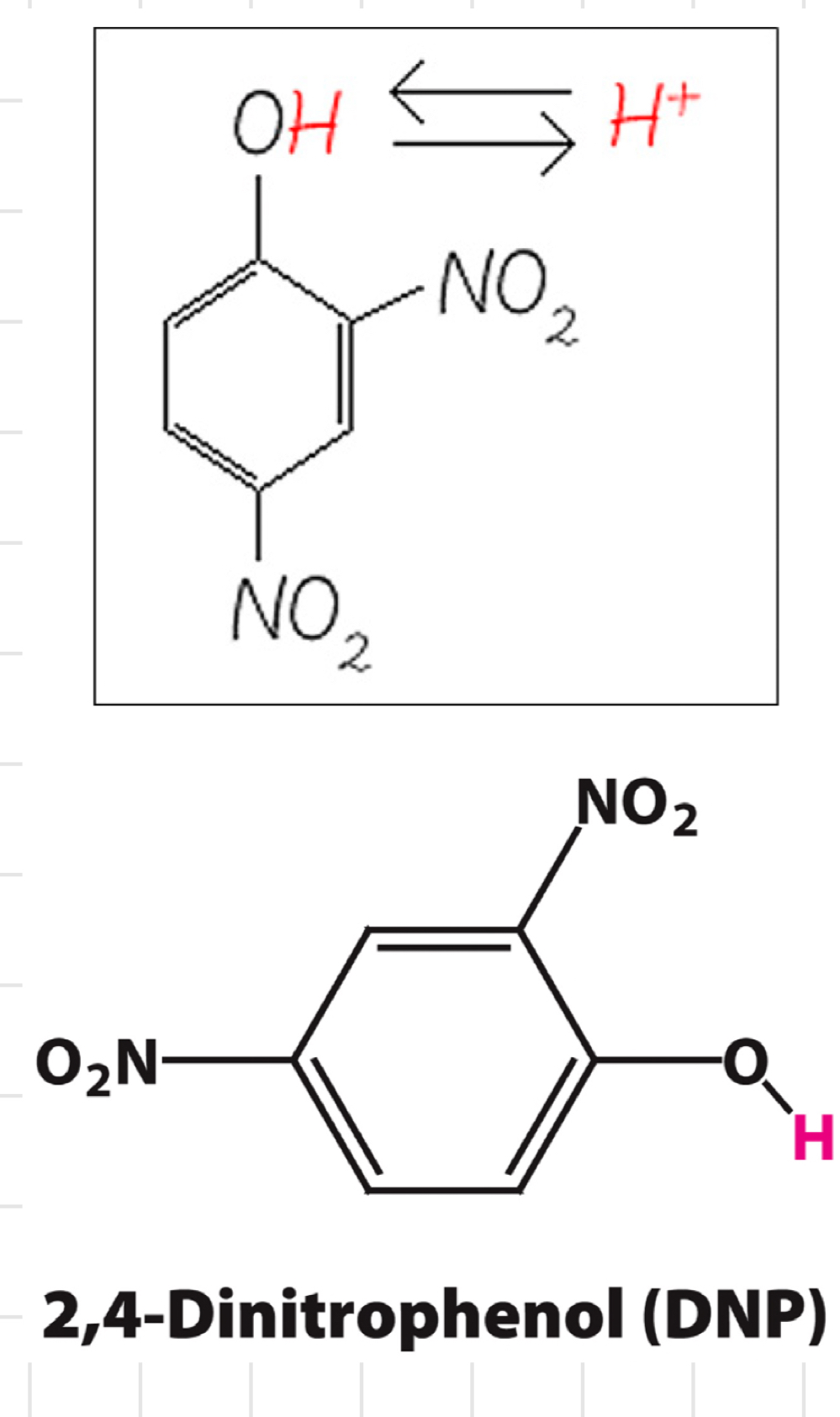
Dinitrophenol (DNP)
* An uncoupler
* Disrupts the normal coupling between ==**electron transport and ATP synthesis**== in oxidative phosphorylation.
* Prevent energy from being stored as fat in the body (instead releasing as heat)
* Hydrophobic when **protonated**
↳ Can move freely across membrane
* Weak acid
> Part of molecule can take up or release H+, depending on surrounding pH
* When H+ comes off → Negative charge can be delocalised (e- shared 2+ in a molecules)
↳ Still hydrophobic
* Disrupts the normal coupling between ==**electron transport and ATP synthesis**== in oxidative phosphorylation.
* Prevent energy from being stored as fat in the body (instead releasing as heat)
* Hydrophobic when **protonated**
↳ Can move freely across membrane
* Weak acid
> Part of molecule can take up or release H+, depending on surrounding pH
* When H+ comes off → Negative charge can be delocalised (e- shared 2+ in a molecules)
↳ Still hydrophobic
45
New cards
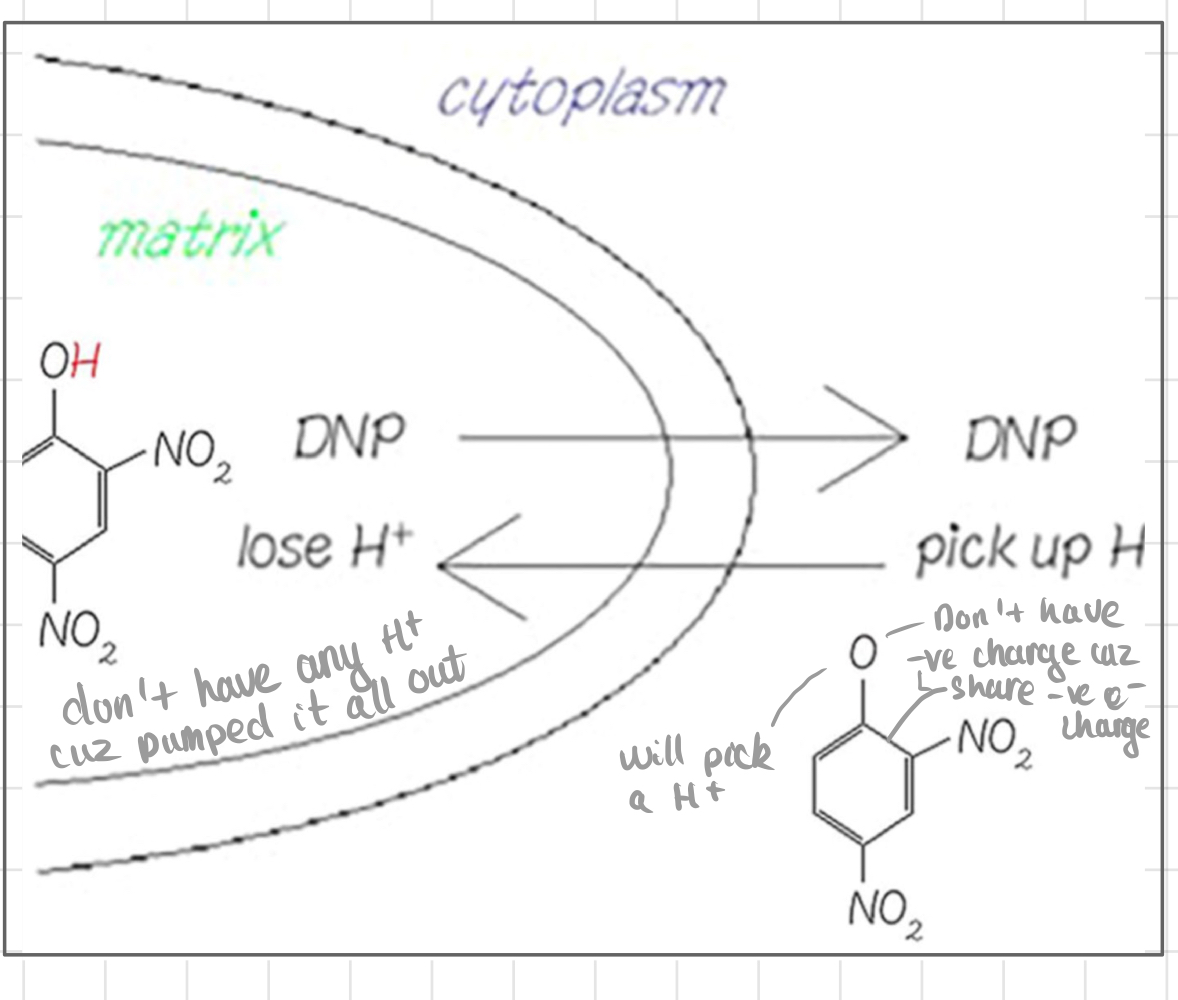
The mechanism of DNP
* DNP is a __**protonophore**__, allowing protons to cross the inner mitochondrial membrane freely.
* Protons leak back into the matrix without passing through ATP synthase, disrupting electron transport and ATP synthesis.
* Dissipation of proton gradient
* ↓ Rate of ATP synthesis (Prevent ATP production)
* Proton gradient dissipates
* ↑ Oxygen consumption.
* ↑ Rate of ß-oxidation
* Massive weight loss and heat production
* Later used as a weight loss agent
* Protons leak back into the matrix without passing through ATP synthase, disrupting electron transport and ATP synthesis.
* Dissipation of proton gradient
* ↓ Rate of ATP synthesis (Prevent ATP production)
* Proton gradient dissipates
* ↑ Oxygen consumption.
* ↑ Rate of ß-oxidation
* Massive weight loss and heat production
* Later used as a weight loss agent
46
New cards
Natural uncoupler - **Uncoupling Protein 1 (UCP-1)**
* UCP-1 or @@**Thermogenin**@@
* Found only in brown adipose tissue
* Function: Generate heat
* Esp in small mammals and hibernating animals
* Under hormonal control
* Noradrenaline binds to ß-receptors (only in white adipose tissue) on the cell surface.
* Stimulates FA secretion
* Open proton channel
➜ Targeted and controllable
> High in neonates, less as we grow up
* Found only in brown adipose tissue
* Function: Generate heat
* Esp in small mammals and hibernating animals
* Under hormonal control
* Noradrenaline binds to ß-receptors (only in white adipose tissue) on the cell surface.
* Stimulates FA secretion
* Open proton channel
➜ Targeted and controllable
> High in neonates, less as we grow up
47
New cards
What are e- transport and H+ pumping?
* The strippers and carriers of H/e-
* Components of the ETC
* H/e- carriers in the chain
* Proteins that support them
* Matrixed fuel system
* Movement of protons out of the matrix
* Components of the ETC
* H/e- carriers in the chain
* Proteins that support them
* Matrixed fuel system
* Movement of protons out of the matrix
48
New cards
What does __**Electron Transport Chain (ETC)**__?
* Contains 4 complexes
* All embedded in the inner mitochondrial membrane
* Complex I skip complex II (I and II are distinct entries)
### I → III → IV, and II → III → IV
* Each complex consists of many proteins
* **Structural -** Maintain the shape of complex
* **Prosthetic group** (a subset of cofactor) **-** Bits that transport H/e-
* Proteins are arranged so that
* **H+ expelling reactions** on the outside
* **H+ consuming reactions** on the matrix side
* ≈ 10 H+ are pumped out for each NADH
* All embedded in the inner mitochondrial membrane
* Complex I skip complex II (I and II are distinct entries)
### I → III → IV, and II → III → IV
* Each complex consists of many proteins
* **Structural -** Maintain the shape of complex
* **Prosthetic group** (a subset of cofactor) **-** Bits that transport H/e-
* Proteins are arranged so that
* **H+ expelling reactions** on the outside
* **H+ consuming reactions** on the matrix side
* ≈ 10 H+ are pumped out for each NADH
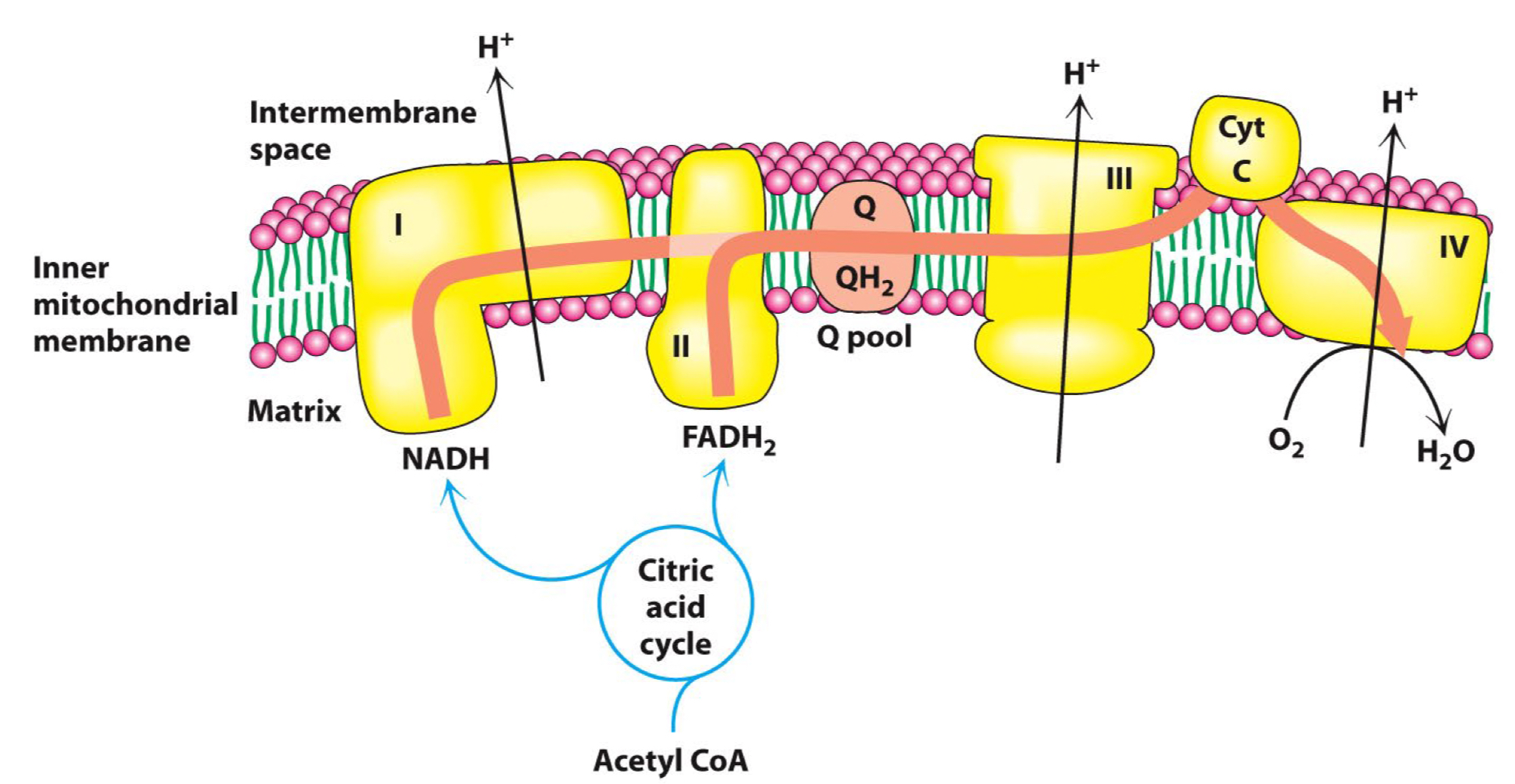
49
New cards
__**Nicotinamide Adenine Dinucleotide (NAD+)**__ in ETC
* __**Donates**__ H/e- to @@**complex I**@@ (re-deoxides NADH to NAD+)
* NAD+ accepts a H+ and 2 e- = A hydride ion H
* NAD+ likes to rip H/e- off from the -CH-OH group converting them to -C=O groups
* Nicotinamide group derived from **nicotic acid (niacin)**
* NAD+ accepts a H+ and 2 e- = A hydride ion H
* NAD+ likes to rip H/e- off from the -CH-OH group converting them to -C=O groups
* Nicotinamide group derived from **nicotic acid (niacin)**
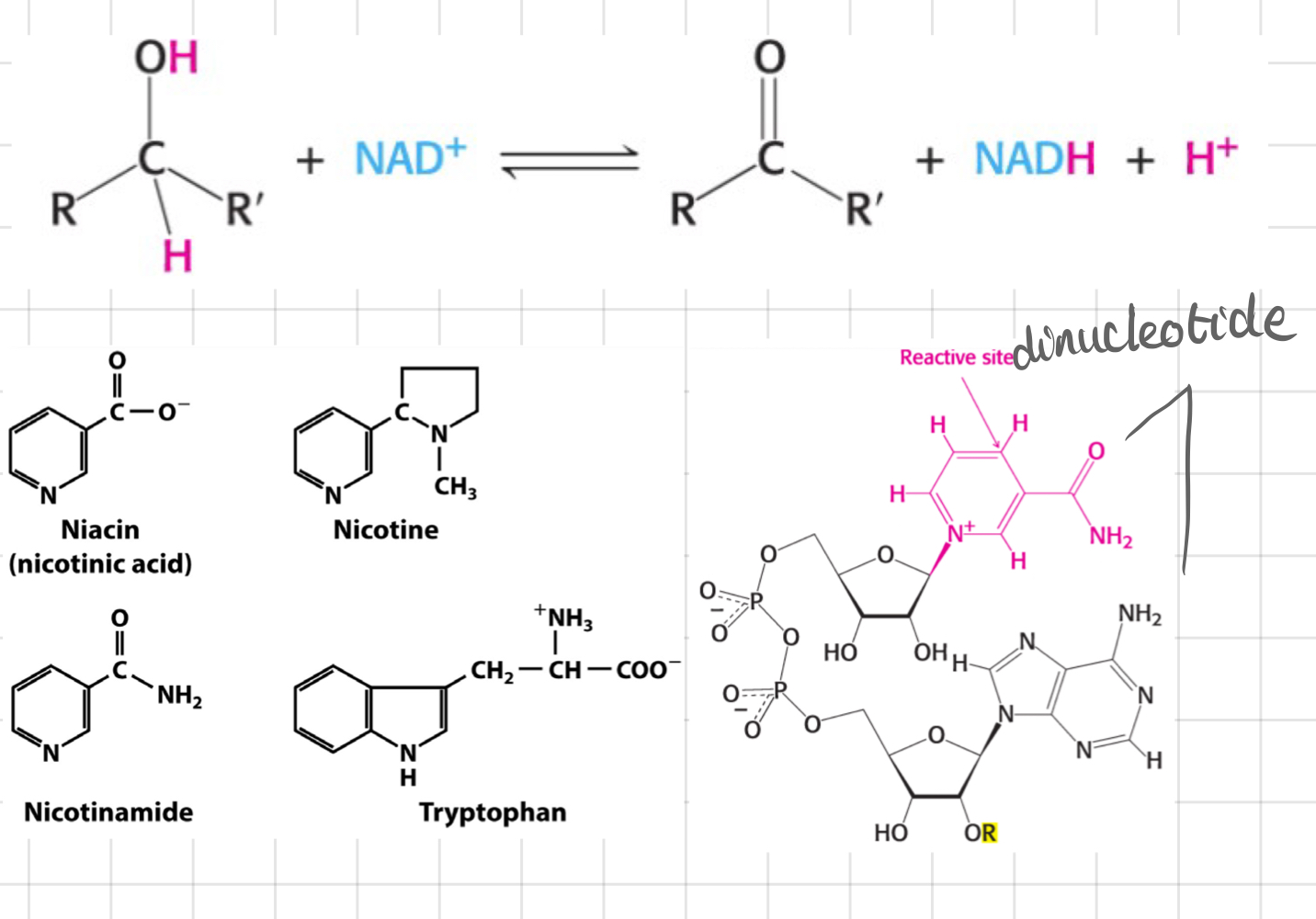
50
New cards
Why NADH but not NADH2?
* NADH is the reduced form of NAD+.
* In cellular respiration, it carries 2 high-energy electrons and 1 proton.
* In cellular respiration, it carries 2 high-energy electrons and 1 proton.
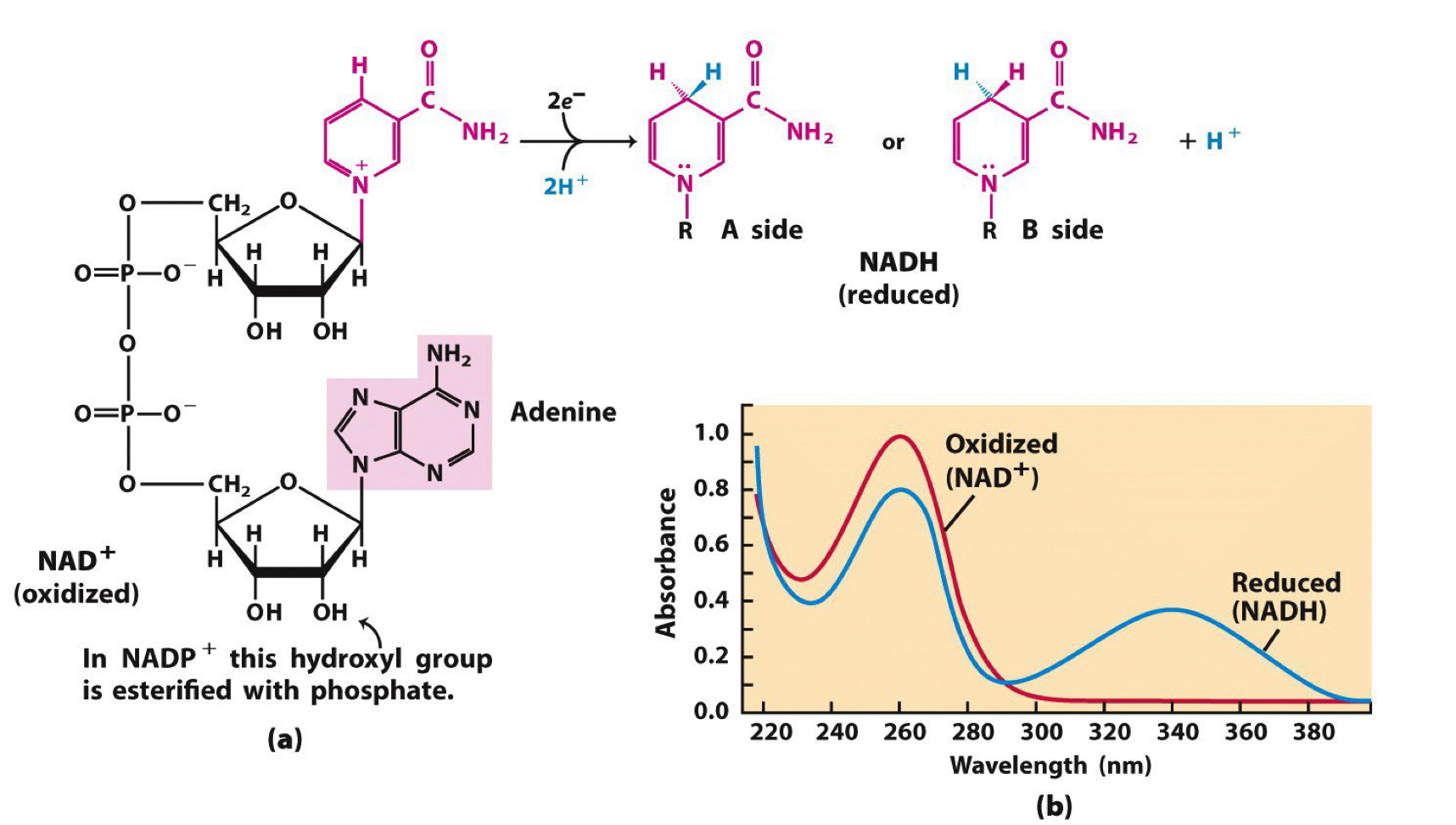
51
New cards
__**Flavin Adenin Nucleotide (FAD)**__ in ETC
* Present inside and __**stuck**__ in @@**Complex II**@@
* Acceptor and donator of Hs
* Rip H from a saturated hydrocarbon chain
* 2 H ripped out and being carried
* Built-up ADP
* Acceptor and donator of Hs
* Rip H from a saturated hydrocarbon chain
* 2 H ripped out and being carried
* Built-up ADP
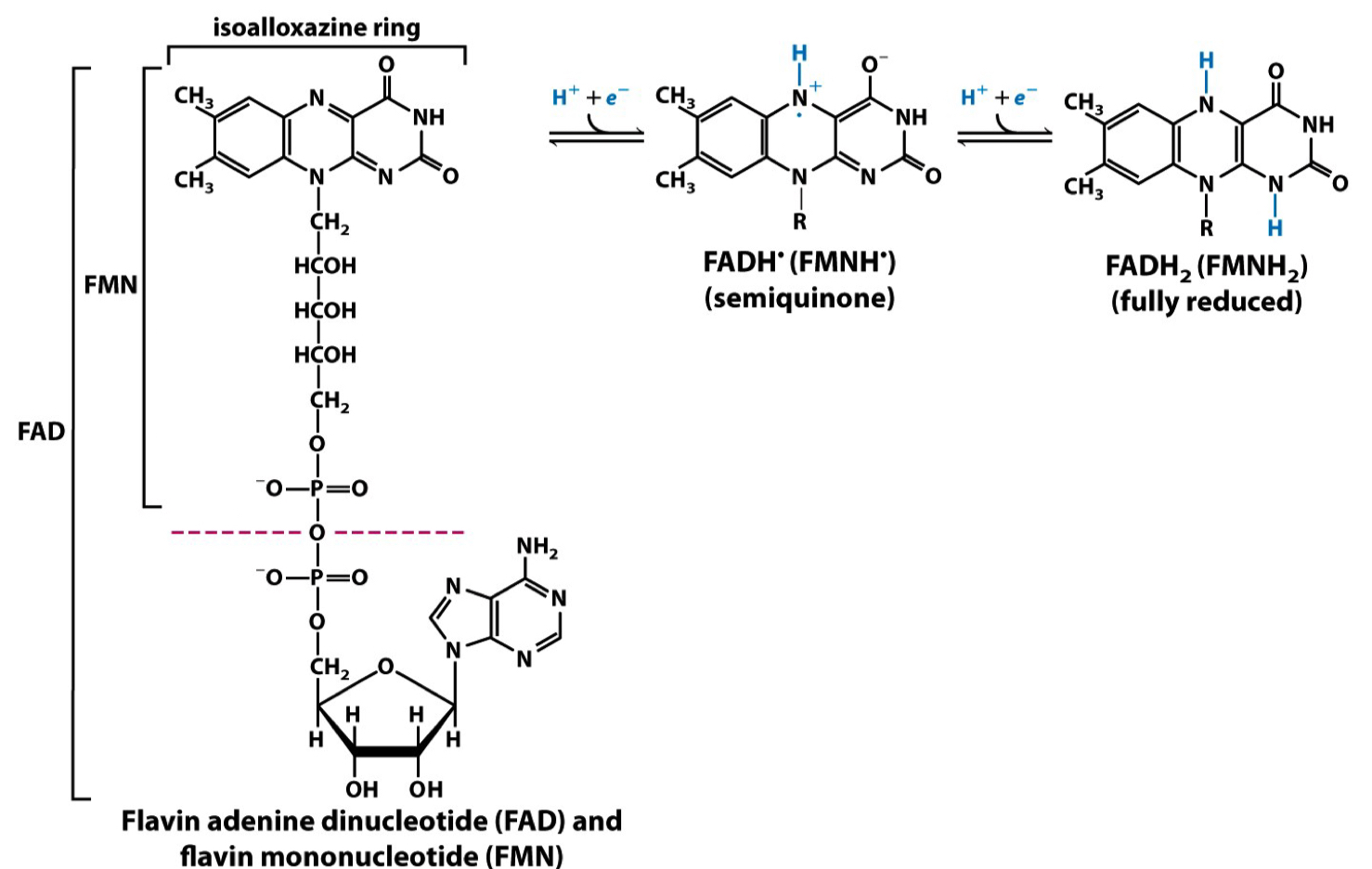
52
New cards
__**Ubiquinone (UQ or Q pool)**__ in ETC
* Reduced form: **UQH2** (__**transfers**__ Hs to @@**Complex III**@@)
* Electrons __**move**__ around in @@**Complex I**@@ from 1 prosthetic group to another until they reach the **Q pool.**
* Very hydrophobic
* Lives in the inner mitochondrial membrane
* Accept **all** H and e- from Complex II
* Never sees the light
* Electrons __**move**__ around in @@**Complex I**@@ from 1 prosthetic group to another until they reach the **Q pool.**
* Very hydrophobic
* Lives in the inner mitochondrial membrane
* Accept **all** H and e- from Complex II
* Never sees the light
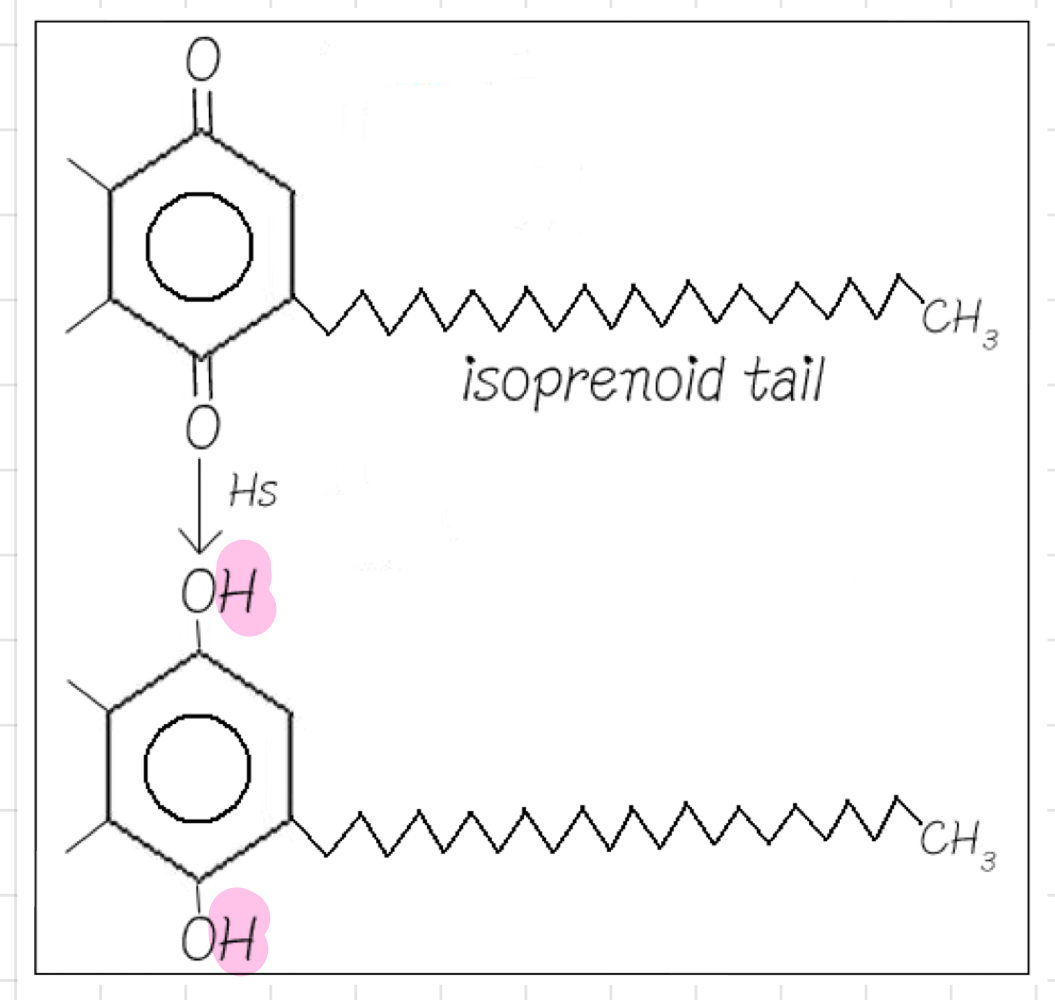
53
New cards
__**Cytochrome C (Cyt C)**__ and __**Iron**__ in ETC
* Cyt C picks up e- from @@**Complex III**@@ and gives e- to @@**Complex IV.**@@
* Cyt C has a prosthetic group that contains a Fe atom
* Changes from ferrous (Fe2+) to ferric (Fe3+) as it loses, and vice versa, as it accepts e-
* Fe does not carry Hs
* Only deal with e-
> Very good at moving e- from 1 place to another
* How are Fe atoms held in place?
* In mid of **porphyrin rings**
* In **Iron-Sulphur complexes**
* Cyt C has a prosthetic group that contains a Fe atom
* Changes from ferrous (Fe2+) to ferric (Fe3+) as it loses, and vice versa, as it accepts e-
* Fe does not carry Hs
* Only deal with e-
> Very good at moving e- from 1 place to another
* How are Fe atoms held in place?
* In mid of **porphyrin rings**
* In **Iron-Sulphur complexes**
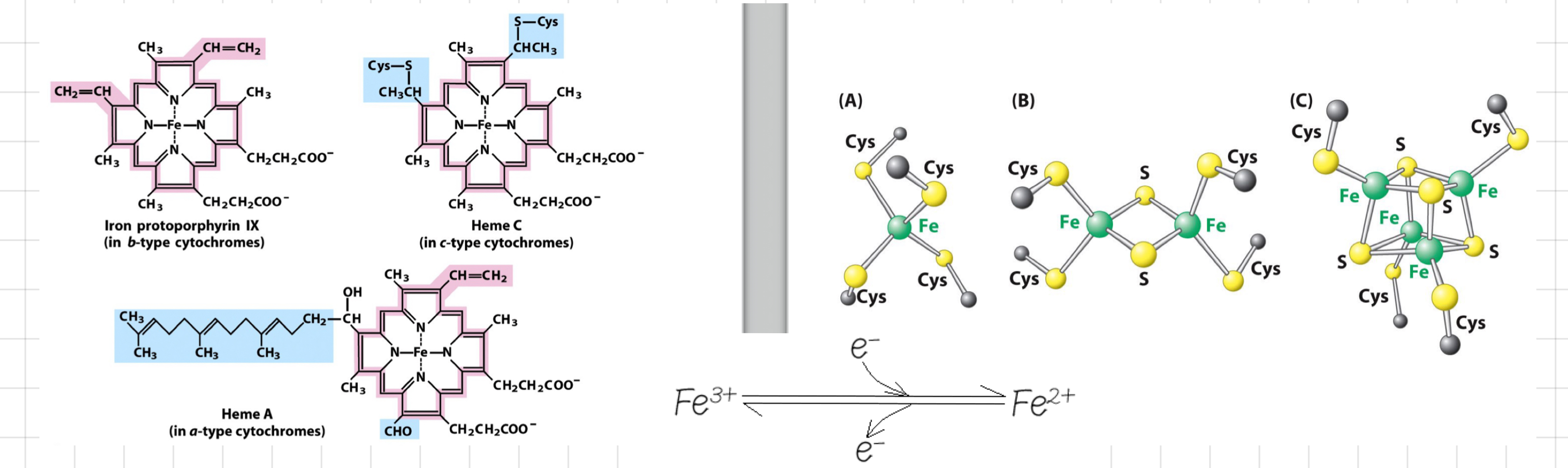
54
New cards
What is the **proton motive force**?
* Local pH is important
* **Proton motive force** has a charge and \[component\]
* Energy in the gradient is based on both charge, conc, chemical and electrical gradient
* 2 components come together to make free energy in gradient that much greater
* **Proton motive force** has a charge and \[component\]
* Energy in the gradient is based on both charge, conc, chemical and electrical gradient
* 2 components come together to make free energy in gradient that much greater
![* Local pH is important
* **Proton motive force** has a charge and \[component\]
* Energy in the gradient is based on both charge, conc, chemical and electrical gradient
* 2 components come together to make free energy in gradient that much greater](https://knowt-user-attachments.s3.amazonaws.com/1b86af7e9b874da18acebbfe76ee3fd4.jpeg)
55
New cards
Getting Cytoplasmic NADH to the ETC
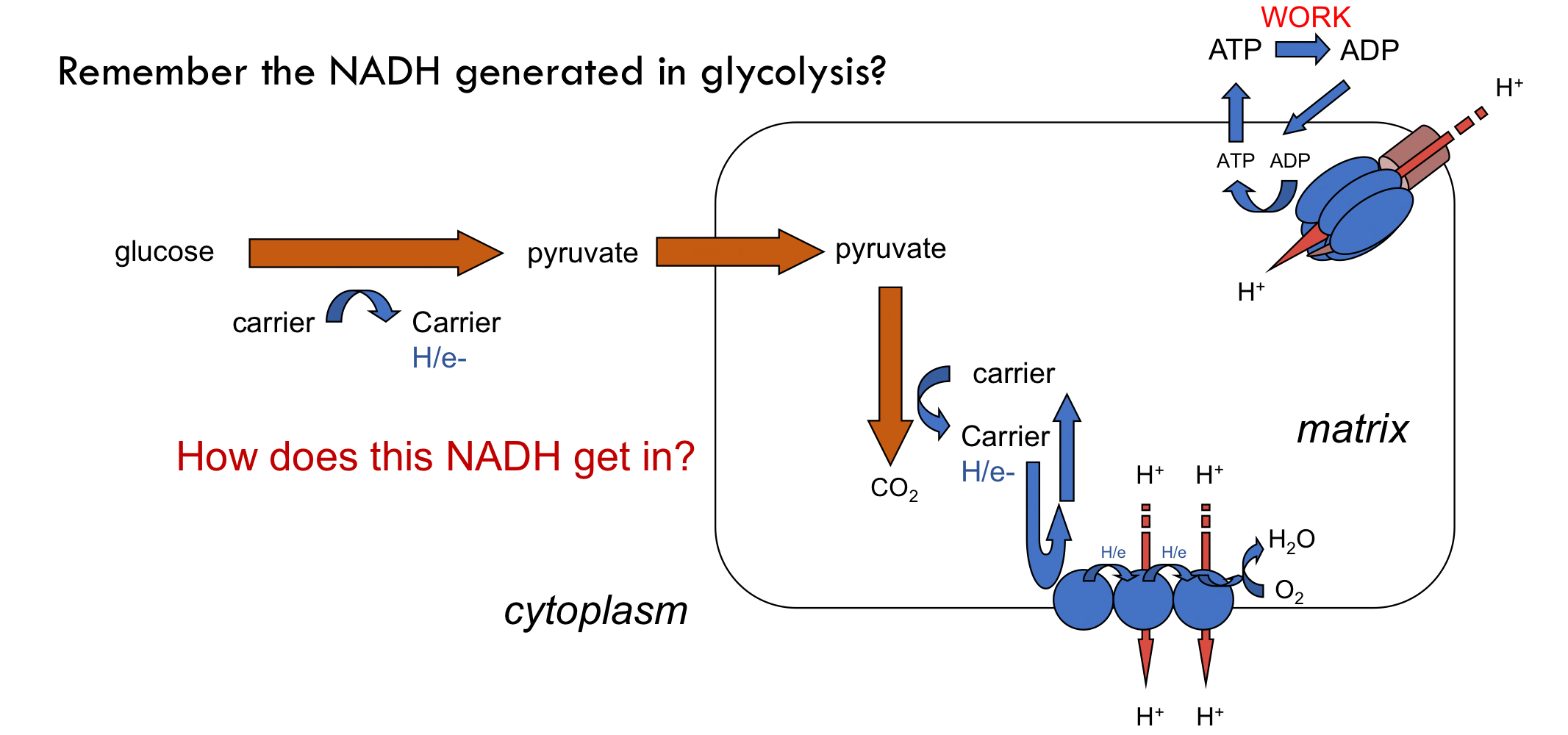
56
New cards
What does __**Glycerol 3-Phosphate Shuttle**__ do?
* Effectively bypassing **Complex I**
* After glycolysis, @@**dihydroxyacetone phosphate**@@ is converted to NAD+ by reacting with NADH → ==**Glycerol 3-Phosphate.**==
* Then oxidised by FAD in the mitochondrial membrane.
* Allow e- to pass through the chain to Q and then through the chain again.
* Losing H+ pumping potential
* **Functions:**
* Transfers e- between cytosolic NADH and mitochondria, producing ATP through oxidative phosphorylation.
* Maintains energy production in tissues where NADH is efficiently produced in the cytosol and transported to the mitochondria.
* After glycolysis, @@**dihydroxyacetone phosphate**@@ is converted to NAD+ by reacting with NADH → ==**Glycerol 3-Phosphate.**==
* Then oxidised by FAD in the mitochondrial membrane.
* Allow e- to pass through the chain to Q and then through the chain again.
* Losing H+ pumping potential
* **Functions:**
* Transfers e- between cytosolic NADH and mitochondria, producing ATP through oxidative phosphorylation.
* Maintains energy production in tissues where NADH is efficiently produced in the cytosol and transported to the mitochondria.
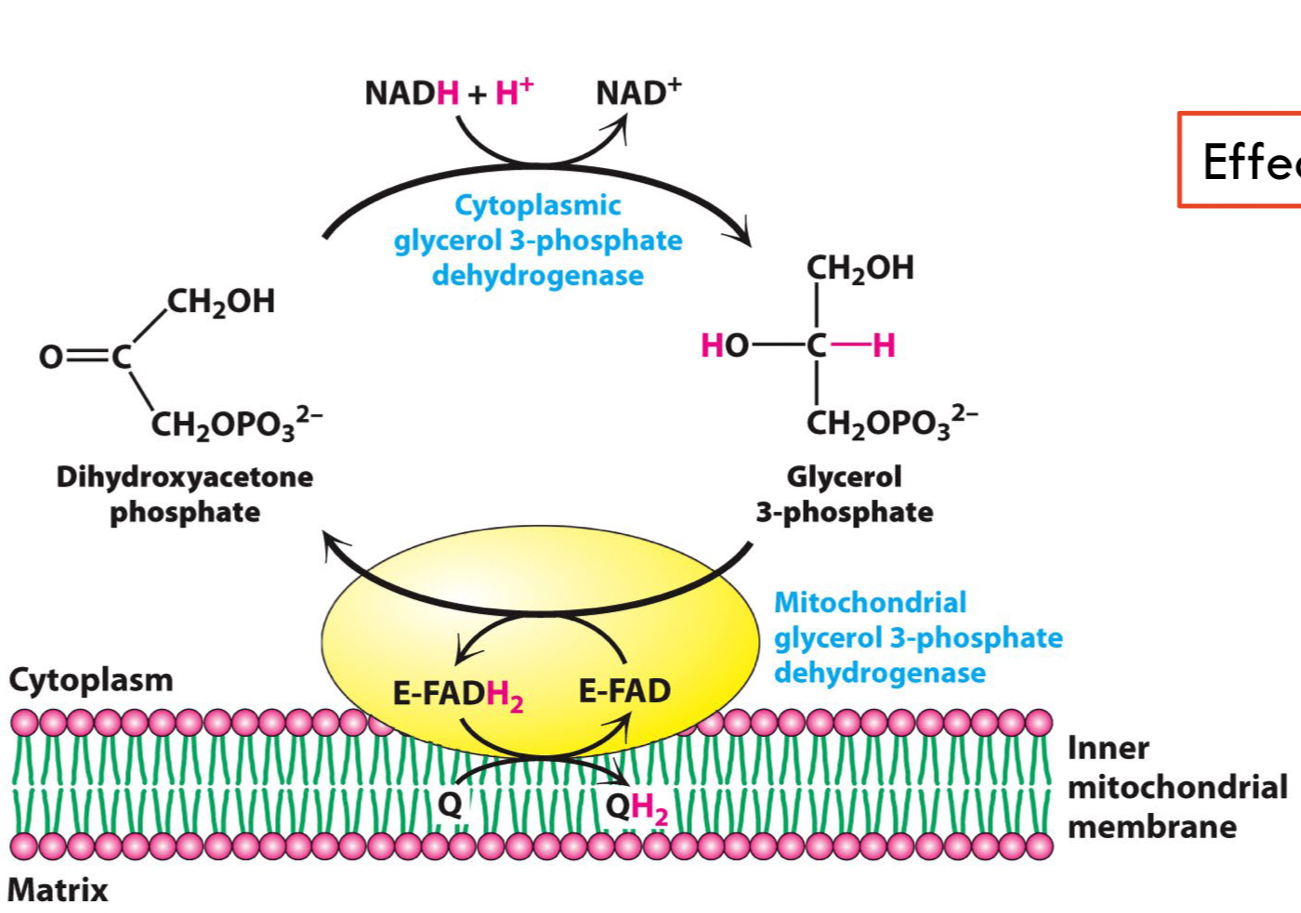
57
New cards
What does __**Malate Aspartate Shuttle**__ do?
* Moves e- around to get them across the inner mitochondrial membrane
* **Purpose:**
* Take NADH from the cytoplasm and make NADH in the matrix
↳ e- transferred into the matrix with __**no**__ loss of H+ potential
* **Function:**
* Transfers reducing equivalents from the cytosol to mitochondria by oxidative phosphorylation, where NADH contributes to ATP production.
* Allows efficient energy utilization and maintains redox balance.
* **Purpose:**
* Take NADH from the cytoplasm and make NADH in the matrix
↳ e- transferred into the matrix with __**no**__ loss of H+ potential
* **Function:**
* Transfers reducing equivalents from the cytosol to mitochondria by oxidative phosphorylation, where NADH contributes to ATP production.
* Allows efficient energy utilization and maintains redox balance.
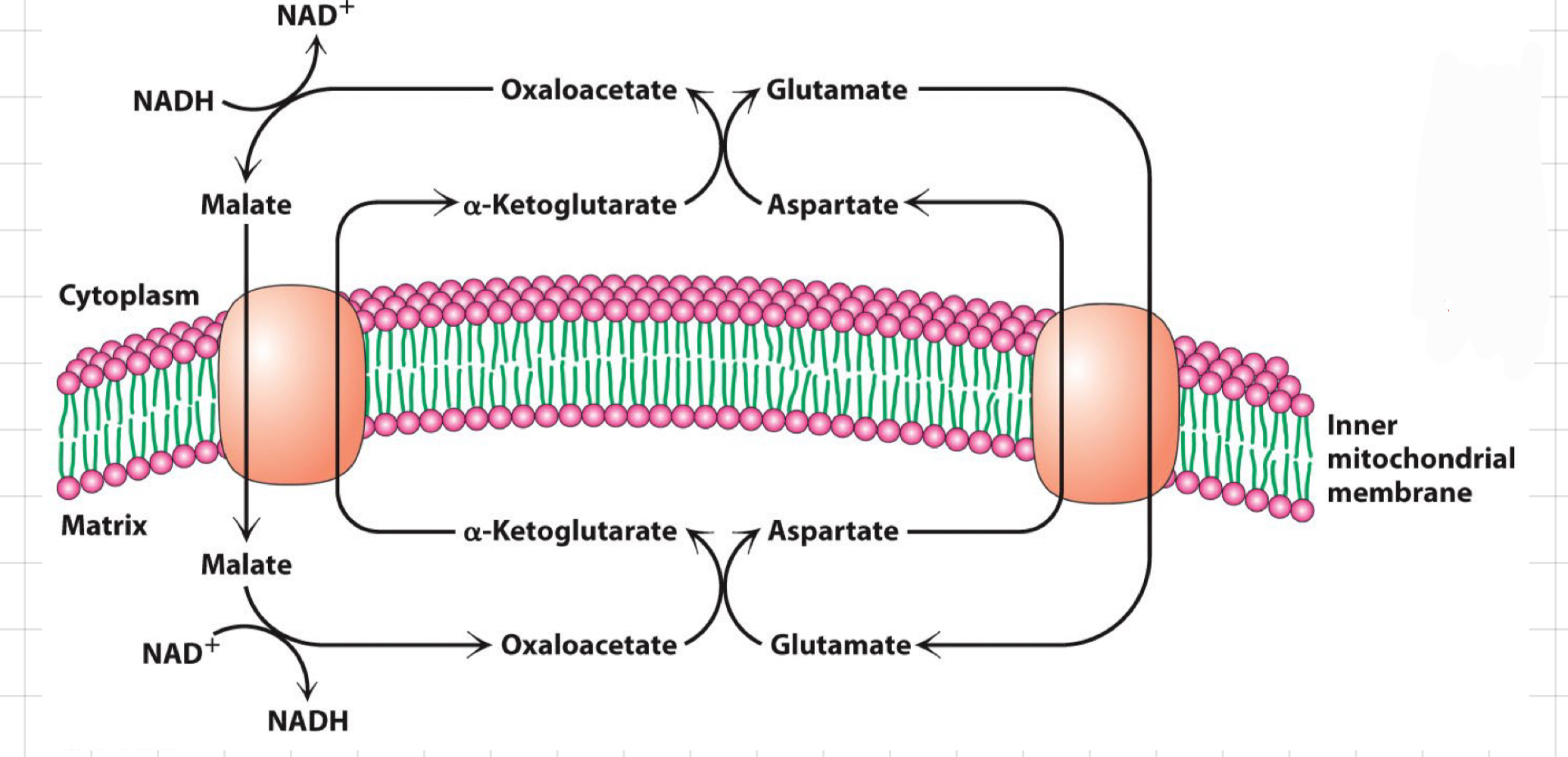
58
New cards
Organise the four separate routes that feed into UQ (Complex I, Complex II, G3P shuttle and beta-oxidation)
1. From **Complex I (NADH dehydrogenase)**
* Transfers electrons from NADH to ubiquinone (UQ) in the ETC.
2. From **Complex II (Succinate dehydrogenase)**
* Directly transfers electrons from succinate to UQ in the ETC.
3. From the 1st step of **ß-oxidation**
4. From the **Glycerol 3-P shuttle**
* Generates NADH and FADH2 during the breakdown of fatty acids, which transfer electrons to UQ in the ETC.
* Once in the **Q pool**, the e- will __**always**__ go to **complex III**
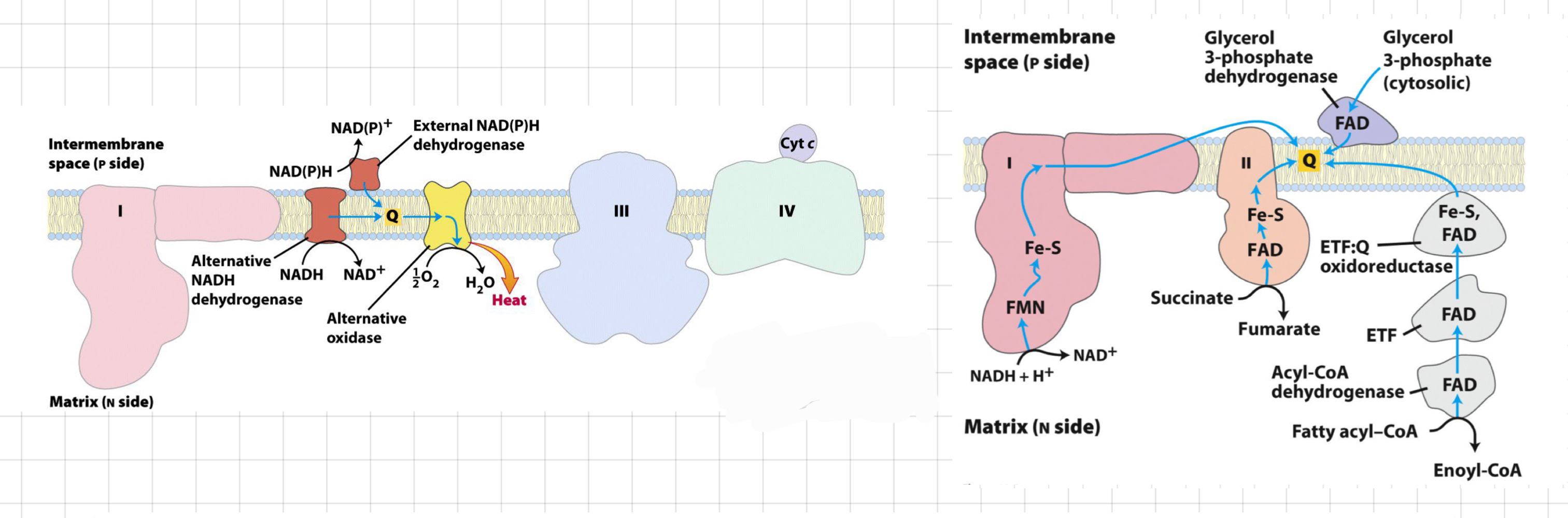
59
New cards
The mechanisms involved in the generation and destruction of free radicals
* **Free radicals:** Very dangerous → Mutations to DNA
* __**Electron Leakage:**__
* Electrons leak and react with molecular oxygen, forming superoxide anion (O2·−).
* This primarily occurs at Complex I and Complex III.
* __**Ubiquinone (Coenzyme Q) Reaction:**__
* After electrons pass through the ETC, the ubiquinone (UQH2) reduced form can react with molecular oxygen to produce superoxide anion (O2)→ Escape ETC → Free radicals
* Things getting out of ETC before getting to O2
↳ Problems bc things in ETC are very reactive (environment in chain keep it safe
* __**Electron Leakage:**__
* Electrons leak and react with molecular oxygen, forming superoxide anion (O2·−).
* This primarily occurs at Complex I and Complex III.
* __**Ubiquinone (Coenzyme Q) Reaction:**__
* After electrons pass through the ETC, the ubiquinone (UQH2) reduced form can react with molecular oxygen to produce superoxide anion (O2)→ Escape ETC → Free radicals
* Things getting out of ETC before getting to O2
↳ Problems bc things in ETC are very reactive (environment in chain keep it safe
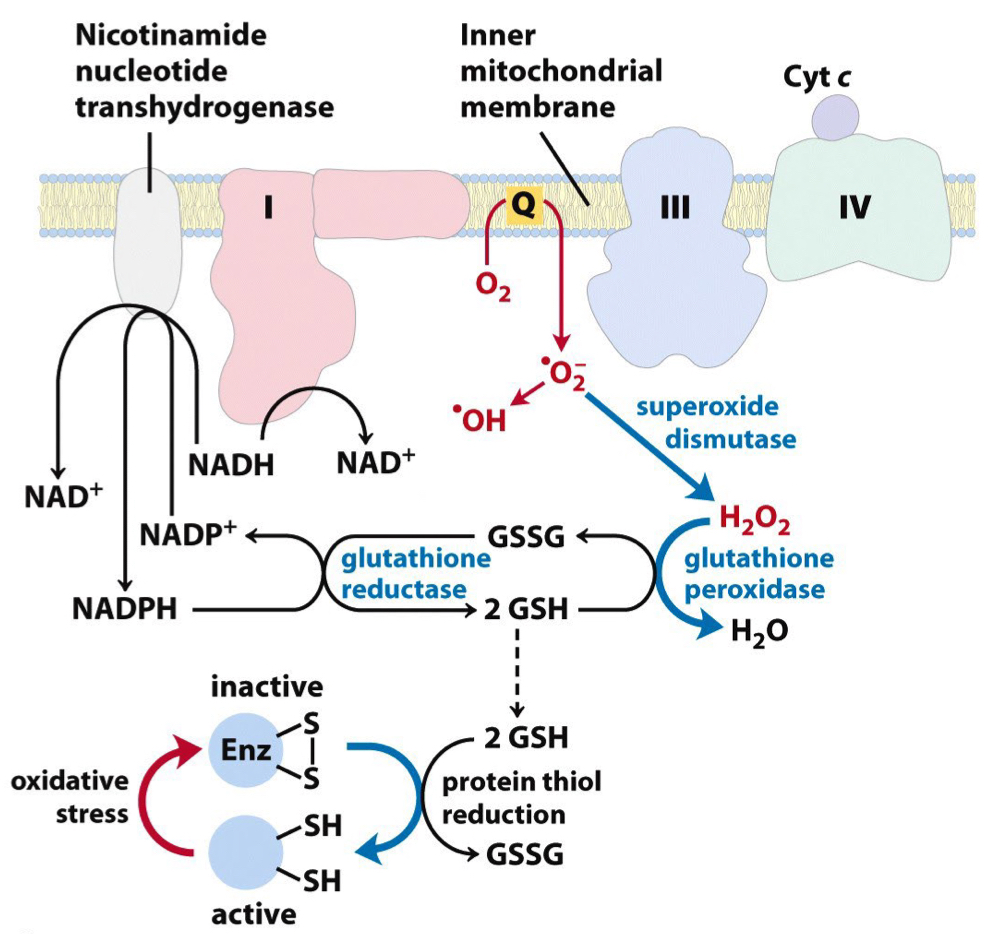
60
New cards
ATP Synthase and its structure
* Using H+ gradient to make ATP
* Movement of 3 H+ → 1 ATP per 1 rotation
* __**F0 channel:**__ composed of 12 cylindrical proteins
* As protons enter → **γ subunit** rotates
* Causes **ß subunit** of __**F1**__ to change its conformation in 3 ways:
* Accepting ADP + Pi
* Reacting them together to give ATP
* Releasing ATP
* Movement of 3 H+ → 1 ATP per 1 rotation
* __**F0 channel:**__ composed of 12 cylindrical proteins
* As protons enter → **γ subunit** rotates
* Causes **ß subunit** of __**F1**__ to change its conformation in 3 ways:
* Accepting ADP + Pi
* Reacting them together to give ATP
* Releasing ATP
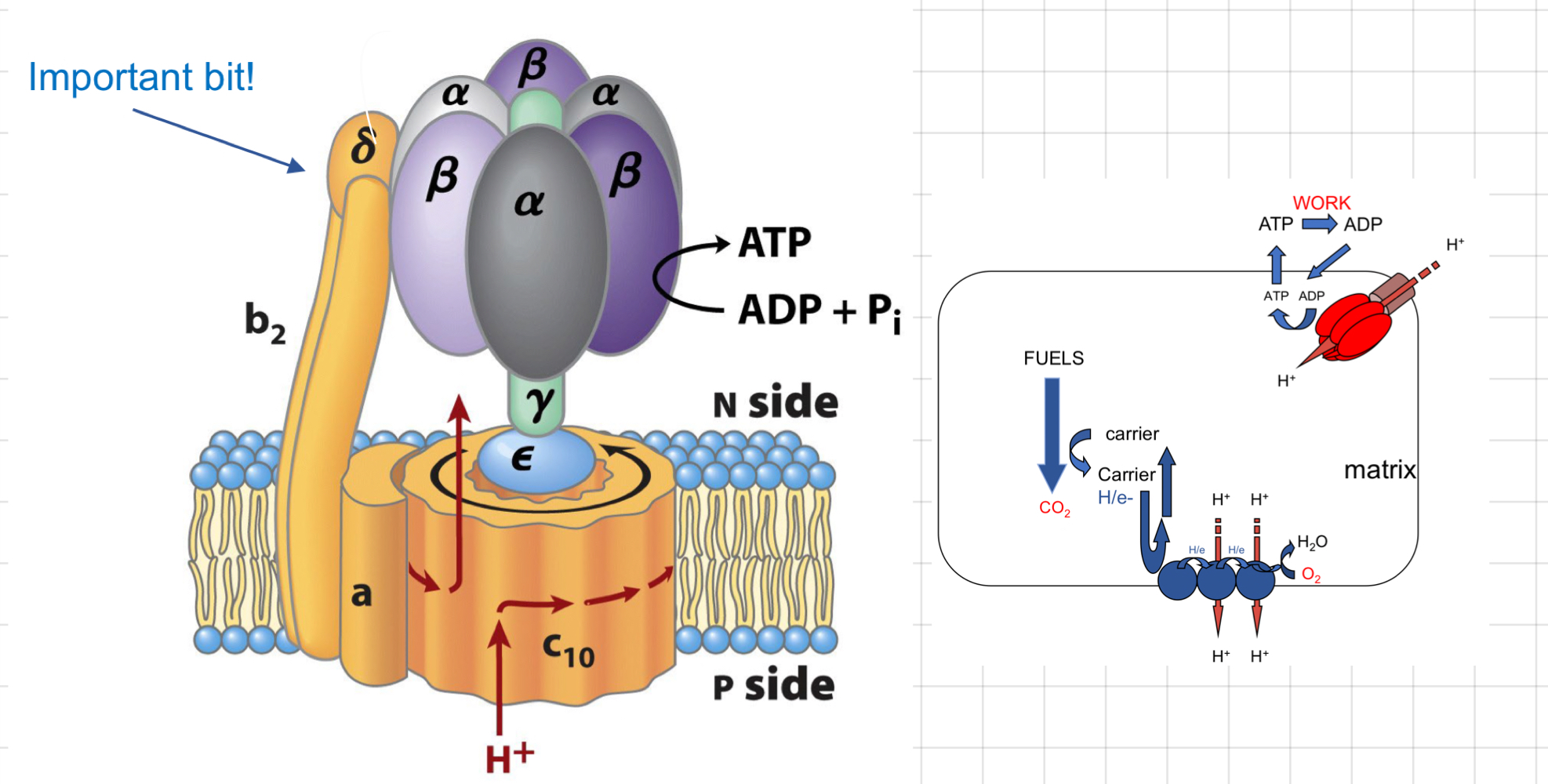
61
New cards
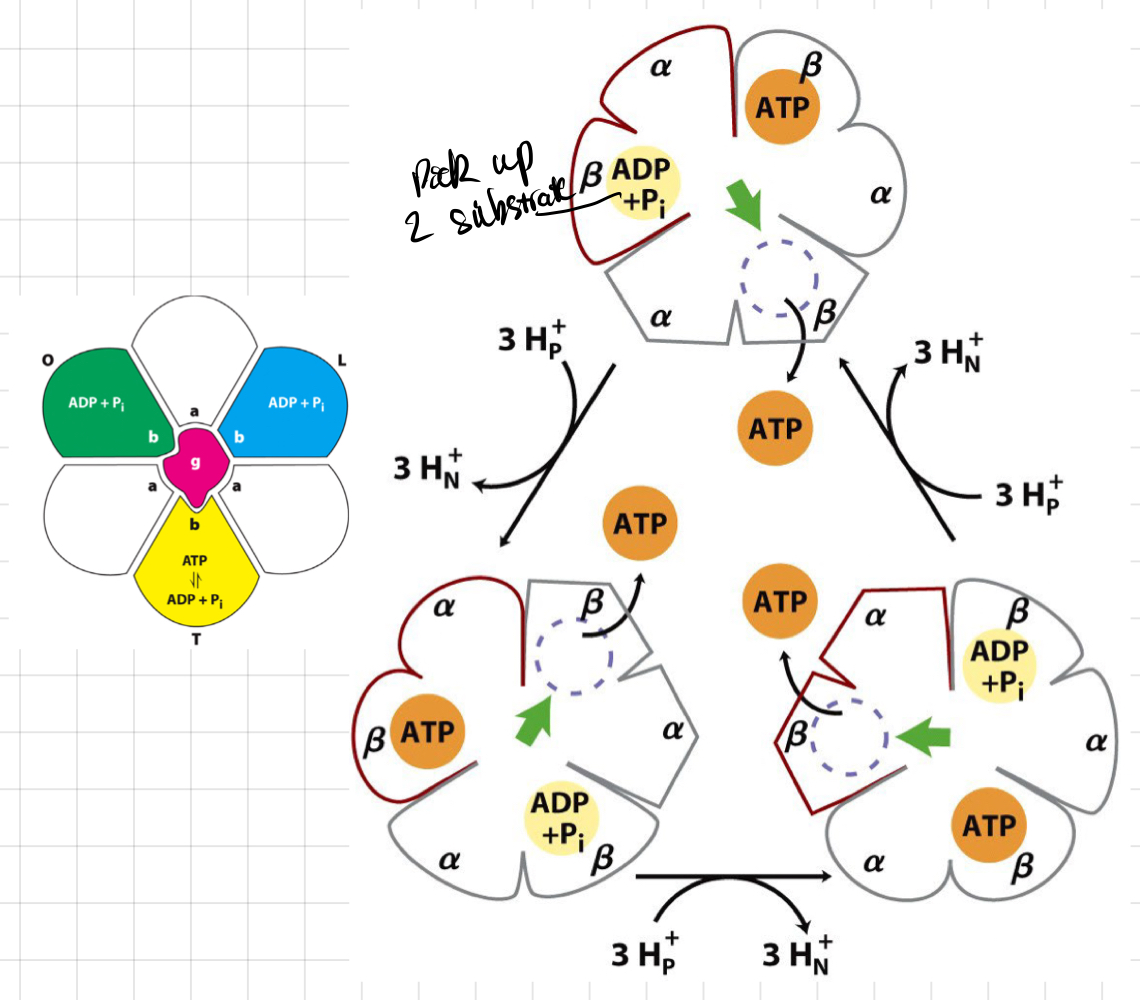
The alternate states of the ß-subunit
* Every time 3 H+ come in → ß-subunit change conformation
* Start at any point and follow the ß-subunit ways
* 3 ß-subunits
* Start at any point and follow the ß-subunit ways
* 3 ß-subunits
62
New cards
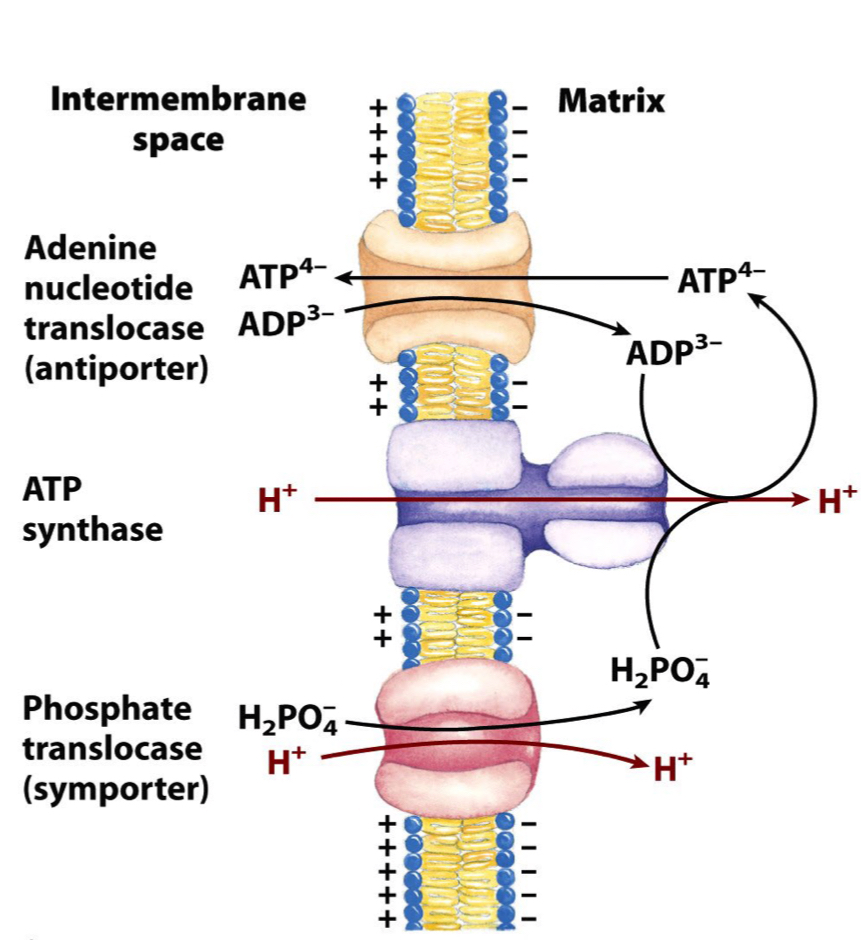
The contribution of the proton gradient to processes other than the ATP synthase
* Swapping of ATP/ADP (brings ADP + Pi) takes negative charge outside
* ATP goes out of into the cytoplasm
* 3- charges come in, 4- go out
* Need positive charge to do movement → Use a H+ (proton gradient)
* The import of Pi consumes H+
* ATP goes out of into the cytoplasm
* 3- charges come in, 4- go out
* Need positive charge to do movement → Use a H+ (proton gradient)
* The import of Pi consumes H+
63
New cards
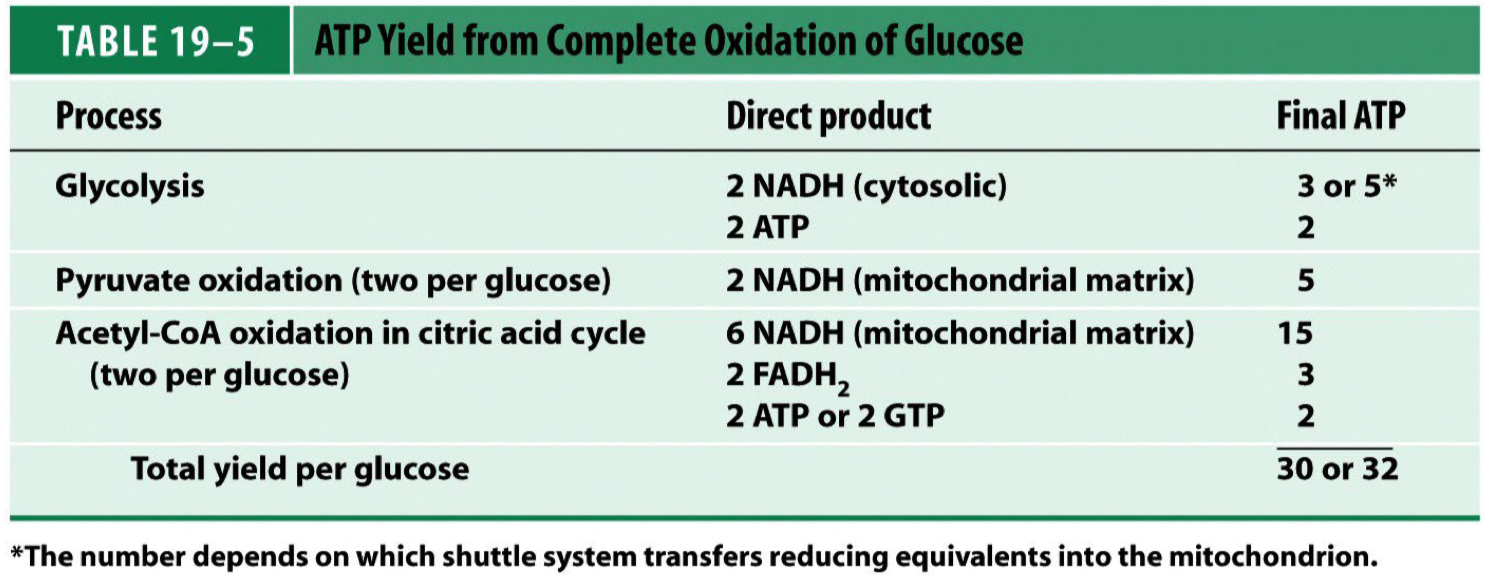
Counting ATP
64
New cards
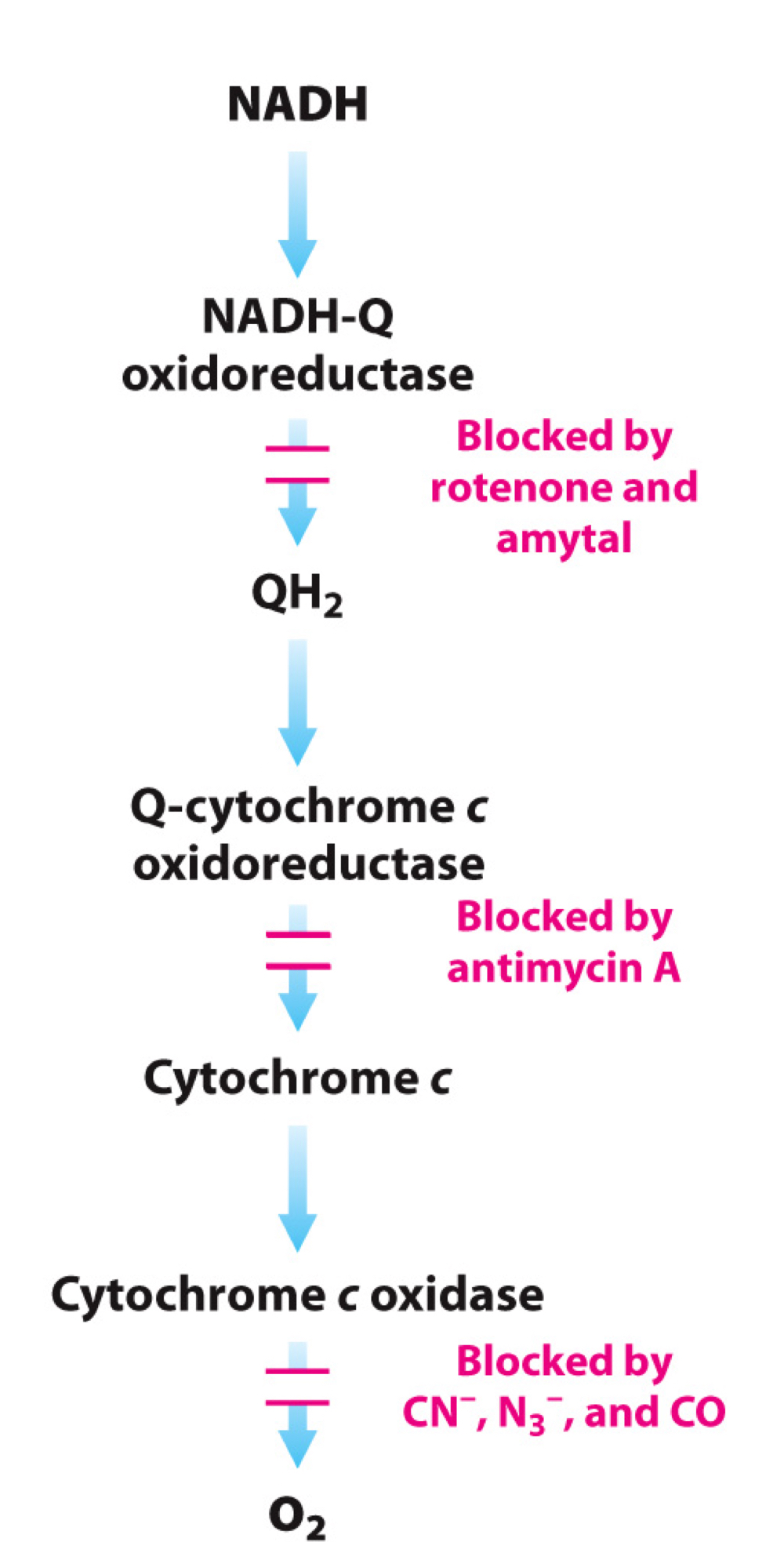
Inhibitors and Acceptors in ETC
* __**Rotenone**__
* Inhibit at Complex I
* Whole chain stops → H+ pumping stops
* Everything **downstream** is **oxidised** (stop consuming O2)
* __**Cyanize, Azide and CO**__
* Inhibit at Complex IV
* Whole chain stops → H+ pumping stops
* Everything **upstream** is **reduced**
* __**Alternative receptors**__, e.g. Methylene blue
* Accepts e- from Complex IV before cyanine blockage point
* Allow transport to continue
* Inhibit at Complex I
* Whole chain stops → H+ pumping stops
* Everything **downstream** is **oxidised** (stop consuming O2)
* __**Cyanize, Azide and CO**__
* Inhibit at Complex IV
* Whole chain stops → H+ pumping stops
* Everything **upstream** is **reduced**
* __**Alternative receptors**__, e.g. Methylene blue
* Accepts e- from Complex IV before cyanine blockage point
* Allow transport to continue
65
New cards
Starvation and Some rules
### **Starvation**
* Begin at the start of the **post-absorptive period**
* When all food digested
* No substrates coming in from gut
* Reliant on blood and stored fuel
### **Some Rules**
* Need to keep \[blood glucose\] ≈ 5 mM (> 4 mM)
* **Euglycemia** or **Normoglycemia** (normal \[blood glucose\])
* Under normal circumstances, brain can only use glucose
* Cannot use FAs which cannot cross **Blood Brain Barrier (BBB)**
* Uses ≈ 120g glucose/day
* Transported by **GLUT-1**
* Although we store most of our energy as fat, we cannot convert FA into CHO (carbohydrate/glucose)
* Acyl CoA can’t be made into **Gluconeogenic precursors.**
* Pyruvate (3C) → Acetyl CoA (2C → Glucose is lost) is Irreversible
* Begin at the start of the **post-absorptive period**
* When all food digested
* No substrates coming in from gut
* Reliant on blood and stored fuel
### **Some Rules**
* Need to keep \[blood glucose\] ≈ 5 mM (> 4 mM)
* **Euglycemia** or **Normoglycemia** (normal \[blood glucose\])
* Under normal circumstances, brain can only use glucose
* Cannot use FAs which cannot cross **Blood Brain Barrier (BBB)**
* Uses ≈ 120g glucose/day
* Transported by **GLUT-1**
* Although we store most of our energy as fat, we cannot convert FA into CHO (carbohydrate/glucose)
* Acyl CoA can’t be made into **Gluconeogenic precursors.**
* Pyruvate (3C) → Acetyl CoA (2C → Glucose is lost) is Irreversible
66
New cards
Glucose requirements during the first few hours and what happens to them
* Parts of the kidney, skin and RBCs have obligatory requirements for glucose
↳ Cannot use anything else but glucose
* Other tissues (primarily muscle)
↳ Can switch to FAs as an alternate fuel during starvation
### **General Strategy**
* __**Glucose conservation:**__ Don’t use it unless you must to
* __**Glucose recycling:**__ Don’t fully oxidise it - Generate from **Lactate**
* __**New glucose formation:**__ Make it from other things
### **First Few Hours**
* Tissues are using glucose → ↓ \[blood glucose\]
* Prevent hypoglycemia, liver releases glucose into bloodstream
* \[blood glucose\] stays constant to at least euglycemia at ≈ 4 mM
↳ Cannot use anything else but glucose
* Other tissues (primarily muscle)
↳ Can switch to FAs as an alternate fuel during starvation
### **General Strategy**
* __**Glucose conservation:**__ Don’t use it unless you must to
* __**Glucose recycling:**__ Don’t fully oxidise it - Generate from **Lactate**
* __**New glucose formation:**__ Make it from other things
### **First Few Hours**
* Tissues are using glucose → ↓ \[blood glucose\]
* Prevent hypoglycemia, liver releases glucose into bloodstream
* \[blood glucose\] stays constant to at least euglycemia at ≈ 4 mM
![* Parts of the kidney, skin and RBCs have obligatory requirements for glucose
↳ Cannot use anything else but glucose
* Other tissues (primarily muscle)
↳ Can switch to FAs as an alternate fuel during starvation
### **General Strategy**
* __**Glucose conservation:**__ Don’t use it unless you must to
* __**Glucose recycling:**__ Don’t fully oxidise it - Generate from **Lactate**
* __**New glucose formation:**__ Make it from other things
### **First Few Hours**
* Tissues are using glucose → ↓ \[blood glucose\]
* Prevent hypoglycemia, liver releases glucose into bloodstream
* \[blood glucose\] stays constant to at least euglycemia at ≈ 4 mM](https://knowt-user-attachments.s3.amazonaws.com/f3fe17db0b944b79be4d61c0c9fb0b2e.jpeg)
67
New cards
What happen at first 24 hours?
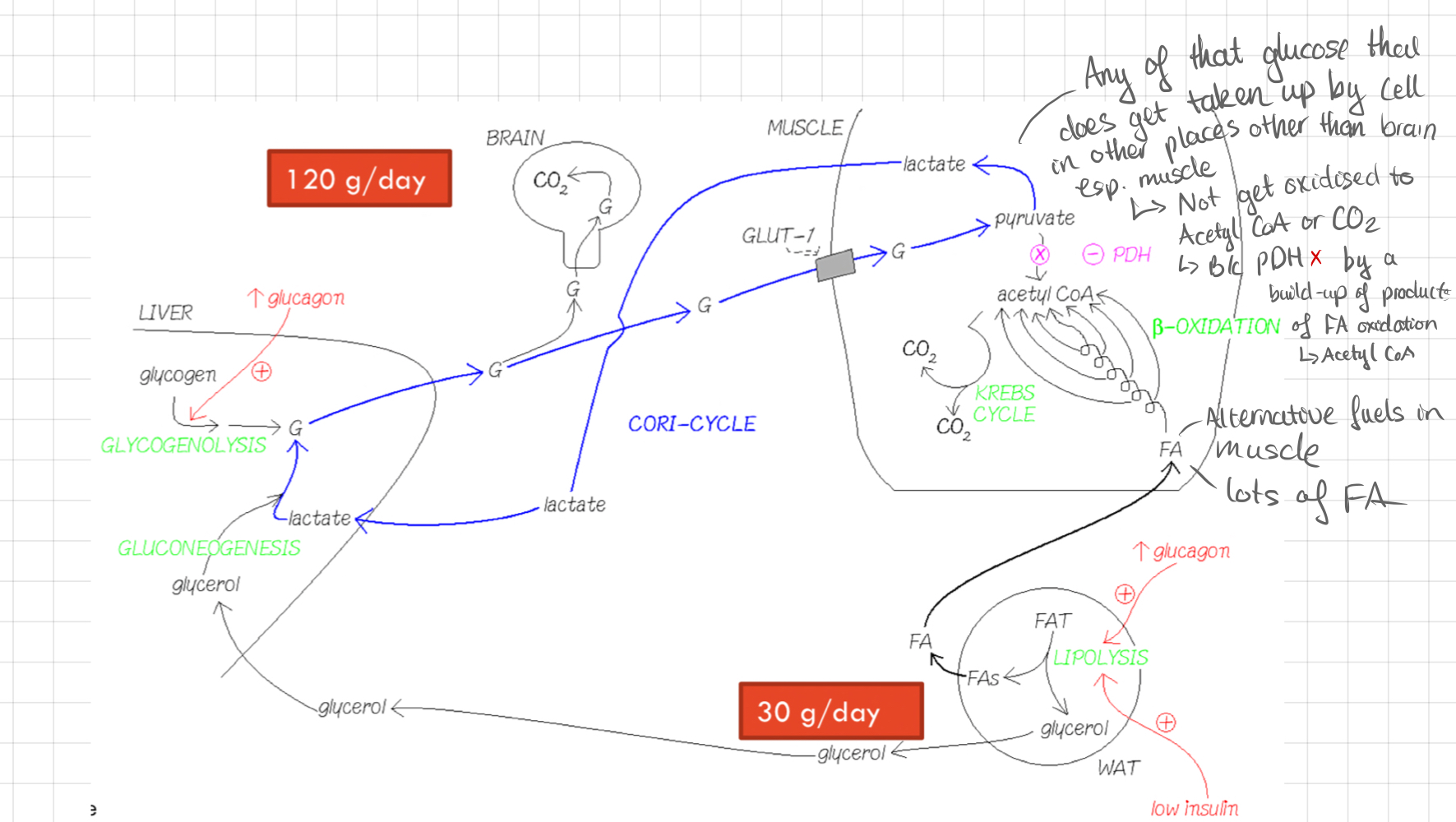
68
New cards
Glycogen Mobilisation or Glycogenolysis
* Breakdown of glycogen to release glucose.
* __**Signal:**__ The binding of **glucagon** to the receptor on liver cell membrane
* **Glycogen phosphorylase**
* Cleaves glucose units from the glycogen molecule, and a branching enzyme removes branch points using **Phosphates.**
* Produce **G-1P**
* Rapidly converted into **G 6-P**
* __**Signal:**__ The binding of **glucagon** to the receptor on liver cell membrane
* **Glycogen phosphorylase**
* Cleaves glucose units from the glycogen molecule, and a branching enzyme removes branch points using **Phosphates.**
* Produce **G-1P**
* Rapidly converted into **G 6-P**
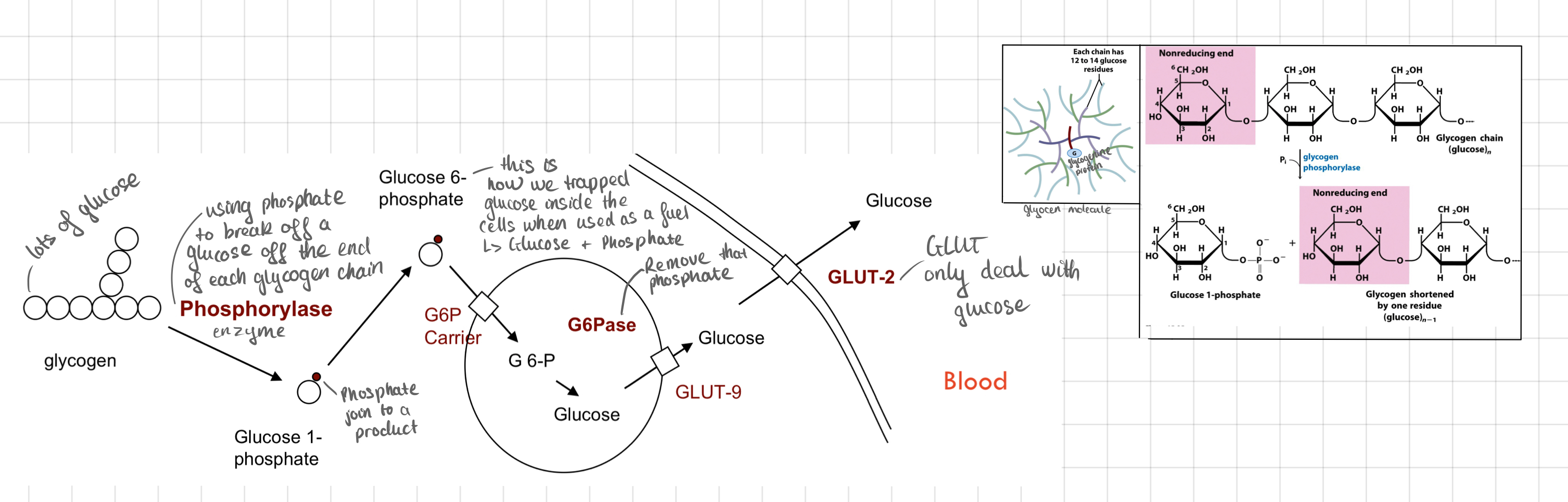
69
New cards
Phosphorylase Activation by Glucagon
* Glucagon activates **glycogen phosphorylase** via cAMP and protein kinase A (PKA).
* The PKA activates glycogen phosphorylase, leading to glycogen breakdown and glucose release.
* The PKA activates glycogen phosphorylase, leading to glycogen breakdown and glucose release.
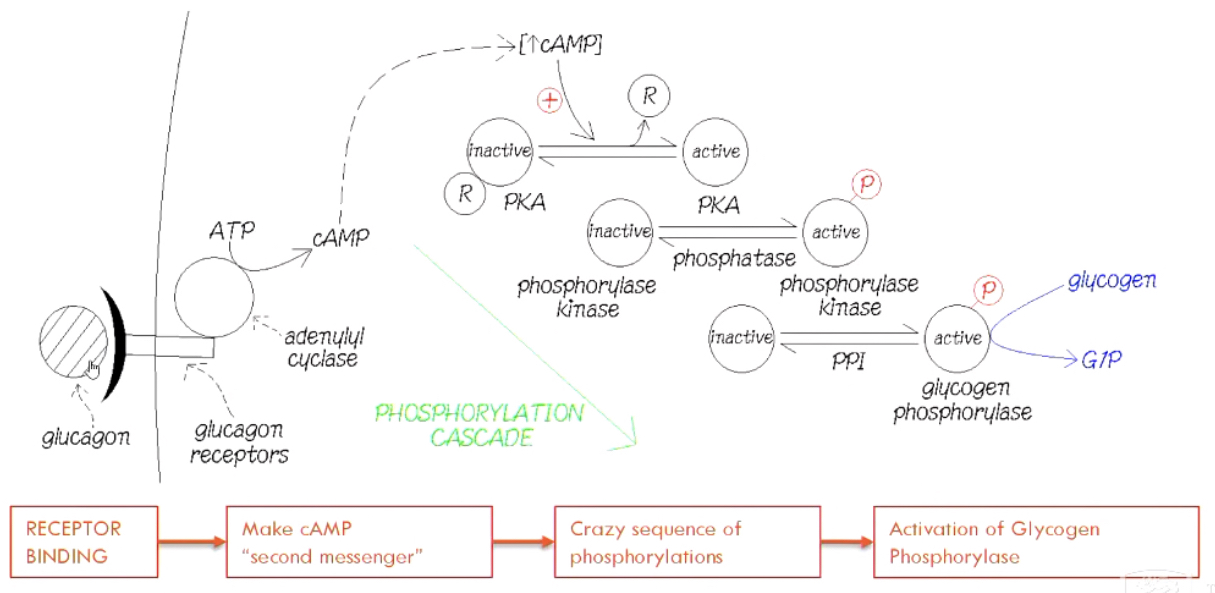
70
New cards
How much ATP is used?
* Not much ATP is consumed
* The amount of ATP being used and amount of cAMP being made are very tiny → Not really affect \[ATP\]cell
* cAMP is the 2nd messenger in the pathway
* Tiny changes in conc are detected by __**PKA (Protein Kinase A)**__
* PKA is activated by removing a regulatory inhibitory subunit
* The amount of ATP being used and amount of cAMP being made are very tiny → Not really affect \[ATP\]cell
* cAMP is the 2nd messenger in the pathway
* Tiny changes in conc are detected by __**PKA (Protein Kinase A)**__
* PKA is activated by removing a regulatory inhibitory subunit
71
New cards
Why it’s so complicated?
* Amplification through 2nd messenger and cascade, rather than directed binding
* Massive response from small signal
↳ Each step catalysed by an enzyme
* More control over the whole process
↳ Each enzyme can be further influenced by other factors (e.g. Ca2+ and AMP)
* ≠ in muscle → Adrenaline is the stimulant
* Massive response from small signal
↳ Each step catalysed by an enzyme
* More control over the whole process
↳ Each enzyme can be further influenced by other factors (e.g. Ca2+ and AMP)
* ≠ in muscle → Adrenaline is the stimulant
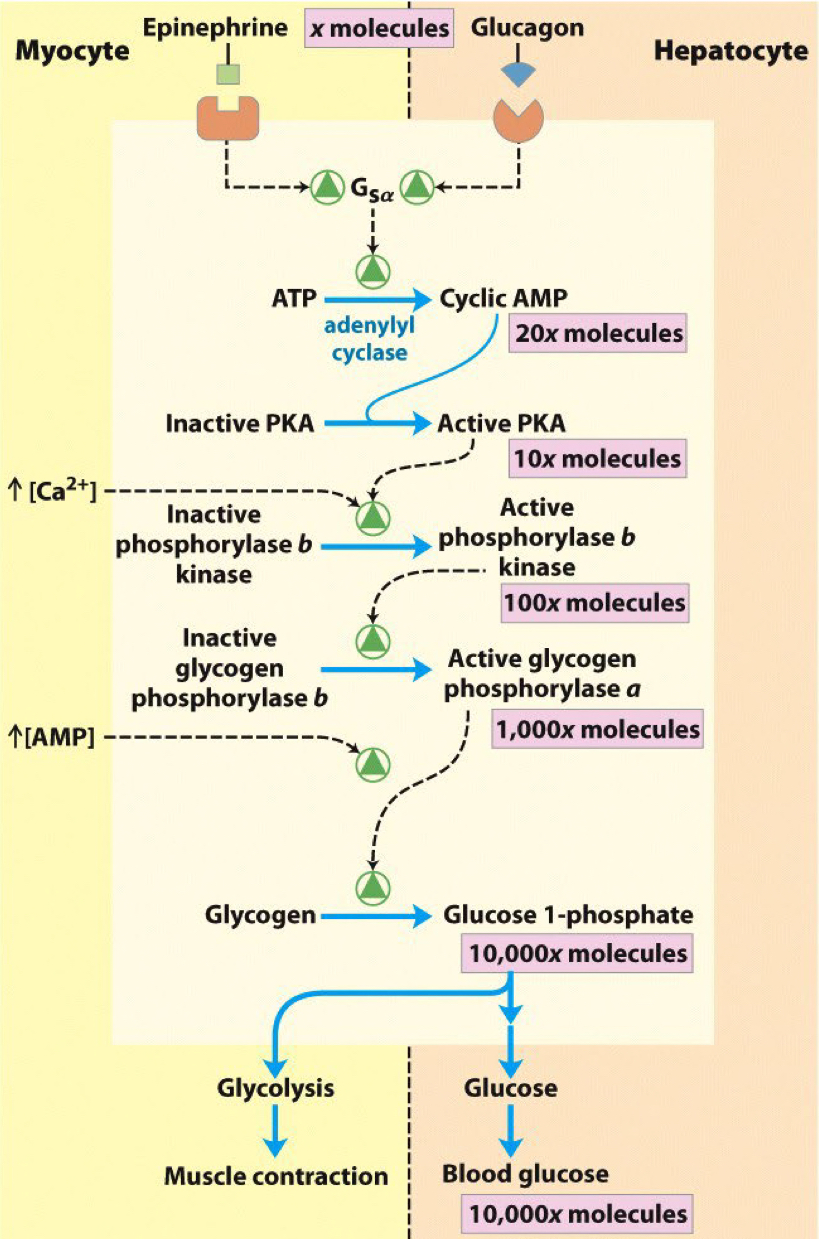
72
New cards
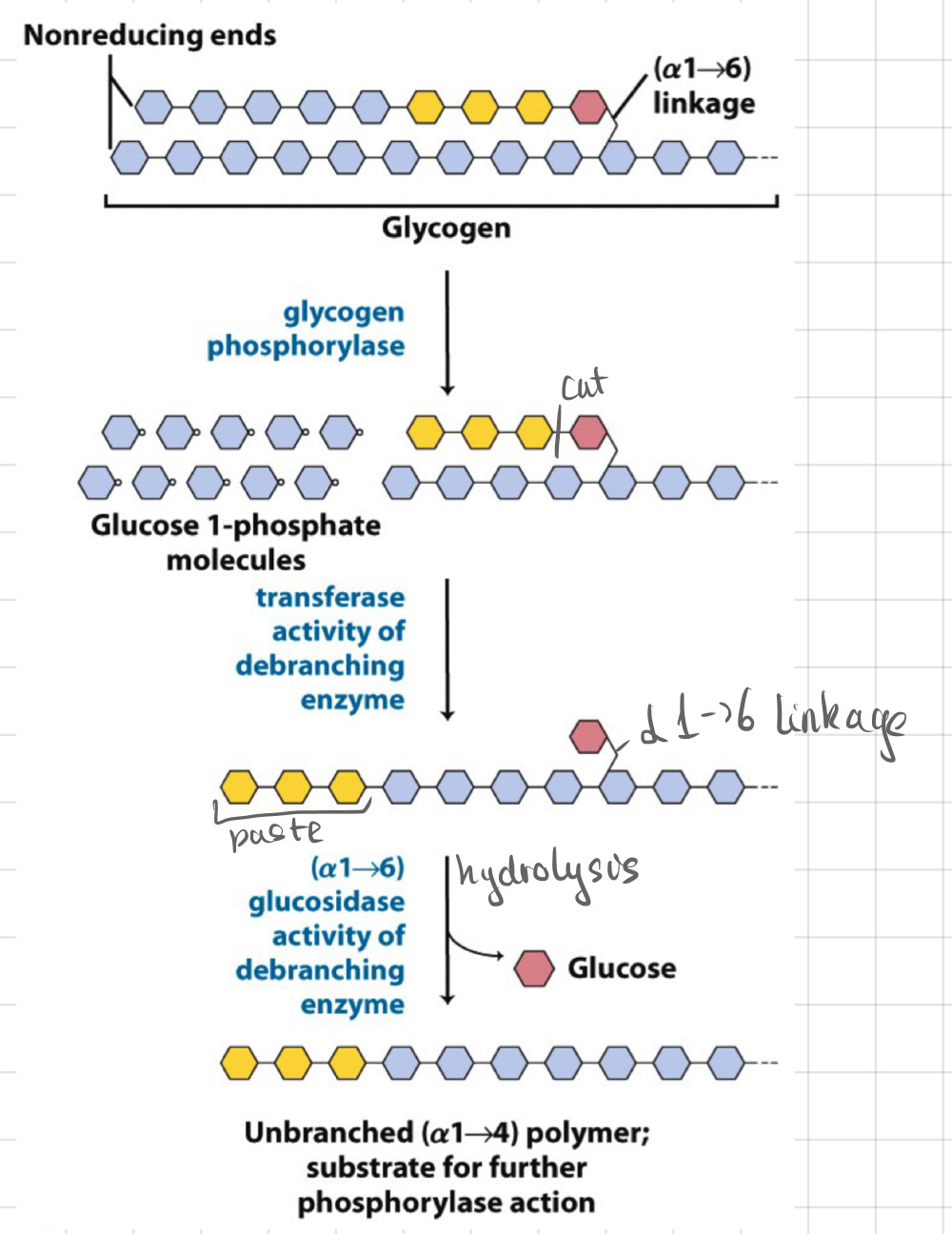
Branch points of Glucose
* __**Debranching enzyme**__
* At the branch points, a simple hydrolysis is used
* ≈ 10% of the glucose residues are released as glucose (__not__ glucose 1-P)
* At the branch points, a simple hydrolysis is used
* ≈ 10% of the glucose residues are released as glucose (__not__ glucose 1-P)
73
New cards
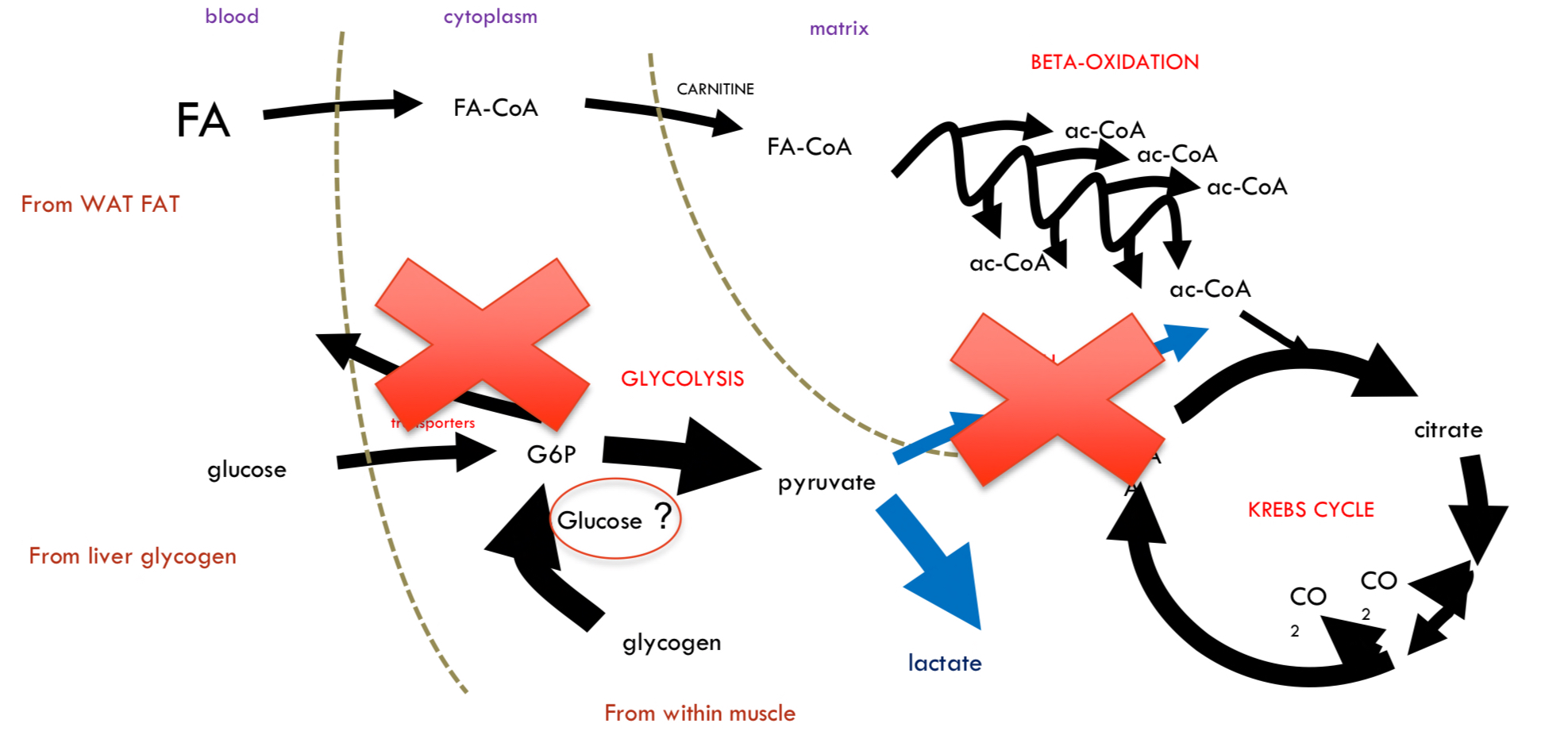
Does muscle contribute to blood glucose?
* Muscle __doesn’t__ breakdown glycogen much in starvation, bc
* NO glucagon receptors
* NO **G6Pase** (only liver has)
↳ Cannot convert G6P to glucose → Cannot release glucose into blood.
* However, some glucose residues in glycogen are released as neat glucose
* B/c debranching enzyme uses water to hydrolysed the **glycosidic linkages**, not phosphate
* ≈ 10% potentially released
* Muscle is selfish with its glucagon, but what if PDH is inhibited, G6P will go to lactate
* NO glucagon receptors
* NO **G6Pase** (only liver has)
↳ Cannot convert G6P to glucose → Cannot release glucose into blood.
* However, some glucose residues in glycogen are released as neat glucose
* B/c debranching enzyme uses water to hydrolysed the **glycosidic linkages**, not phosphate
* ≈ 10% potentially released
* Muscle is selfish with its glucagon, but what if PDH is inhibited, G6P will go to lactate
74
New cards
White Adipose Tissue (WAT) Lipolysis
* WAT lipolysis is the breakdown of stored fat in white adipose tissue.
* Accessing the large reserves of fat in WAT
* Glucagon → ↑ \[cAMP\] → ↑ activity of PKA
* PKA then phosphorylates __**Hormone Sensitive Lipase (HSL)**__ (breakdown fat) → Cleaves **triglycerides** into fatty acids and glycerol.
* PKA also phosphorylates **perilipin** (shell surrounding the vacuole)
↳ Allow the activated HSL to interact with the fat
* FAs released into the bloodstream
* Glycerol can return to liver → Convert back to glucose (small amount)
* Accessing the large reserves of fat in WAT
* Glucagon → ↑ \[cAMP\] → ↑ activity of PKA
* PKA then phosphorylates __**Hormone Sensitive Lipase (HSL)**__ (breakdown fat) → Cleaves **triglycerides** into fatty acids and glycerol.
* PKA also phosphorylates **perilipin** (shell surrounding the vacuole)
↳ Allow the activated HSL to interact with the fat
* FAs released into the bloodstream
* Glycerol can return to liver → Convert back to glucose (small amount)
![* WAT lipolysis is the breakdown of stored fat in white adipose tissue.
* Accessing the large reserves of fat in WAT
* Glucagon → ↑ \[cAMP\] → ↑ activity of PKA
* PKA then phosphorylates __**Hormone Sensitive Lipase (HSL)**__ (breakdown fat) → Cleaves **triglycerides** into fatty acids and glycerol.
* PKA also phosphorylates **perilipin** (shell surrounding the vacuole)
↳ Allow the activated HSL to interact with the fat
* FAs released into the bloodstream
* Glycerol can return to liver → Convert back to glucose (small amount)](https://knowt-user-attachments.s3.amazonaws.com/569879d0944449e6ba6b16d2255c8487.jpeg)
75
New cards
What are the effects of FA oxidation?
* FAs will be oxidised to provide the acetyl CoA for the Krebs Cycle
* But need to avoid oxidation
* PDH ( pyruvate → AcCoA) is __Irreversible__
* **GLUT-1** is still present in muscle
* Even though GLUT-4s were endocytosed due to a lack of insulin
↳ Muscle can still take up glucose
* Need to preserve glucose
* Get tissues to stop oxidising glucose
* But need to avoid oxidation
* PDH ( pyruvate → AcCoA) is __Irreversible__
* **GLUT-1** is still present in muscle
* Even though GLUT-4s were endocytosed due to a lack of insulin
↳ Muscle can still take up glucose
* Need to preserve glucose
* Get tissues to stop oxidising glucose
76
New cards
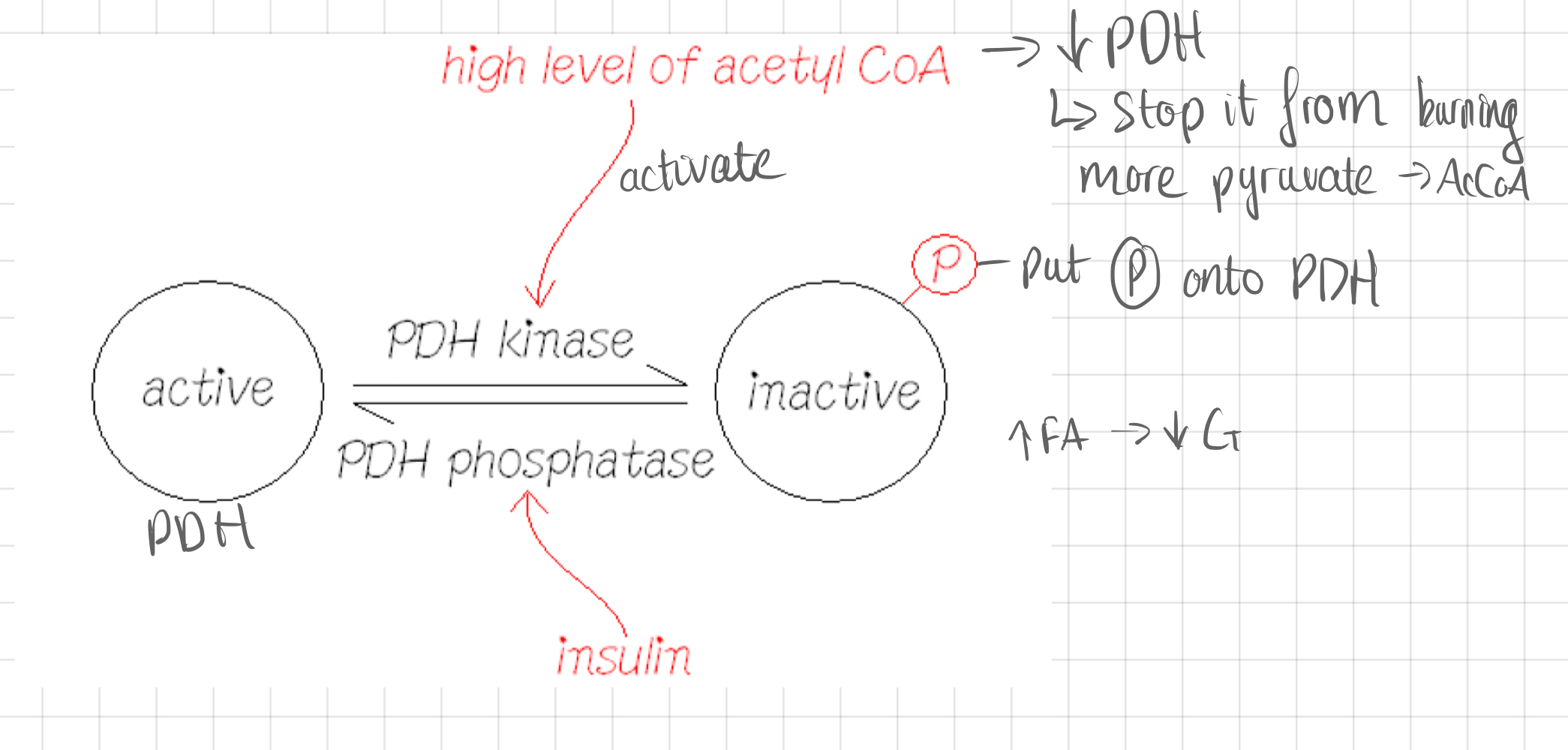
Activating PDH
77
New cards
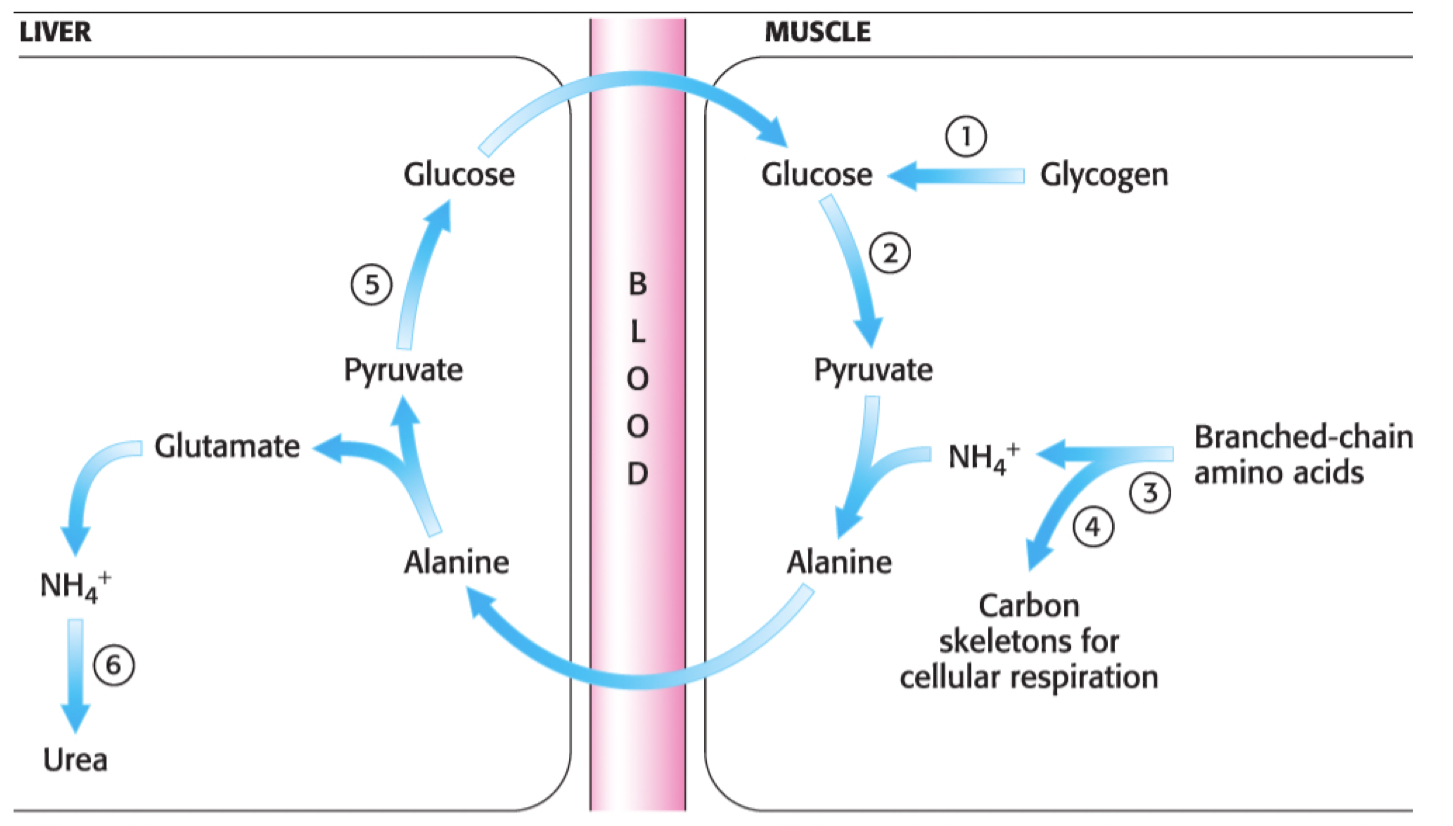
Glucose-Fatty Acid Cycle
### **In starvation, PDH needs to be off**
* PDH Kinase activity >> PDH phosphate activity
* Acetyl CoA stimulates PDH
* PDH is inactive when phosphorylated
* Prevents wasteful oxidation of pyruvate
* Pyruvate only converted into lactate
### **When PDH is off**
* Pyruvate cannot be oxidised to acetyl CoA
* Then, there is only 1 fate for pyruvate in the muscle to be converted into lactate by LDH.
* Lactate can be taken up by liver.
* Remade into glucose by **gluconeogenesis**
* Called __**Cori-cycle**__
* Muscle glucose → Pyruvate → Lactate → Liver → Glucose (via gluconeogenesis) → Glucose into bloodstream again
* **Gluconeogenesis** can also happen from **glycerol**
* Made by 30g glucose per day
* **Glycerol** (from lipolysis) is the only source of de novo gluconeogenesis
* Lactate-fueled gluconeogenesis is recycling
* PDH Kinase activity >> PDH phosphate activity
* Acetyl CoA stimulates PDH
* PDH is inactive when phosphorylated
* Prevents wasteful oxidation of pyruvate
* Pyruvate only converted into lactate
### **When PDH is off**
* Pyruvate cannot be oxidised to acetyl CoA
* Then, there is only 1 fate for pyruvate in the muscle to be converted into lactate by LDH.
* Lactate can be taken up by liver.
* Remade into glucose by **gluconeogenesis**
* Called __**Cori-cycle**__
* Muscle glucose → Pyruvate → Lactate → Liver → Glucose (via gluconeogenesis) → Glucose into bloodstream again
* **Gluconeogenesis** can also happen from **glycerol**
* Made by 30g glucose per day
* **Glycerol** (from lipolysis) is the only source of de novo gluconeogenesis
* Lactate-fueled gluconeogenesis is recycling
78
New cards
What is __**Proteolysis?**__
* The process of breaking down proteins into smaller peptide fragments or individual amino acids.
* After a few a hrs, \[blood glucose\] < 5 mM → Insulin secretion stops
* Important in stimulating lipolysis
* **Hypoinsulinemia** → **Proteolysis**
* Release of amino acids from tissues (mainly muscles)
* Many amino acid ‘carbon skeletons’ are used for gluconeogenesis
* Need to get amino acids to the liver
* Need to do sth with an amine group (Ammonia is poisonous)
* **Carbon skeletons** – the portion of the molecule remaining after the removal of nitrogen
* After a few a hrs, \[blood glucose\] < 5 mM → Insulin secretion stops
* Important in stimulating lipolysis
* **Hypoinsulinemia** → **Proteolysis**
* Release of amino acids from tissues (mainly muscles)
* Many amino acid ‘carbon skeletons’ are used for gluconeogenesis
* Need to get amino acids to the liver
* Need to do sth with an amine group (Ammonia is poisonous)
* **Carbon skeletons** – the portion of the molecule remaining after the removal of nitrogen
79
New cards
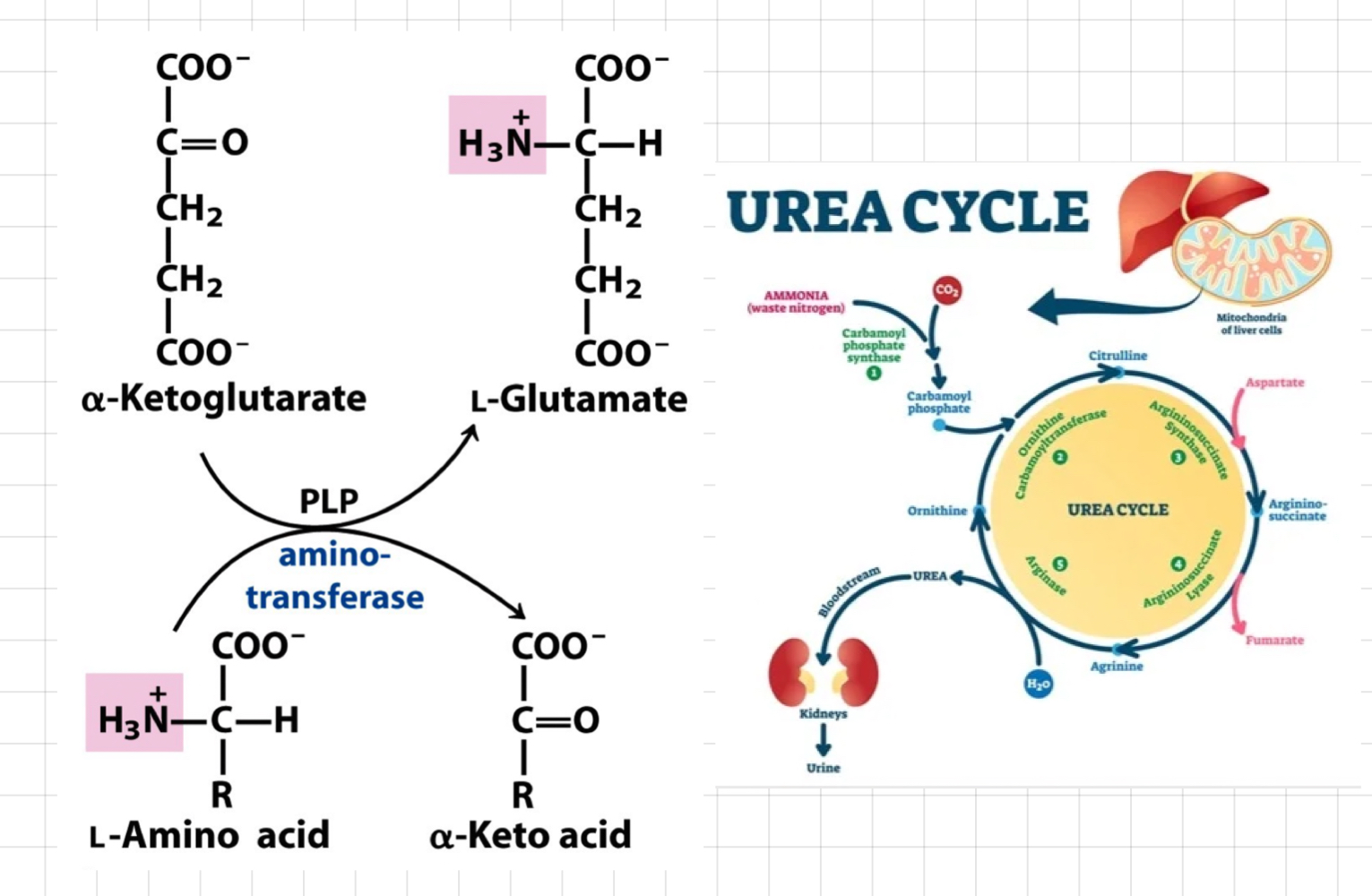
What are **Processing Amino Acids** and the **Fate of Amine Groups?**
### **Processing Amino Acids**
* Channel amine groups to 3 amino acids
↳ **Alanine**, **Glutamate** and **Aspartate**
* 3 acceptors are all found in the main pathways
* ==__**Pyruvate**__== **→** ==**Alanine**==
* __**α-ketoglutarate**__ **→** **Glutamate**
* @@__**Oxaloacetate**__@@ **→** @@**Aspartate**@@
* Result in **α-keto acids** used in gluconeogenesis
### **Fate of Amine Groups**
* __**Urea Cycle**__ – Only in liver
* The body’s way of converting toxic ammonia into urea.
* Ammine groups are channelled into urea.
* Synthesised from aspartate and glutamate
* Consume lots of ATP
* Urea is non-toxic
* Channel amine groups to 3 amino acids
↳ **Alanine**, **Glutamate** and **Aspartate**
* 3 acceptors are all found in the main pathways
* ==__**Pyruvate**__== **→** ==**Alanine**==
* __**α-ketoglutarate**__ **→** **Glutamate**
* @@__**Oxaloacetate**__@@ **→** @@**Aspartate**@@
* Result in **α-keto acids** used in gluconeogenesis
### **Fate of Amine Groups**
* __**Urea Cycle**__ – Only in liver
* The body’s way of converting toxic ammonia into urea.
* Ammine groups are channelled into urea.
* Synthesised from aspartate and glutamate
* Consume lots of ATP
* Urea is non-toxic
80
New cards
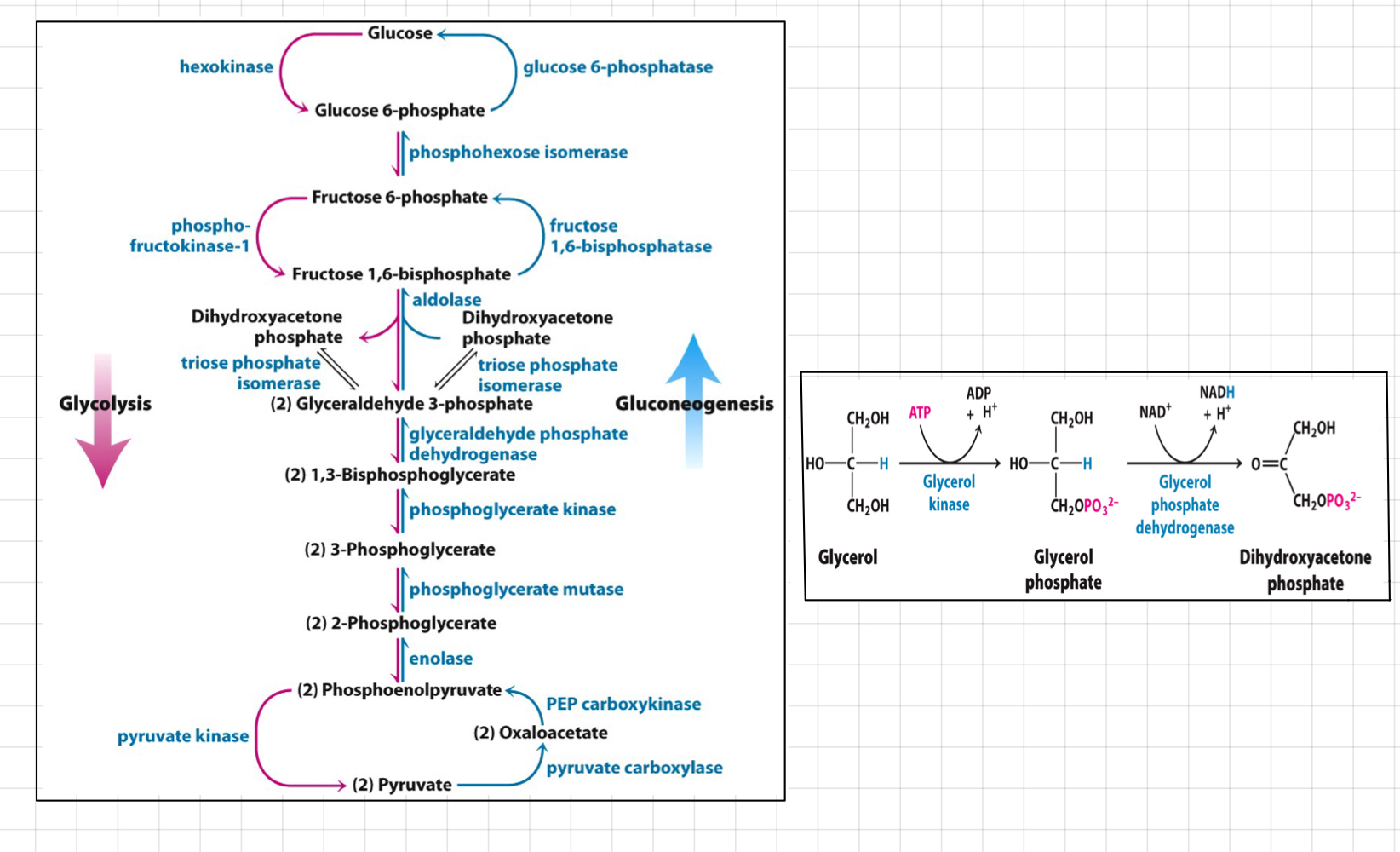
What is __**Gluconeogenesis?**__
* A reversal of Glycolysis
* Amino acids and glycerol are converted into glucose.
* Maintains blood glucose levels during fasting or low carbohydrate intake.
* **Except** 3 ‘rate limiting ‘ steps bypassed
* ==__**Hexokinase**__== **→** ==**Glucose trapping step**==
* __**Phosphofructokinase**__ **→** **Rate limiting step**
* @@__**Pyruvate Kinase**__@@ **→** **Final** and @@**Energy releasing step**@@
* Completing the pathway __only__ in liver
* Mainly cytoplasmic → **Pyruvate carboxylase**
* __Substrates:__
* __**Lactate**__ **→** Enter as **pyruvate** at the bottom
* __**Glycerol**__ **→** Enter as **dihydroxyacetone phosphate**
* __**Amino acid carbon skeletons**__ **→** Enter as various places
* Amino acids and glycerol are converted into glucose.
* Maintains blood glucose levels during fasting or low carbohydrate intake.
* **Except** 3 ‘rate limiting ‘ steps bypassed
* ==__**Hexokinase**__== **→** ==**Glucose trapping step**==
* __**Phosphofructokinase**__ **→** **Rate limiting step**
* @@__**Pyruvate Kinase**__@@ **→** **Final** and @@**Energy releasing step**@@
* Completing the pathway __only__ in liver
* Mainly cytoplasmic → **Pyruvate carboxylase**
* __Substrates:__
* __**Lactate**__ **→** Enter as **pyruvate** at the bottom
* __**Glycerol**__ **→** Enter as **dihydroxyacetone phosphate**
* __**Amino acid carbon skeletons**__ **→** Enter as various places
81
New cards
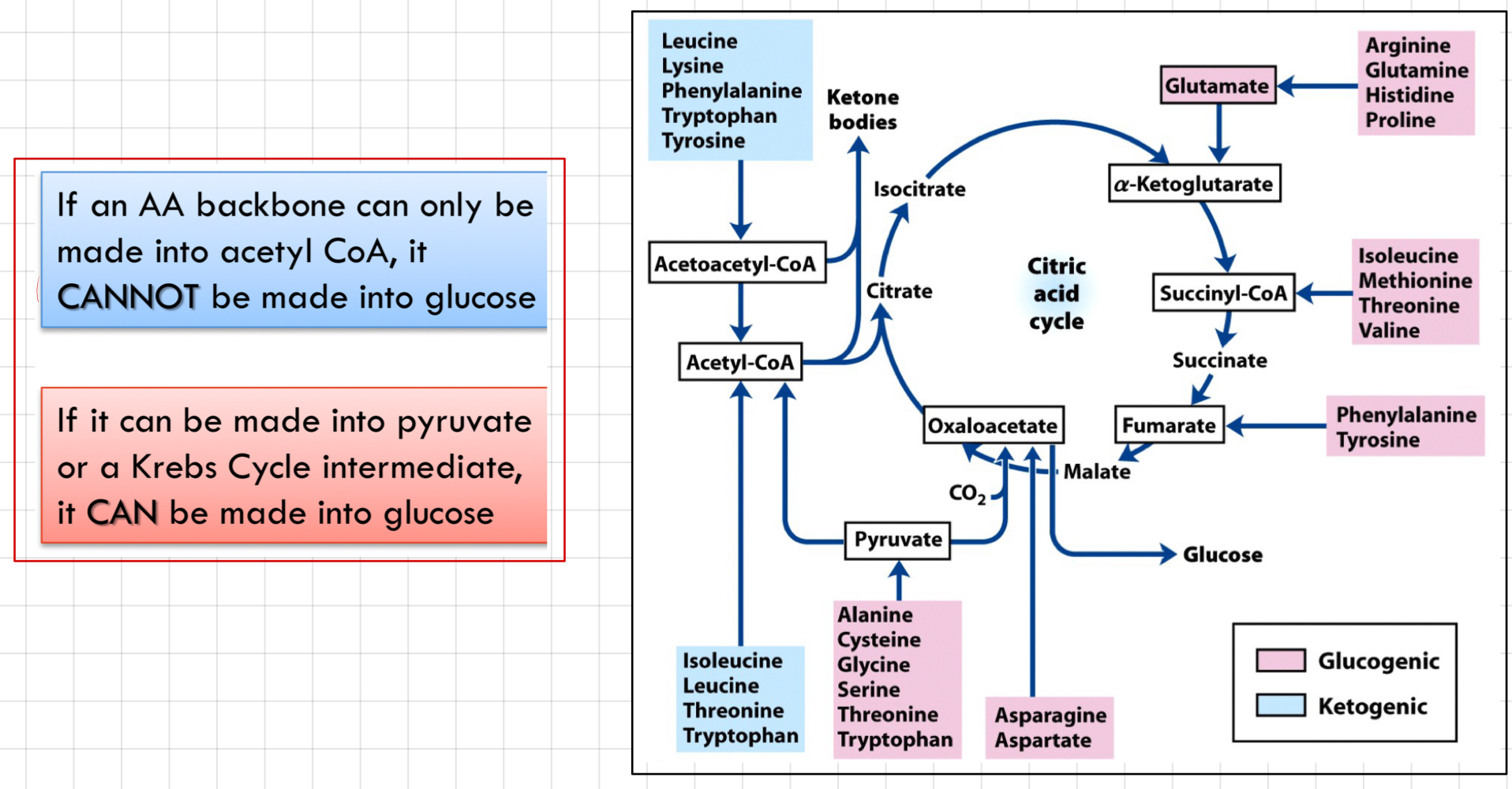
Can all amino acid skeleton make glucose?
* Not all AA skeletons can make glucose
* A carbon skeleton can be converted into an intermediate for gluconeogenesis.
* Cost for lots of energy to make proteins (made for reasons)
* Lots of ATP is required to dispose of the amine groups
* Not all amino acids made into glucose
* Many amino acids from proteolysis are burnt before release from tissue.
* Need extra 90g glucose (breakdown 180g) per day
* A carbon skeleton can be converted into an intermediate for gluconeogenesis.
* Cost for lots of energy to make proteins (made for reasons)
* Lots of ATP is required to dispose of the amine groups
* Not all amino acids made into glucose
* Many amino acids from proteolysis are burnt before release from tissue.
* Need extra 90g glucose (breakdown 180g) per day
82
New cards
Lipolysis + ß-Oxidation
* After 2-3 days of starvation, the rate of lipolysis will be at max.
* FA released into bloodstream → ↑ \[FA\] in blood → More FA than is needed
* ß-Oxidation in Liver
* Rate depends on the demand of ATP by the tissues
* Generation of CoA by Krebs Cycle needed to keep FA oxidation going
* Rate of Krebs Cycle strictly regulate by demand for ATP
* BUT ß-oxidation can occur even if ATP isn't required
* Other pathways can regenerate CoA from acetyl-CoA
* FA released into bloodstream → ↑ \[FA\] in blood → More FA than is needed
* ß-Oxidation in Liver
* Rate depends on the demand of ATP by the tissues
* Generation of CoA by Krebs Cycle needed to keep FA oxidation going
* Rate of Krebs Cycle strictly regulate by demand for ATP
* BUT ß-oxidation can occur even if ATP isn't required
* Other pathways can regenerate CoA from acetyl-CoA
![* After 2-3 days of starvation, the rate of lipolysis will be at max.
* FA released into bloodstream → ↑ \[FA\] in blood → More FA than is needed
* ß-Oxidation in Liver
* Rate depends on the demand of ATP by the tissues
* Generation of CoA by Krebs Cycle needed to keep FA oxidation going
* Rate of Krebs Cycle strictly regulate by demand for ATP
* BUT ß-oxidation can occur even if ATP isn't required
* Other pathways can regenerate CoA from acetyl-CoA](https://knowt-user-attachments.s3.amazonaws.com/ccf9f63271ad45019737ddd1fa01353f.jpeg)
83
New cards
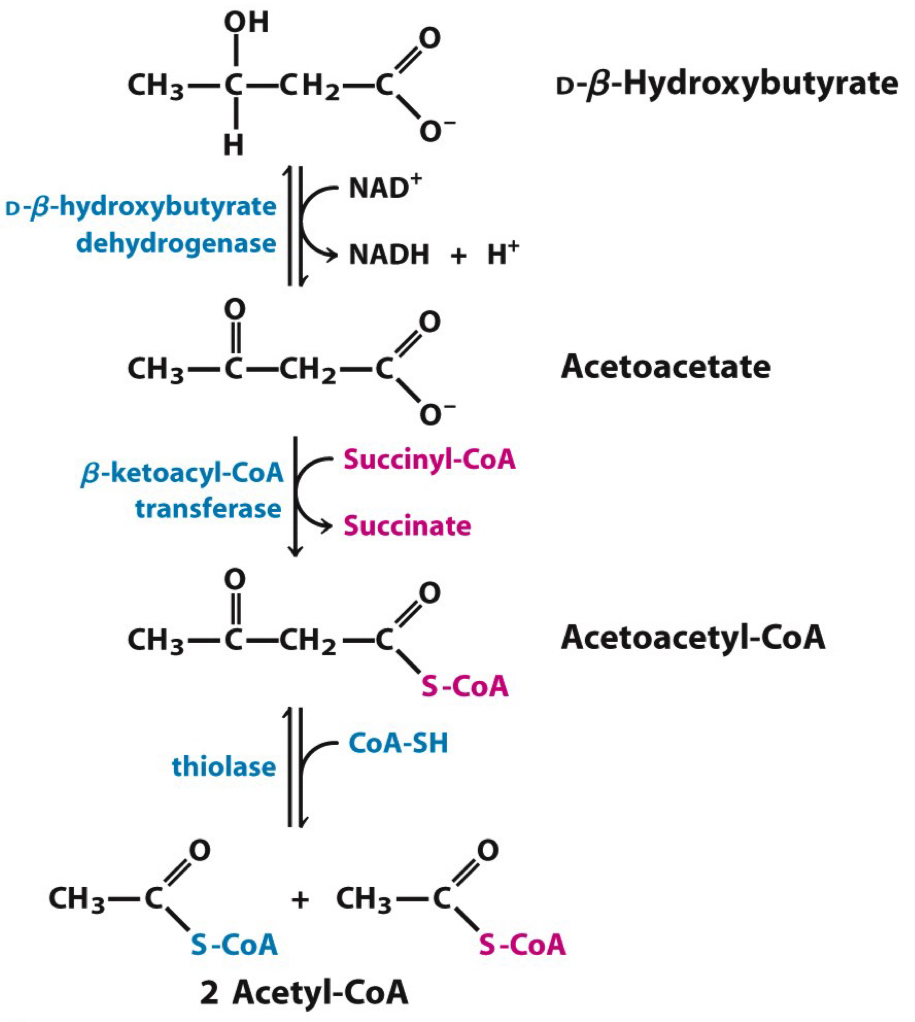
What is the function of **Fate of Acetoacetate (Ketone body)**?
* __**Acetoacetate,**__ or a __**Ketone body**__, is used as an alternative energy source by various tissues for energy.
* It can be converted
* Back into **acetyl-CoA** to generate ATP or
* Into other ketone bodies like **beta-hydroxybutyrate** and **acetone**.
* Split in the mitochondria to acetyl CoA
* An instant source of fuel for the Krebs Cycle
* AcCoA inhibits PDH and stimulates PDH kinase
* Reduces brain glucose consumption
* It can be converted
* Back into **acetyl-CoA** to generate ATP or
* Into other ketone bodies like **beta-hydroxybutyrate** and **acetone**.
* Split in the mitochondria to acetyl CoA
* An instant source of fuel for the Krebs Cycle
* AcCoA inhibits PDH and stimulates PDH kinase
* Reduces brain glucose consumption
84
New cards
Functions of Acetoacetate (Ketone body)
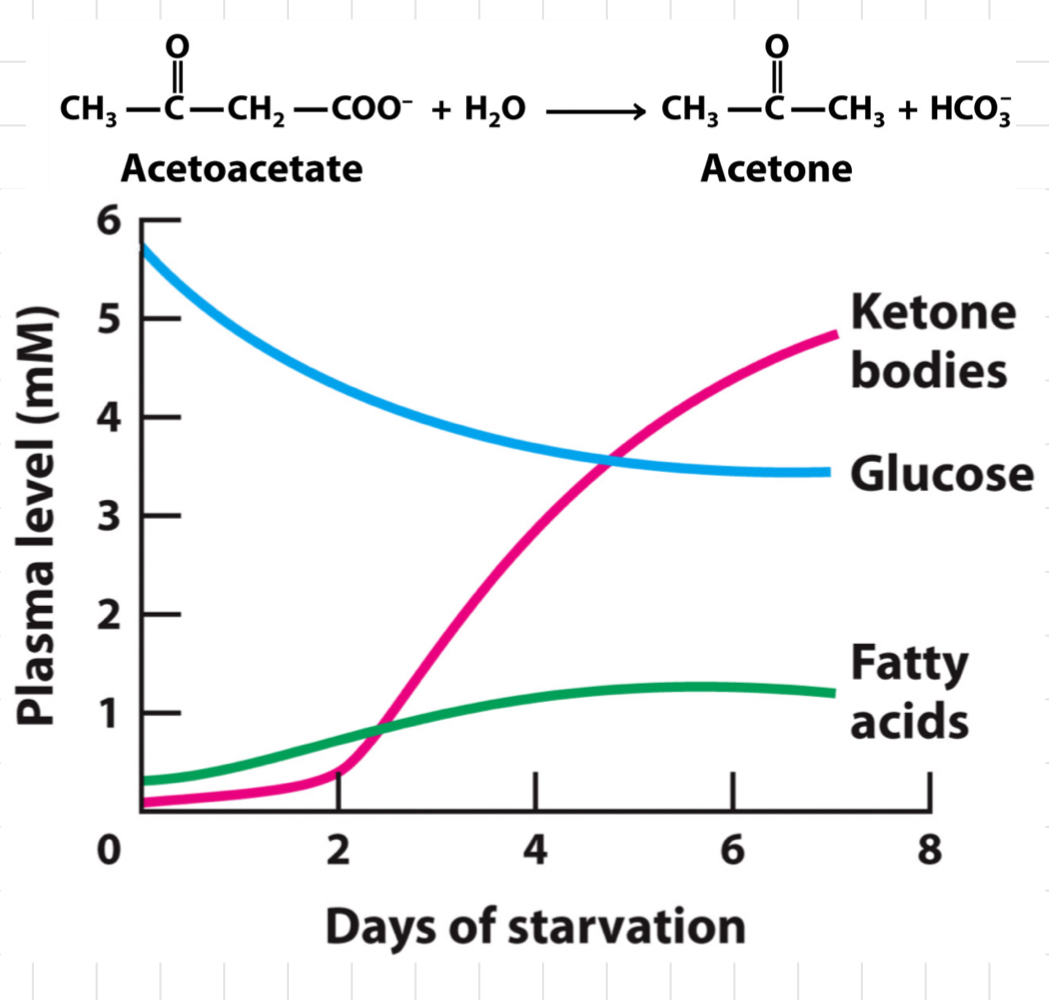
### **Inefficiency in Ketone Body Metabolism**
* Nothing inefficient about the oxidation
* But ketone body
* Lost in the urine
* Spontaneously decarboxylate
### **Have Ketone Bodies Saved Us?**
* Make 30g glucose per day from glycerol
* After 2 days of starvation
* Brain using 120g glucose a day
* Protein loss > 100g protein/day
* After 3-4 days
* Ketone bodies are lowering the brain’s need for glucose
* Protein losses ≈ 75g/day
* By day 5
* Brain using 30g glucose/day
* Nothing inefficient about the oxidation
* But ketone body
* Lost in the urine
* Spontaneously decarboxylate
### **Have Ketone Bodies Saved Us?**
* Make 30g glucose per day from glycerol
* After 2 days of starvation
* Brain using 120g glucose a day
* Protein loss > 100g protein/day
* After 3-4 days
* Ketone bodies are lowering the brain’s need for glucose
* Protein losses ≈ 75g/day
* By day 5
* Brain using 30g glucose/day
85
New cards
How is the protein related to tissues' demand performing?
* Proteins are lost from all tissues
* Inactive muscles slightly preferentially degraded
* Will reach equilibrium
* The loss of body protein is ultimately what kills us
* Loss of function
* Much more prone to infection
### Amount of protein breakdown = (amount of glucose needed - 30) x ≈ 2
* Inactive muscles slightly preferentially degraded
* Will reach equilibrium
* The loss of body protein is ultimately what kills us
* Loss of function
* Much more prone to infection
### Amount of protein breakdown = (amount of glucose needed - 30) x ≈ 2
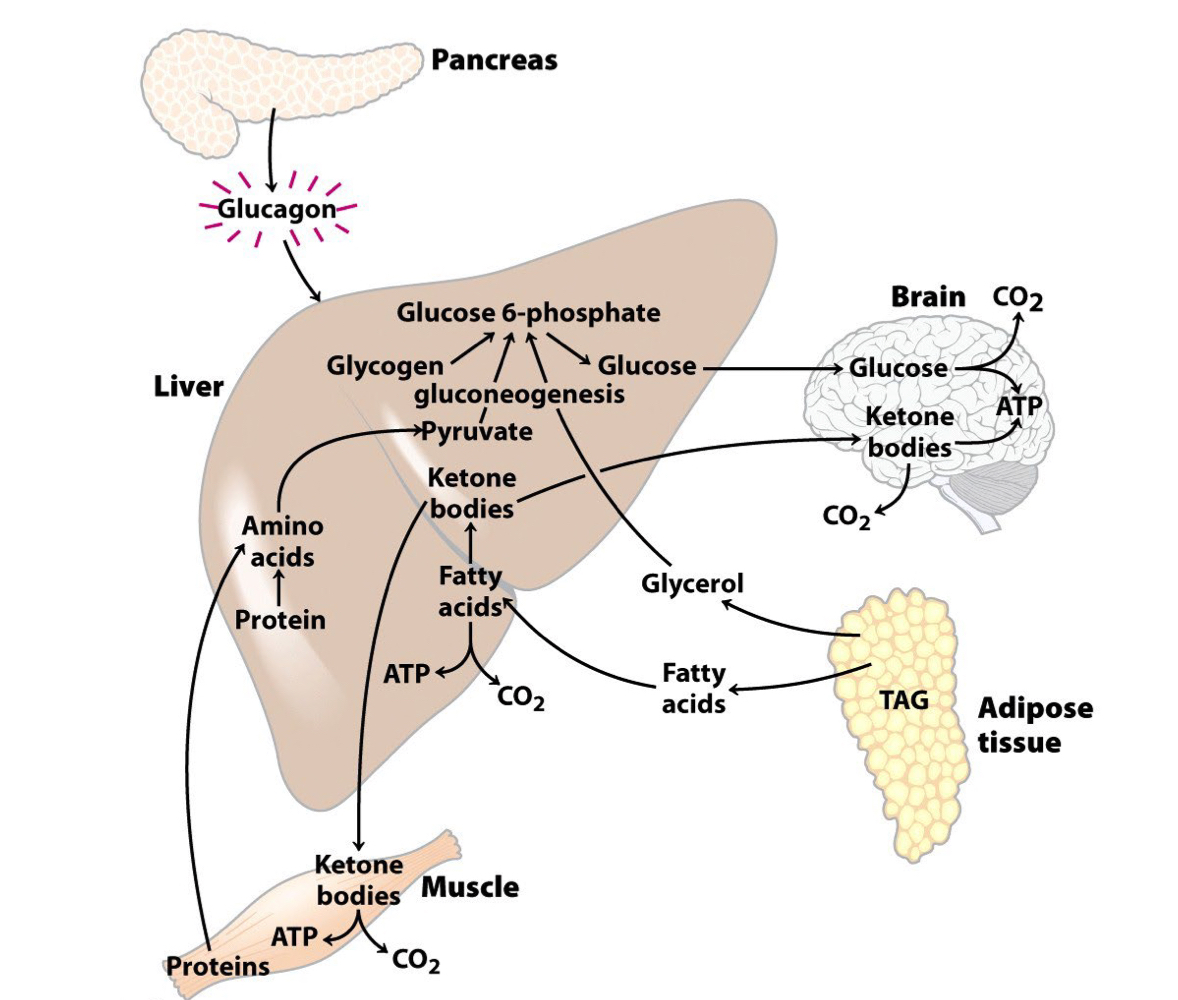
86
New cards
The importance of Glucagon
* Glucagon promotes glycogen breakdown (glycogenolysis) and stimulates gluconeogenesis, which raises blood glucose.
* Maintains glucose homeostasis during fasting.
* Maintains glucose homeostasis during fasting.
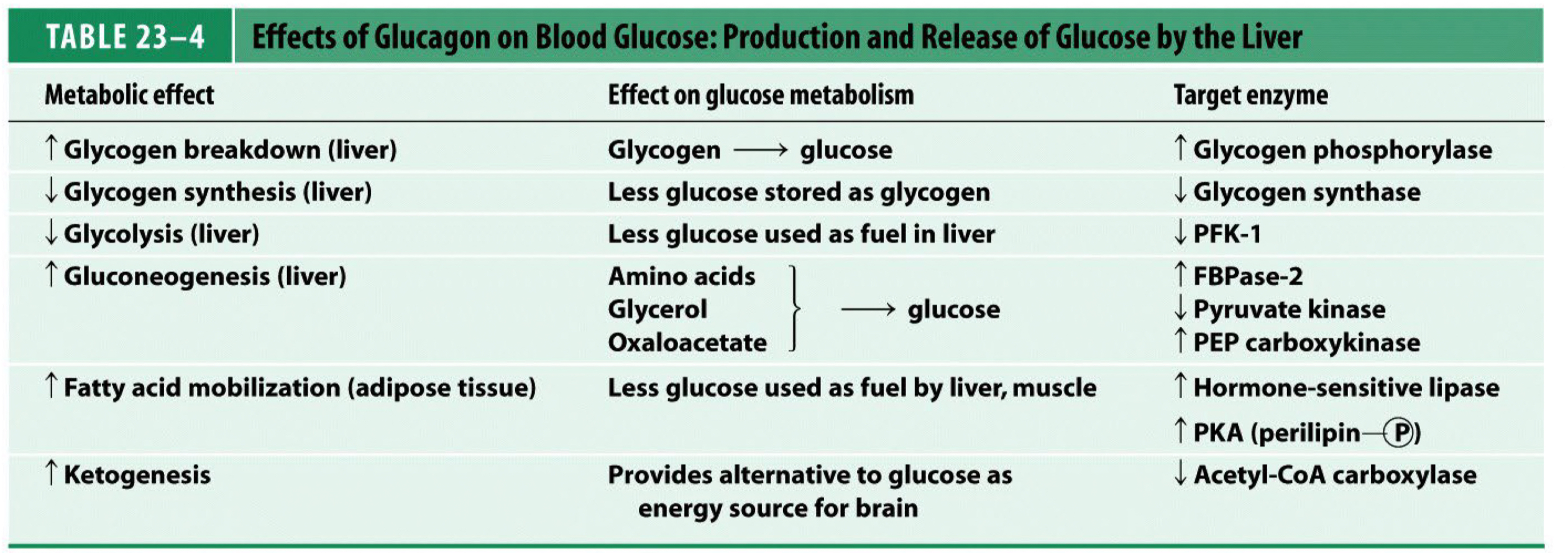
87
New cards
Energy Charge and ATP
* ATP is __**not**__ the most energy-containing molecule in metabolism
* Need to keep at 5 mM
* Instant reserves
* Compounds that phosphorylate substrates
* Only few seconds supply
* Still need ↑ catabolic pathway
### **ATP**
* Energy released when any of the terminal phosphates are hydrolysed
* ATP → ADP or ADP → AMP releasing energy
### **Adenylate Kinase (AK)**
* Converts ATP to ADP, helping to buffer energy fluctuations.
* 2ADP ↔ ATP + AMP
* Translate small change in ATP and large change in AMP
* Ratio of \[Adenine molecule\] = **Energy charge**
* __Key molecule:__ AMP
* ATP:ADP kept high
* Key enzymes very sensitive to \[ADP\]
* Need to keep at 5 mM
* Instant reserves
* Compounds that phosphorylate substrates
* Only few seconds supply
* Still need ↑ catabolic pathway
### **ATP**
* Energy released when any of the terminal phosphates are hydrolysed
* ATP → ADP or ADP → AMP releasing energy
### **Adenylate Kinase (AK)**
* Converts ATP to ADP, helping to buffer energy fluctuations.
* 2ADP ↔ ATP + AMP
* Translate small change in ATP and large change in AMP
* Ratio of \[Adenine molecule\] = **Energy charge**
* __Key molecule:__ AMP
* ATP:ADP kept high
* Key enzymes very sensitive to \[ADP\]
![* ATP is __**not**__ the most energy-containing molecule in metabolism
* Need to keep at 5 mM
* Instant reserves
* Compounds that phosphorylate substrates
* Only few seconds supply
* Still need ↑ catabolic pathway
### **ATP**
* Energy released when any of the terminal phosphates are hydrolysed
* ATP → ADP or ADP → AMP releasing energy
### **Adenylate Kinase (AK)**
* Converts ATP to ADP, helping to buffer energy fluctuations.
* 2ADP ↔ ATP + AMP
* Translate small change in ATP and large change in AMP
* Ratio of \[Adenine molecule\] = **Energy charge**
* __Key molecule:__ AMP
* ATP:ADP kept high
* Key enzymes very sensitive to \[ADP\]](https://knowt-user-attachments.s3.amazonaws.com/f08283ea243749e18f5096174ba6d5c2.jpeg)
88
New cards
Which enzymes are controlled these?
### **Rate-limiting step (RLS)**
* Slowest enzyme-catalysed reaction in a metabolic pathway.
* Control the overall rate of metabolic activity
* Irreversible
* Need alternative enzymes to go back
* NOT equilibrium under physiological conditions
* Saturated (bao hoa) with substrate.
* Low Km or \[S\] > Km
* Working at Vmax
### **Flux generating step**
* Contributes significantly to the overall flux or rate of metabolite flow through the pathway.
* Often associated with the rate-limiting step, as it determines the overall speed of the pathway.
* 3 major ways to regulate
* Make the rate-limiting enzyme go faster/slower
* Turn the rate-limiting enzyme on/off or make it work the other way
* ↑ rate of transcription/translation of the RLS or change its rate of degradation
* Slowest enzyme-catalysed reaction in a metabolic pathway.
* Control the overall rate of metabolic activity
* Irreversible
* Need alternative enzymes to go back
* NOT equilibrium under physiological conditions
* Saturated (bao hoa) with substrate.
* Low Km or \[S\] > Km
* Working at Vmax
### **Flux generating step**
* Contributes significantly to the overall flux or rate of metabolite flow through the pathway.
* Often associated with the rate-limiting step, as it determines the overall speed of the pathway.
* 3 major ways to regulate
* Make the rate-limiting enzyme go faster/slower
* Turn the rate-limiting enzyme on/off or make it work the other way
* ↑ rate of transcription/translation of the RLS or change its rate of degradation
![### **Rate-limiting step (RLS)**
* Slowest enzyme-catalysed reaction in a metabolic pathway.
* Control the overall rate of metabolic activity
* Irreversible
* Need alternative enzymes to go back
* NOT equilibrium under physiological conditions
* Saturated (bao hoa) with substrate.
* Low Km or \[S\] > Km
* Working at Vmax
### **Flux generating step**
* Contributes significantly to the overall flux or rate of metabolite flow through the pathway.
* Often associated with the rate-limiting step, as it determines the overall speed of the pathway.
* 3 major ways to regulate
* Make the rate-limiting enzyme go faster/slower
* Turn the rate-limiting enzyme on/off or make it work the other way
* ↑ rate of transcription/translation of the RLS or change its rate of degradation](https://knowt-user-attachments.s3.amazonaws.com/3e086438a0c544c18a1a29013f338e32.jpeg)
89
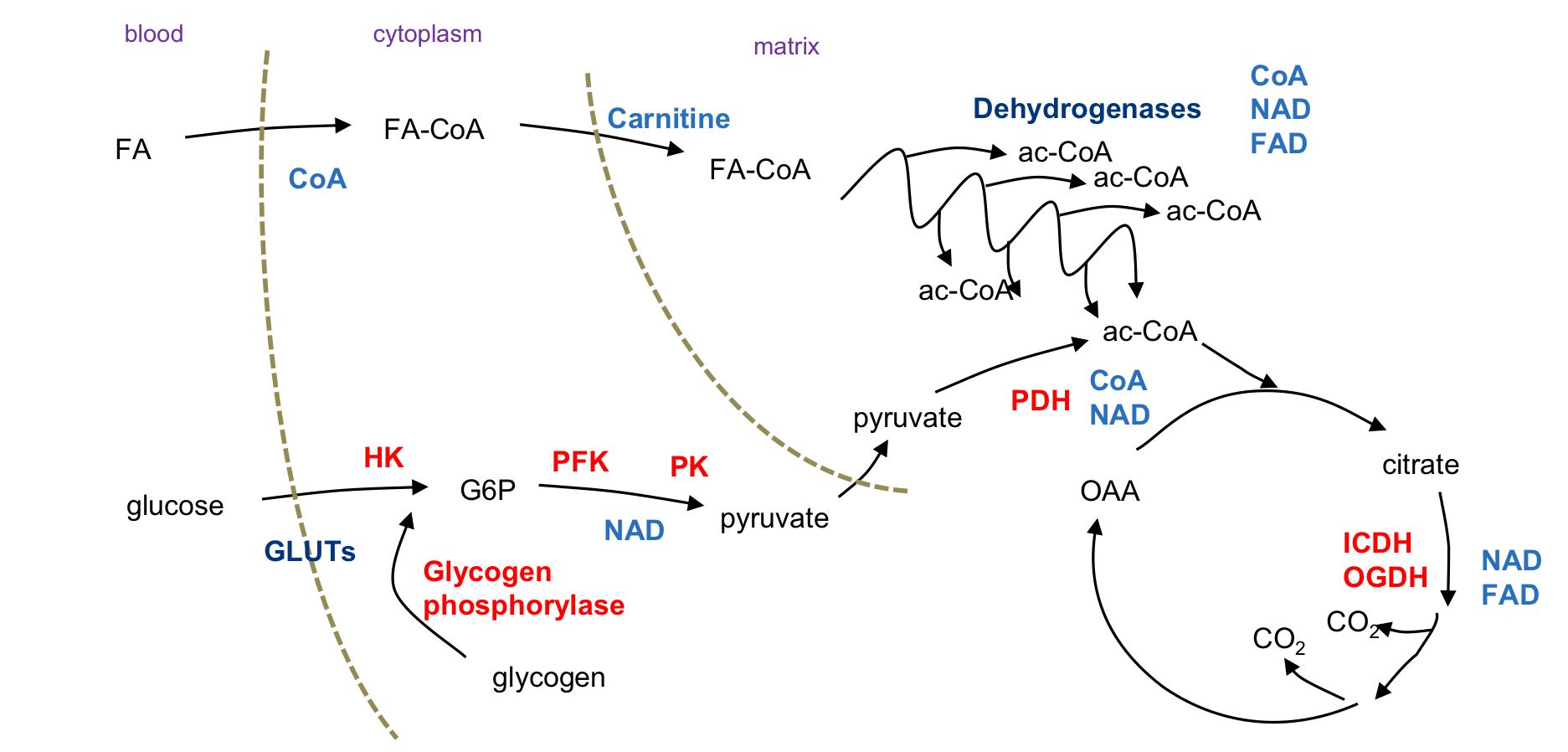
New cards
Overall catabolic pathway
90
New cards
Allosteric PFK (Phosphofructokinase)
* Involved in the anabolic pathway of glycolysis.
* Catalyses the breakdown of glucose for energy by converting **fructose-6-phosphate** to **fructose-1,6-bisphosphate**.
> F6P + ATP → F1,6BP
* In the catabolic pathway, PFK acts as an **allosteric regulator** of glycolysis and ATP production.
* The binding of ATP and AMP to PFK ensures efficient energy use.
* Has binding sites for AMP away from the active site.
↳ Biniding AMP changes the way PFK responds to ATP
* Also binds __**Citrate allosterically**__
* Citrate inhibits PFK
* \[Citrate\] high → ↑ PFK → ↓ Glycolysis
* Catalyses the breakdown of glucose for energy by converting **fructose-6-phosphate** to **fructose-1,6-bisphosphate**.
> F6P + ATP → F1,6BP
* In the catabolic pathway, PFK acts as an **allosteric regulator** of glycolysis and ATP production.
* The binding of ATP and AMP to PFK ensures efficient energy use.
* Has binding sites for AMP away from the active site.
↳ Biniding AMP changes the way PFK responds to ATP
* Also binds __**Citrate allosterically**__
* Citrate inhibits PFK
* \[Citrate\] high → ↑ PFK → ↓ Glycolysis
91
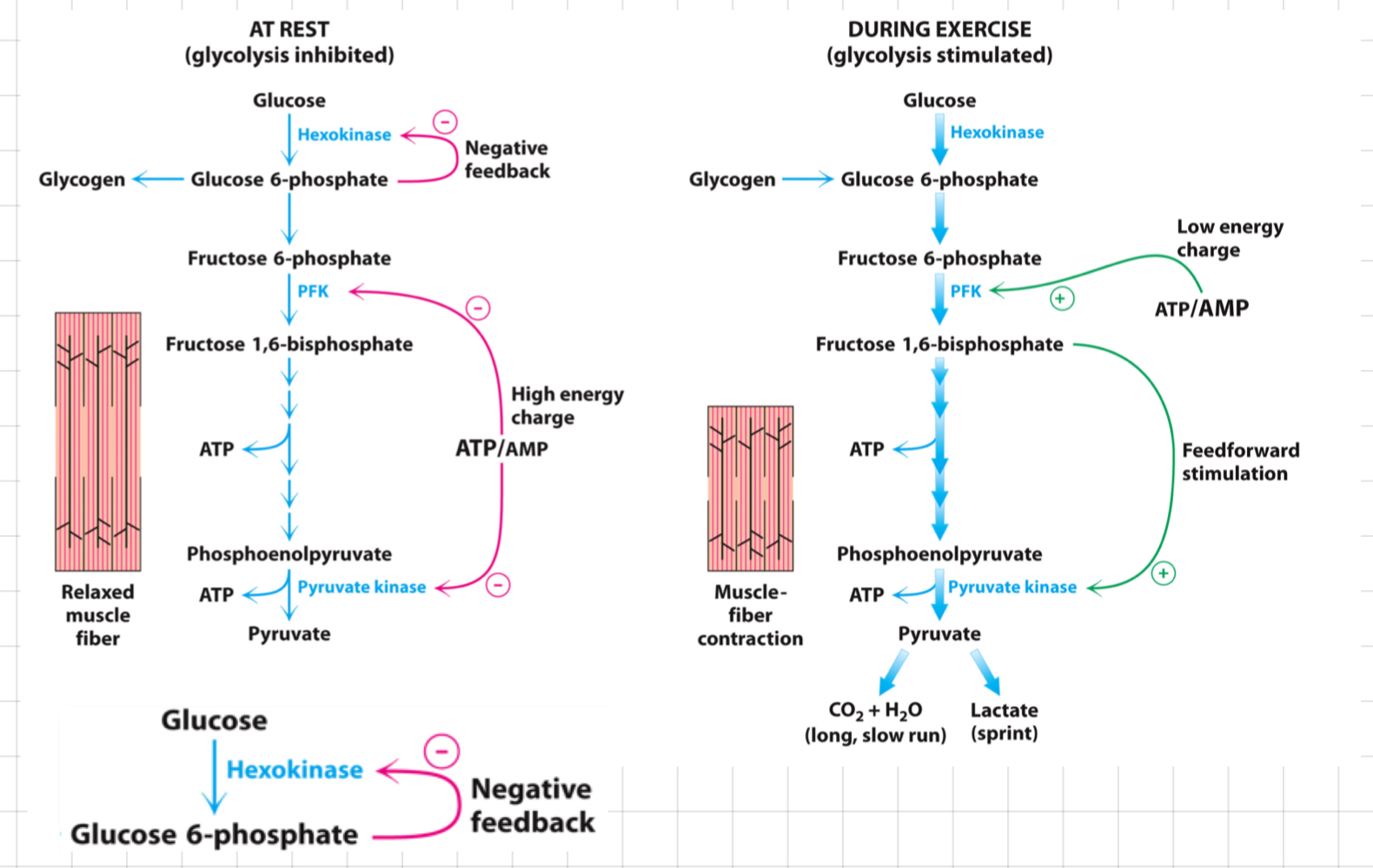
New cards
Hexokinase – Feedback Inhibition
* Involved in the first step of glycolysis, it catalyses glucose phosphorylation to produce **glucose-6-phosphate (G6P)**.
* G6P levels control the activity of __**hexokinase**__ through **feedback inhibition**.
* In feedback inhibition, G6P acts as an allosteric inhibitor of hexokinase.
* ↑ G6P levels = Sufficient glucose or saturated downstream metabolic pathways.
↳ Hexokinase is inhibited → Slowing down the phosphorylation of glucose and ↓ the flux of glucose through glycolysis → ↓ Glycolysis
* Prevent unnecessary accumulation of G6P and ensures that glucose is utilized efficiently.
* Prevent waste of ATP
* Allow glucose to go back out of the cell
* If G6P is not used, glucose is not trapped
* G6P levels control the activity of __**hexokinase**__ through **feedback inhibition**.
* In feedback inhibition, G6P acts as an allosteric inhibitor of hexokinase.
* ↑ G6P levels = Sufficient glucose or saturated downstream metabolic pathways.
↳ Hexokinase is inhibited → Slowing down the phosphorylation of glucose and ↓ the flux of glucose through glycolysis → ↓ Glycolysis
* Prevent unnecessary accumulation of G6P and ensures that glucose is utilized efficiently.
* Prevent waste of ATP
* Allow glucose to go back out of the cell
* If G6P is not used, glucose is not trapped
92
New cards
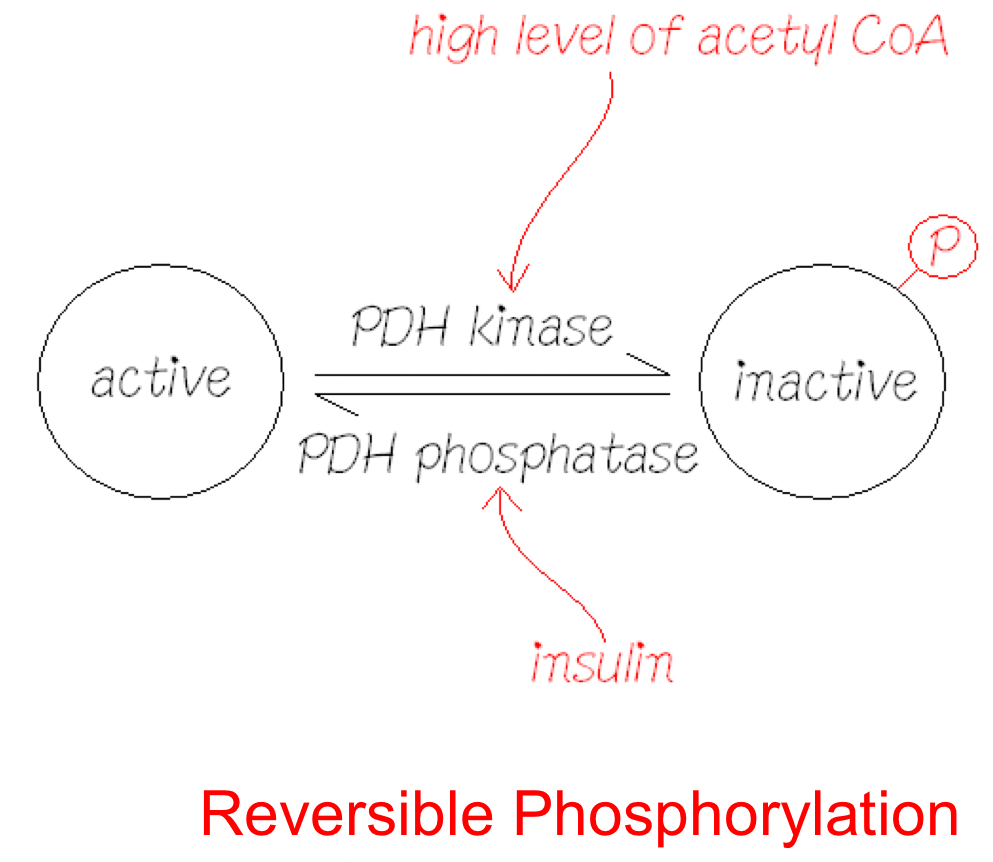
PDH – Covalent Modification
* Inactivated by phosphorylation (totally OFF)
* Phosphorylation inhibits PDH activity, while dephosphorylation activates it.
* Help control the conversion of pyruvate to acetyl-CoA based on energy demands.
* The total amount of enzyme doesn’t change
* **Phosphorylated : Dephosphorylated ratio**
* Reactivation by phosphate
* Release of phosphate
* Totally ON
* PDH activity balance between kinase and phosphate
* Phosphorylation inhibits PDH activity, while dephosphorylation activates it.
* Help control the conversion of pyruvate to acetyl-CoA based on energy demands.
* The total amount of enzyme doesn’t change
* **Phosphorylated : Dephosphorylated ratio**
* Reactivation by phosphate
* Release of phosphate
* Totally ON
* PDH activity balance between kinase and phosphate
93
New cards
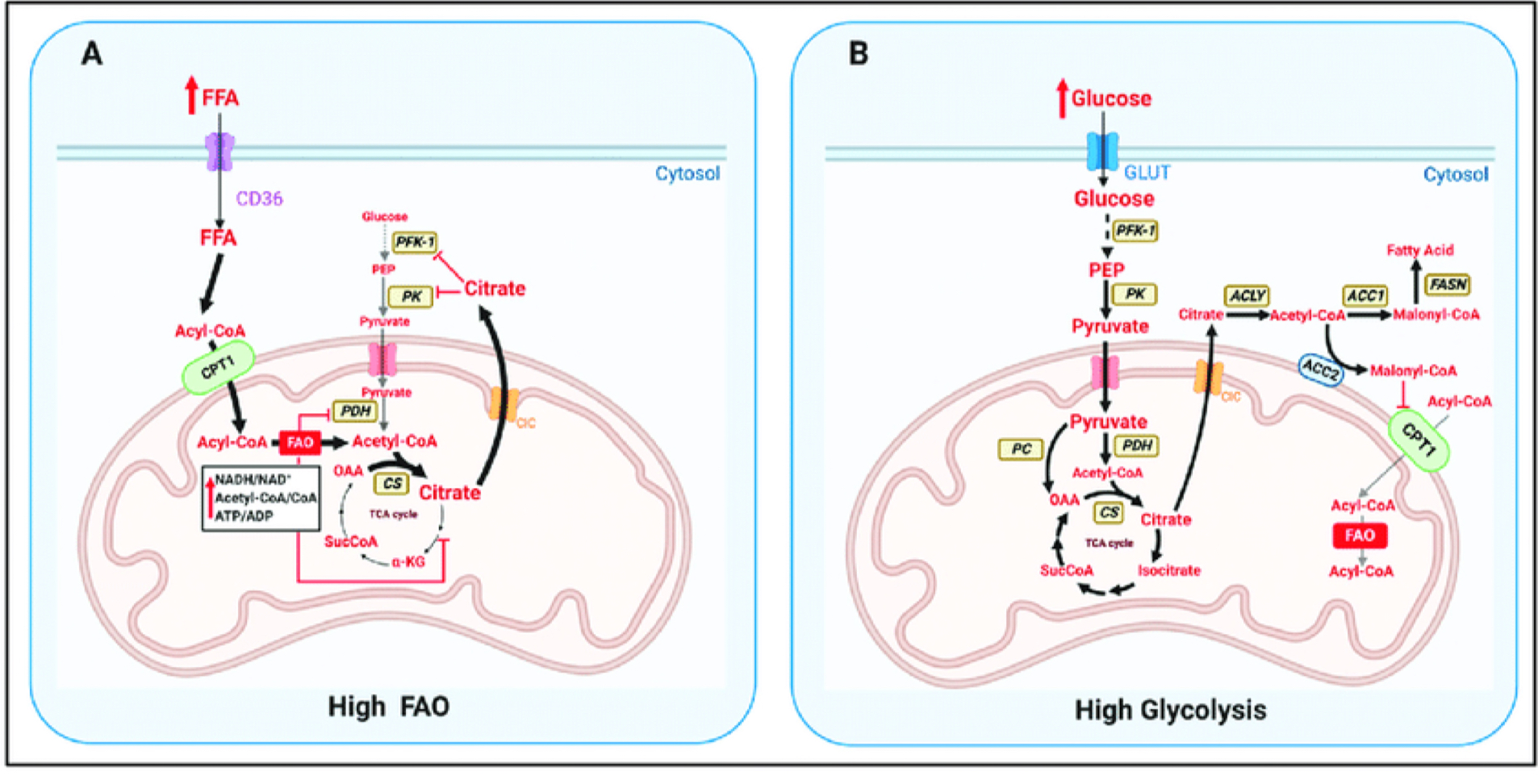
Fuel Selection
* **Catabolism vs. Anabolism**
* Glycolysis vs. Gluconeogenesis
* ß-Oxidation vs. FA synthesis
* When 1 pathway is stimulated, the opposite is inhibited
* When they both occur at the same time → **Futile Cycle**
* A net loss of energy without any productive outcome.
* Regulatory purposes but generally leads to wasteful energy expenditure.
* Glycolysis vs. Gluconeogenesis
* ß-Oxidation vs. FA synthesis
* When 1 pathway is stimulated, the opposite is inhibited
* When they both occur at the same time → **Futile Cycle**
* A net loss of energy without any productive outcome.
* Regulatory purposes but generally leads to wasteful energy expenditure.
94
New cards
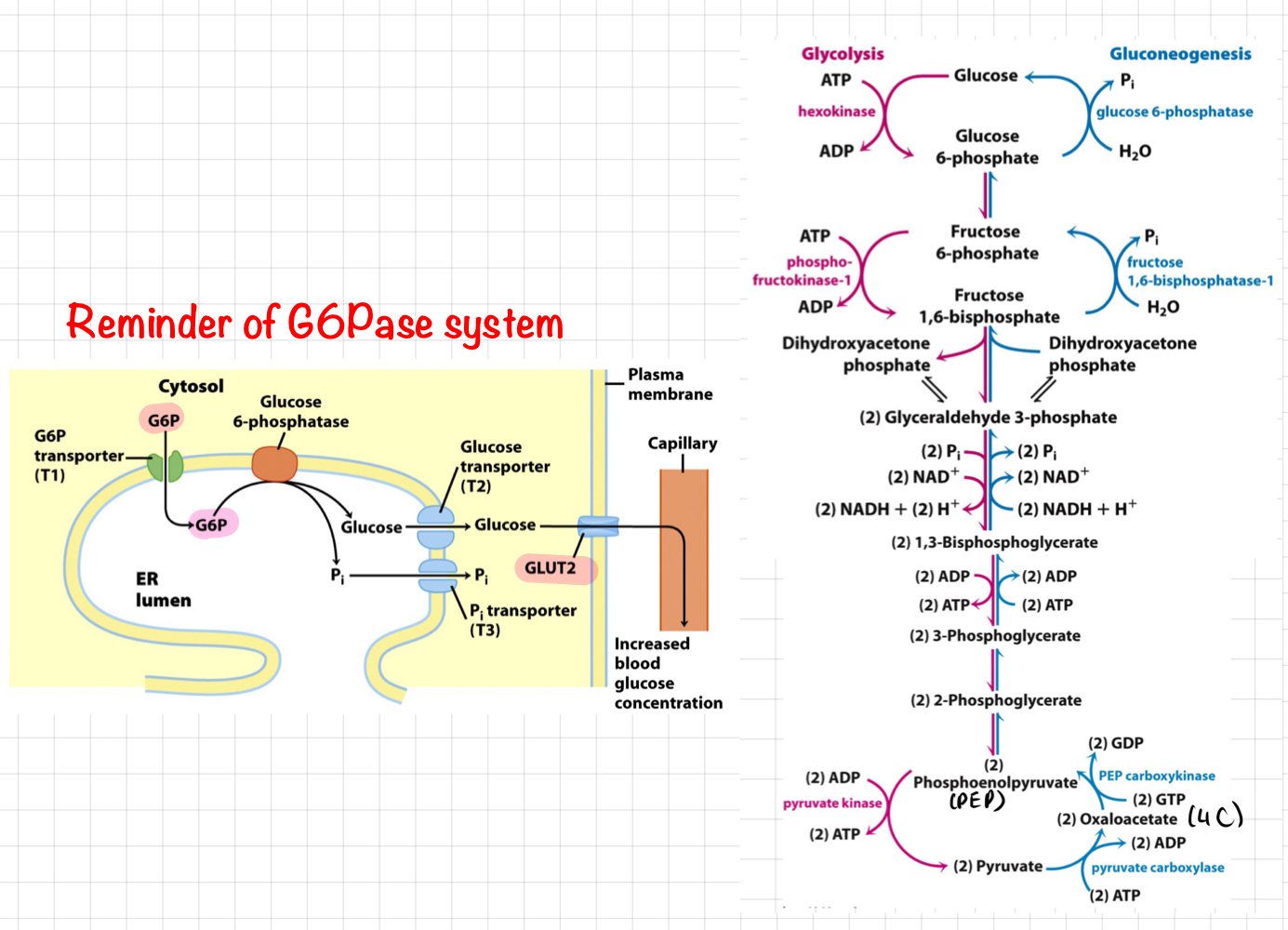
**Gluconeogenesis** and its pathway
* Getting from 3C to 6C
* Bypassing hexokinase and PFK → **PFK-1**
* __**G6Pase**__ (only in liver) and __**F1**__
* NO ATP gained from the loss of phosphate at these steps
* @@__**PC (Pyruvate Carboxylase)**__@@
* In the mitochondria.
* Crucial role in gluconeogenesis, converting pyruvate into oxaloacetate
↳ Further converted into glucose.
* Stimulated by **acetyl-CoA (FA oxidation)**
* Acetyl-CoA levels high when ß-Oxidation prominent
* Inhibition of PDH → Prevenr wasteful oxidation of glucose
* @@__**PEPCK (phosphoenolpyruvate carboxykinase)**__@@
* Involved in gluconeogenesis, converting oxaloacetate to **phosphoenolpyruvate (PEP)**.
* Crucial role in maintaining blood glucose levels during fasting
* Regulated by hormones such as glucagon and cortisol.
* Stimulated by ↑ transcription/translation of gene
* These enzymes can exist in tissues other than liver
* Enables glycerol to be made from pyruvate
* Bypassing hexokinase and PFK → **PFK-1**
* __**G6Pase**__ (only in liver) and __**F1**__
* NO ATP gained from the loss of phosphate at these steps
* @@__**PC (Pyruvate Carboxylase)**__@@
* In the mitochondria.
* Crucial role in gluconeogenesis, converting pyruvate into oxaloacetate
↳ Further converted into glucose.
* Stimulated by **acetyl-CoA (FA oxidation)**
* Acetyl-CoA levels high when ß-Oxidation prominent
* Inhibition of PDH → Prevenr wasteful oxidation of glucose
* @@__**PEPCK (phosphoenolpyruvate carboxykinase)**__@@
* Involved in gluconeogenesis, converting oxaloacetate to **phosphoenolpyruvate (PEP)**.
* Crucial role in maintaining blood glucose levels during fasting
* Regulated by hormones such as glucagon and cortisol.
* Stimulated by ↑ transcription/translation of gene
* These enzymes can exist in tissues other than liver
* Enables glycerol to be made from pyruvate
95
New cards
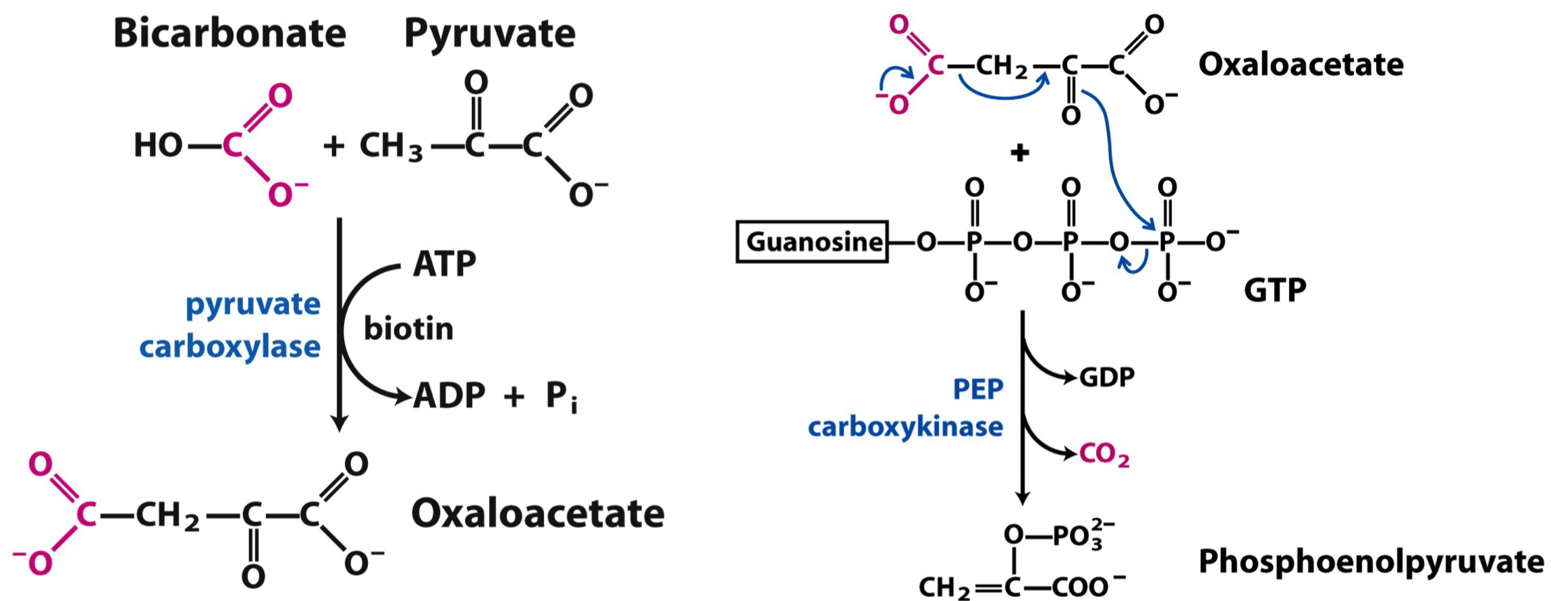
Synthesis of PEP from Pyruvate
* Pyruvate is carboxylated to form oxaloacetate by ==__**pyruvate carboxylase (PC)**__==.
* Oxaloacetate is then converted to PEP by ==__**phosphoenolpyruvate carboxykinase (PEPCK)**__==.
* Essential for gluconeogenesis, allowing the body to generate glucose **from non-carbohydrate sources**.
* Oxaloacetate is then converted to PEP by ==__**phosphoenolpyruvate carboxykinase (PEPCK)**__==.
* Essential for gluconeogenesis, allowing the body to generate glucose **from non-carbohydrate sources**.
96
New cards
Why **2-deoxy glucose** can’t be used in glycolysis
* B/c it lacks a hydroxyl group (OH-) at the C2 position
* Preventing it from being efficiently phosphorylated by hexokinase.
* A key step in glycolysis.
* Acts as a **competitive inhibitor of hexokinase** and **blocks** the entry of glucose into the glycolytic pathway.
* Preventing it from being efficiently phosphorylated by hexokinase.
* A key step in glycolysis.
* Acts as a **competitive inhibitor of hexokinase** and **blocks** the entry of glucose into the glycolytic pathway.
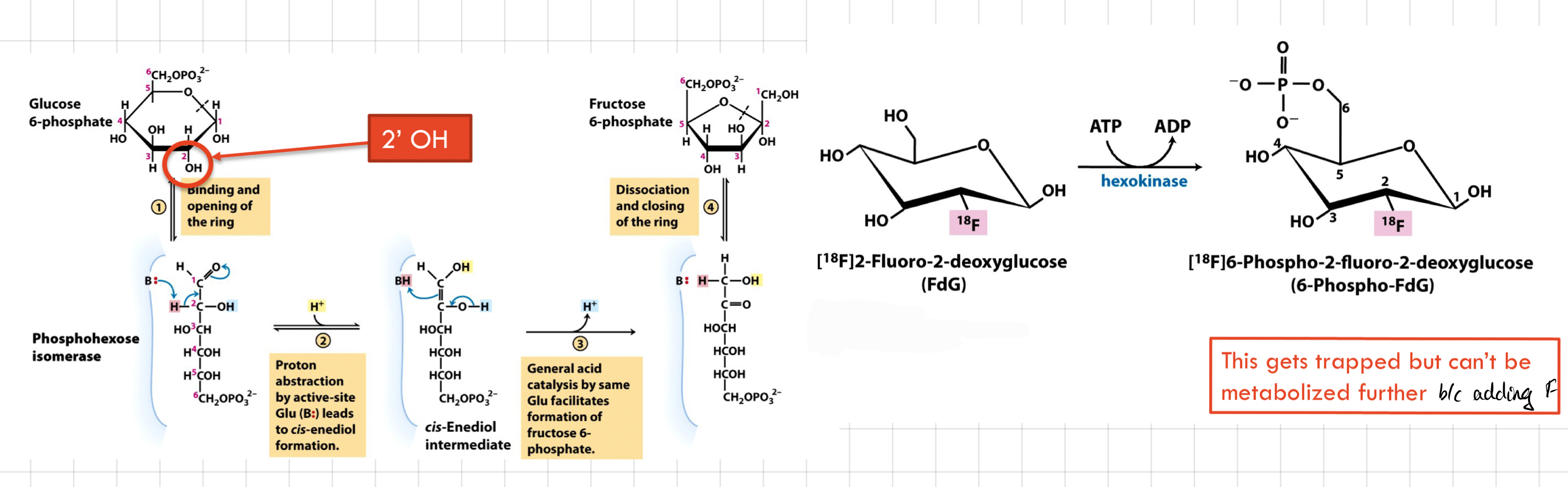
97
New cards
F26BP affects PFK-1 and FBPase-1
* Reversal of **F6P → F16BP**
* **PFK-2** and **FBPase-2** are the same enzyme
* Swapping from 1 form to another after reversible phosphorylation
* Interconversion catalysed by **Protein Kinase A (PKA)** and \[glucagon\] and \[insulin\]
* Sensitive to cAMP
* **PFK-2** and **FBPase-2** are the same enzyme
* Swapping from 1 form to another after reversible phosphorylation
* Interconversion catalysed by **Protein Kinase A (PKA)** and \[glucagon\] and \[insulin\]
* Sensitive to cAMP
![* Reversal of **F6P → F16BP**
* **PFK-2** and **FBPase-2** are the same enzyme
* Swapping from 1 form to another after reversible phosphorylation
* Interconversion catalysed by **Protein Kinase A (PKA)** and \[glucagon\] and \[insulin\]
* Sensitive to cAMP](https://knowt-user-attachments.s3.amazonaws.com/181ffafbe6ef48cd9f71c6b90778128d.jpeg)
98
New cards
Gluconeogenesis & Glycolysis
* During starvation
* ↑ Glucagon → ↑ \[cAMP)
* ↓ \[F2,6\]
* NO stimulus for PFK → NO glycolysis
* NO inhibition of F1,6BPase → Gluconeogenesis
* **F6O → F16BP** stimulated by allosteric effector __**F26BP**__
* F26BP made by PFK-2
* F26BP inhibits F16BPase and stimulates PFK
* When F26BP is @@**high**@@ → @@**Glycolysis**@@ is favoured
* Phosphorylation of PFK-2 converts it into __**F26BPase**__
* ↓ \[F26BP\]
* PFK is inhibited
* ↑ F16BPase activity
* When F26BP is **low** → **Gluconeogenesis** is favoured
* Phosphorylation is catalysed by cAMP-dependant protein kinase (or PKA)
* cAMP stimulates PKA
* cAMP are high when glucagon bound to receptors on liver cell
* F16BPase is more active when \[glucagon\] high
* As in starvation
* ↑ Glucagon → ↑ \[cAMP)
* ↓ \[F2,6\]
* NO stimulus for PFK → NO glycolysis
* NO inhibition of F1,6BPase → Gluconeogenesis
* **F6O → F16BP** stimulated by allosteric effector __**F26BP**__
* F26BP made by PFK-2
* F26BP inhibits F16BPase and stimulates PFK
* When F26BP is @@**high**@@ → @@**Glycolysis**@@ is favoured
* Phosphorylation of PFK-2 converts it into __**F26BPase**__
* ↓ \[F26BP\]
* PFK is inhibited
* ↑ F16BPase activity
* When F26BP is **low** → **Gluconeogenesis** is favoured
* Phosphorylation is catalysed by cAMP-dependant protein kinase (or PKA)
* cAMP stimulates PKA
* cAMP are high when glucagon bound to receptors on liver cell
* F16BPase is more active when \[glucagon\] high
* As in starvation
![* During starvation
* ↑ Glucagon → ↑ \[cAMP)
* ↓ \[F2,6\]
* NO stimulus for PFK → NO glycolysis
* NO inhibition of F1,6BPase → Gluconeogenesis
* **F6O → F16BP** stimulated by allosteric effector __**F26BP**__
* F26BP made by PFK-2
* F26BP inhibits F16BPase and stimulates PFK
* When F26BP is @@**high**@@ → @@**Glycolysis**@@ is favoured
* Phosphorylation of PFK-2 converts it into __**F26BPase**__
* ↓ \[F26BP\]
* PFK is inhibited
* ↑ F16BPase activity
* When F26BP is $$**low**$$ → $$**Gluconeogenesis**$$ is favoured
* Phosphorylation is catalysed by cAMP-dependant protein kinase (or PKA)
* cAMP stimulates PKA
* cAMP are high when glucagon bound to receptors on liver cell
* F16BPase is more active when \[glucagon\] high
* As in starvation](https://knowt-user-attachments.s3.amazonaws.com/2dafff50cc6d421f8c6c0629bc3bd000.jpeg)
99
New cards
**Anaplerosis**
* In the citric acid cycle, __**anaplerotic reactions**__ refill oxaloacetate after consumption.
* Maintain adequate ATP levels for continuous cellular respiration.
* Maintain adequate ATP levels for continuous cellular respiration.
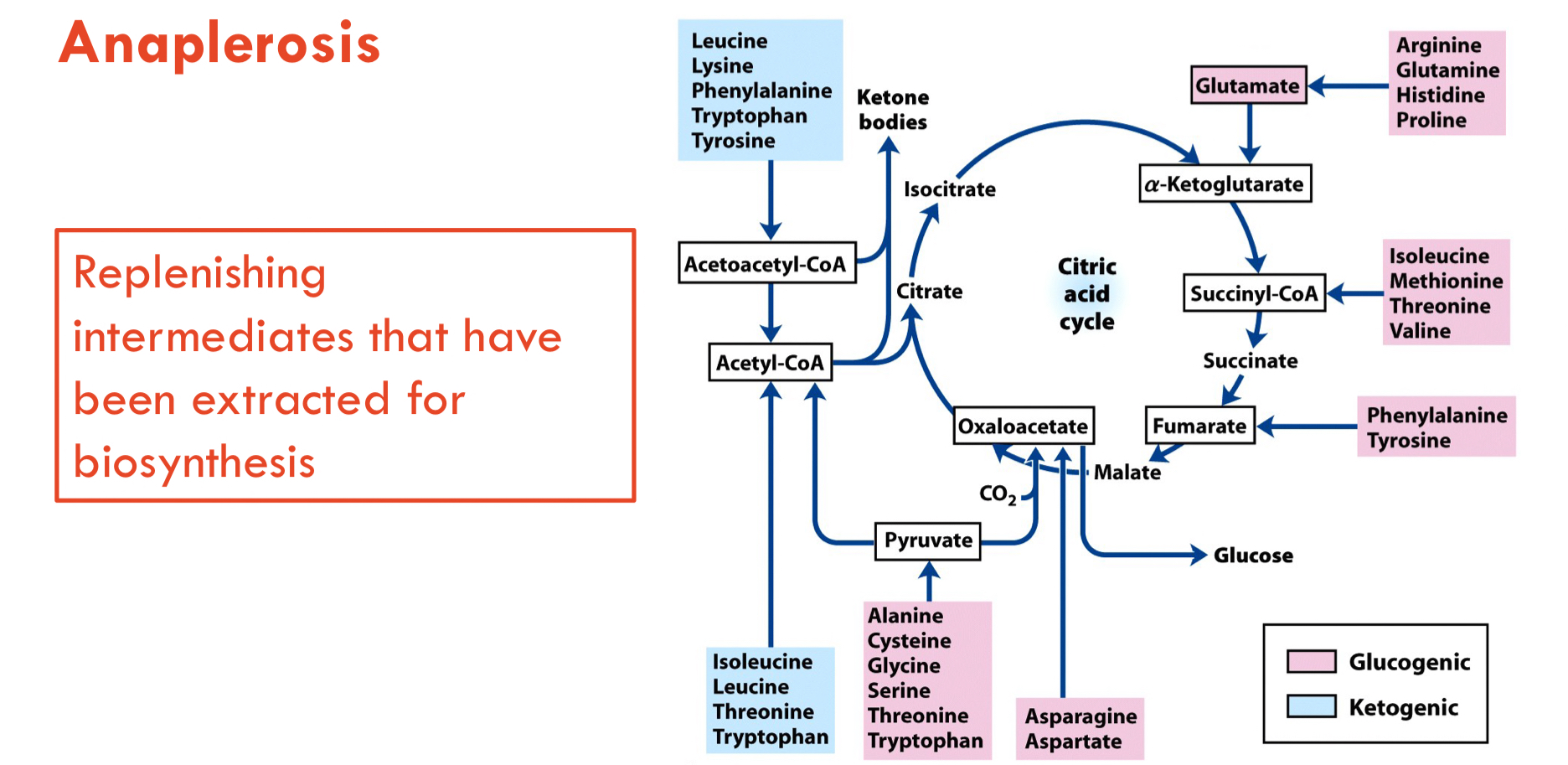
100
New cards
Is Glucose toxic?
* Brain can’t live without it
* Need to keep \[blood glucose\] at 4-5 mM
* Very reactive in vivo
* **Glycation:** __**Non-enzymatic glycosylation**__ of protein
* Destroys protein function
* Rate is directly proportional to \[glucose\]
* Need to keep \[blood glucose\] at 4-5 mM
* Very reactive in vivo
* **Glycation:** __**Non-enzymatic glycosylation**__ of protein
* Destroys protein function
* Rate is directly proportional to \[glucose\]