Module 7
1/102
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
103 Terms
alkane
single cc bonds (saturated)
-ane
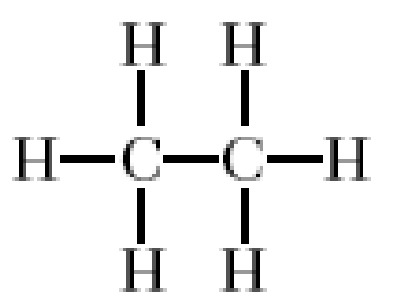
properties of alkanes
covalent molecular substances
non-polar → usually symmetrical & C-H are similar in electronegativity
alkenes and alkynes are similar in structure and in property
low melting & boiling points → dispersion forces
non-conductive • insoluble (non-polar)
size increases, more atoms and more electrons thus the strength of dispersion forces increases hence higher melting point and boiling points
C1-C4 are gases
C16 are liquids
C18 are semi solids
Bulky molecules do not pack neatly together, so less overall dispersion forces form, lowering the melting and boiling points
uses of alkanes
Methane is the main component of natural gas.
Propane is also known as LPG (liquid petroleum gas).
Pentane is used as an industrial solvent.
Octane is the main constituent of automobile fuel.
Nonane and decane are both used in petrol as additives.
longer chain molecules are fuel oil and mineral oil and these are used as lubricants
(C16 to C24) basis of petroleum jelly, greases, paraffin wax and asphalt
starting materials in the syntheses of alcohols, plastics, lacquers, detergents and fuels
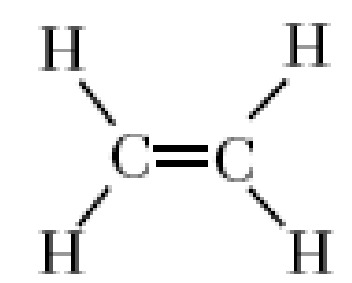
alkene
double cc bond
-ene
alkyne
triple cc bond
-yne
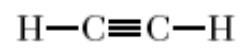
haloalkane
halogen on c (F,Br,I)
fluoro- , bromo- , iodo-
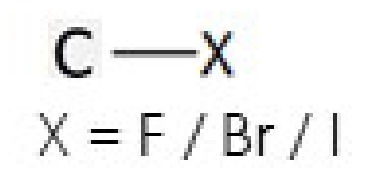
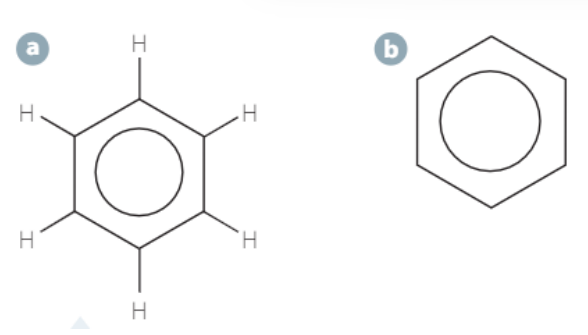
benzene
6-carbon ring with a cloud of delocalised electrons
Parents compound to aromatic compounds
Very stable
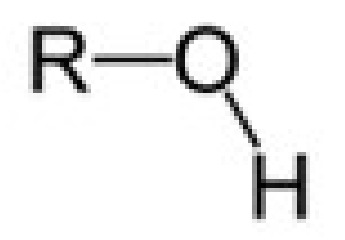
alcohol
hydroxyl group
-ol
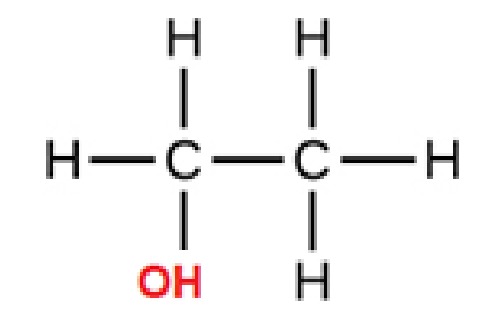
primary alcohols
OH attached to a C with ONE other C
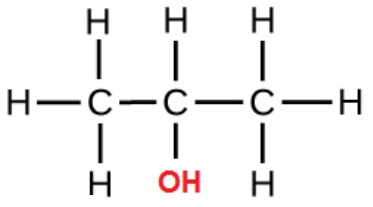
secondary alcohols
OH attached to a C with TWO other Cs
tertiary alcohols
OH attached to a C with THREE other Cs
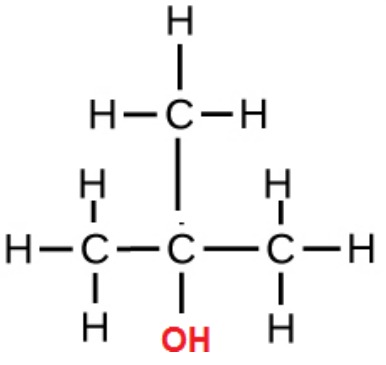
properties of alcohols
Hydrogen bonds
High boiling points
Polar part is - OH, non-polar is hydrocarbon
Smaller alcohols: polar hydroxyl > hydrocarbon
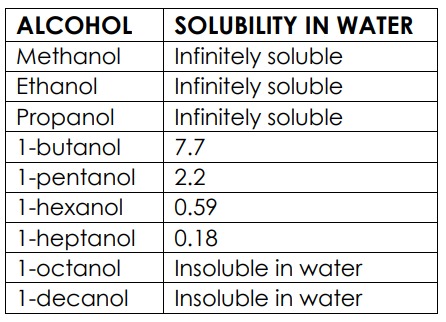
solubility of alcohol in water
as the length of the hydrocarbon chain increases the solubility decreases
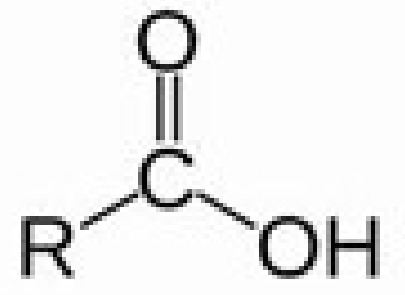
carboxylic acid
carboxyl (hydroxide and double bond oxygen)
-oic acid
properties of carboxylic acids
-OH & C=O make the carboxyl group polar → dipole dipole
Hydrocarbon chain → dispersion forces
Small carboxylic acids are soluble and as Cs increase, solubility decreases
High boiling points
Weak monoprotic acids
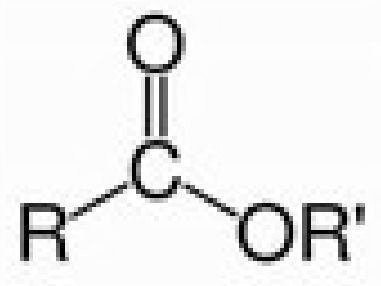
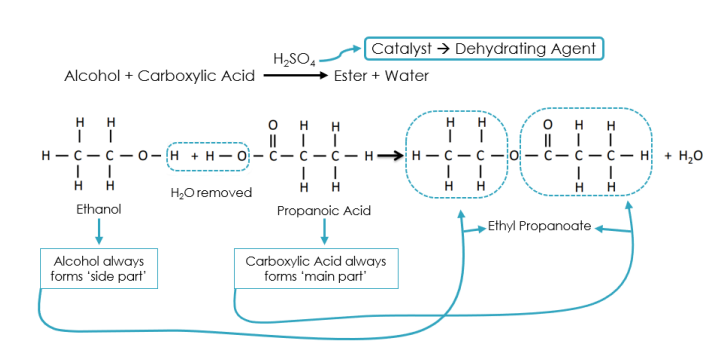
ester
ester group (alcohol + carboxylic acid with water removed)
-ly … -oate
uses of esters
Pleasant fruit odours – flavours & fragrances of fruits & flowers
Solid animal fats & animal and vegetable oils are natural esters
Used in the pharmaceutical industry to ‘mask’ bad tastes (and make the product more palatable).
fats and oils
properties of esters
The presence of the C-O and C=O makes the ester group polar.
However, the alkyl chains on either side, render the molecule non-polar.
Liquid at room temperature & their boiling points are much lower than for carboxylic acids of similar mass. [ main IMF = dipole-dipole forces (weaker than H-bonding in carboxylic acids and alcohols.]
Most not very soluble in water due to their non-polar nature, lack of hydrogen bonding and the presence of large hydrophobic alkyl groups.
However, they are soluble in organic solvents. Many esters are thus used as solvents in industry. For example, ethyl ethanoate is a common solvent in nail polish remover.
esterification
condensation reaction between an alcohol and a carboxylic acid that produces an ester and water → H2SO4 is the catalyst/dehydrating agent
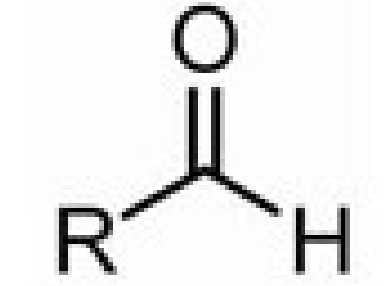
aldehyde
formyl (C=O on end C)
-al
ketone
carbonyl (C=O between two C)
-one
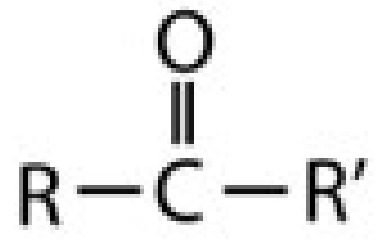
properties of aldehydes and ketones
C=O highly polar, dipole-dipole
higher boiling points than alkanes, alkenes and alkynes, but lower than alcohols of similar size
Aldehydes and ketones are more soluble in water than alkanes, alkenes and alkynes, but lower than alcohols of similar size
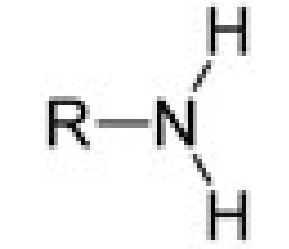
amine
amine (NH2)
-amine
amide
amide (double bonded oxygen and NH2)
-amide
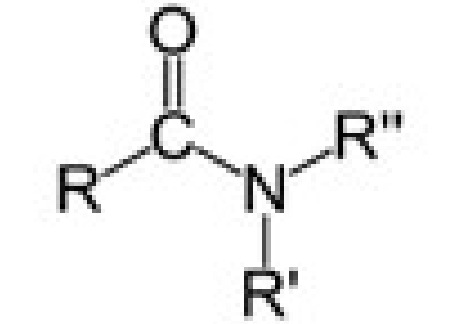
properties of amides
Primary and secondary amides → N—H & C=O (both polar)
Tertiary amides only C=O
N—H hydrogen bonds (strong IMF and high melting points)
Soluble
C chain increases, IMF increases, BP increases but solubility decreases
structural isomers
compounds having the same molecular formula but different structural formula
chain isomers
different chain structures
positional isomers
FG in different position
functional isomers
different functional groups e.g. aldehydes and ketones, carboxylic acids and esters
meth
1 C
eth
2 C
prop
3 C
but
4 C
pent
5 C
hex
6 C
hept
7 C
oct
8 C
potentially dangerous products
paints, lacquers, adhesives, degreasing materials, dry cleaning chemicals and printing materials.
Contain organic substance (ethanal, propanone, pentyl acetate, benzene, tetrachloromethane, dichloromethane, ethanol and 1,2-ethanediol)
safety data sheet (SDS)
all chemicals have an SDS containing:
properties of a particular chemical
identifies possible hazards and precautions for safe use and handling
first aid upon contact, inhalation or ingestion
risks related to physical properties
Many organic compounds are volatile
Weak intermolecular forces - evaporate more readily
Colourless & pungent smell
Flashpoint - the lowest temperature at which a liquid can form an ignite (with a spark or lit match) mixture in air near the surface of the liquid.
Most are highly flammable due to low flashpoint
Flashpoints below 23℃
Cyclohexane, cyclohexene, ethanol, propanone, pentane, propanol & methanol
exposure methods and effects of organic compounds
1. Inhalation (lungs) 2. Absorption (skin) 3. Ingestion (swallowing)
Acute → Immediately or shortly after contact
may result in headaches, dizziness, decreased reaction time and possible loss of consciousness
Chronic → Repeated exposure for years or decades
may result in chronic fatigue, physical weakness, mood changes, liver and kidney damage and central nervous system damage.
using alternatives as a prevention method
Using similar substances to replace more toxic substances.
e.g. benzene was used for a long time before being replaced, causing a multitude of long-term effects on the users. Cyclohexane is its main replacement in modern chemistry
isolation as a prevention method
physical separation from the substance. This can be done by automation of a process or the containment of the substance that can be controlled and used by users on the outside
disposal of organic compounds
Very careful – governed by law
Often neutralised or filtered
General rule, no organic waste should be washed down the sink
Implications of obtaining and using hydrocarbons
Most hydrocarbons are obtained from crude oil, a fossil fuel formed by decomposition of prehistoric organisms.
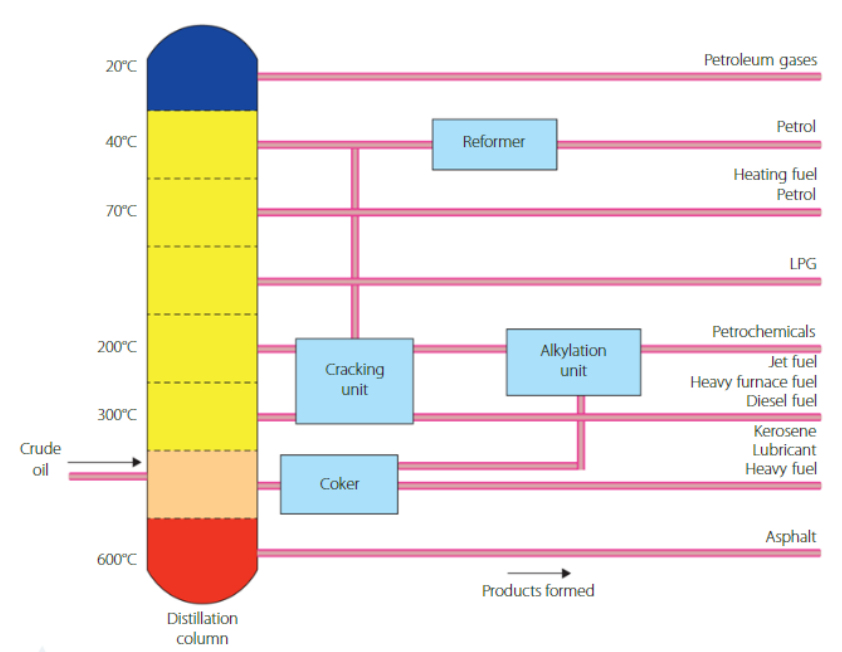
Crude oil is processed using fractional distillation. This is used to separate the hydrocarbons based on boiling point.
Collection trays are found on various sections of the distillation tower that have different temperatures.
The process rarely stops as crude oil is continually fed into the system
Some of the products are refined further, usually heavier ones, which either make certain products or produce more of the lighter products, based on need.
mining crude oil and natural gas
The mining for the crude oil and natural gas is a highly damaging component of the obtaining of hydrocarbons.
The largest impacts occur during spills or accidents involved in obtaining these hydrocarbons.
e.g. The 1989 oil spill in Prince William Sound in Alaska resulted in 41 million litres of crude oil to contaminate 1800km of coastline. The following are the deaths that were directly linked to the spill. As well a large amount of economic stress placed on the country
Greenhouse effect
The common products of the combustion of alkanes are water and carbon dioxide, of which carbon dioxide is a major contributor to the enhanced greenhouse effect.
Data all over the world was collected that correlated to an alarming increase in CO2 concentrations which directly correlate with average global temperature increases.
Some consequences of this include
Significant glacier shrinkage
Earlier break up of ice on both poles
Reduction of arctic ice since 1980 (by ~ 3 million km2)
Consistent sea level rise (200mm since 1870 and 85mm since 1993)
polymer pollution
Many organic compounds are used to create polymers. These Polymer examples include plastics, resins, Teflon, nylon and rayon.
The total mass of plastics currently exceed 300 million tonnes per year and the amount of plastic produced in 2000-10 equalled the plastic produced in 1900-2000.
Only about 10% of this plastic is recycled. o Australia is in the top 5 of waste produced per person. ~71kg per person each year.
With multiple types of plastics that can't be mixed, recycling is difficult for recycling centres.
The packaging often notifies the general public of what can be recycled this is not always completed by the recycling schemes of the area. The easiest plastics to recycle are codes 1, 2 and 3.
combustion of hydrocarbons
often used to take advantage of the release of energy for engines, heating and cooking applications
complete combustion → hydrocarbon reacts with oxygen to produce carbon dioxide and water
incomplete combustion → hydrocarbon reacts with limited oxygen to produce carbon monoxide and water
addition reactions of hydrocarbons
Unsaturated (double & triple) hydrocarbons are very reactive [bonds ‘open’ & accept substances added]
hydrogenation → addition of hydrogen
Unsaturated to saturated (alkanes)
Making margarine
metal catalyst needed [nickel, palladium, platinum or rhodium.]
The hydrogen reacts with the metal, breaking the H2 bond, forming two weak metal-hydrogen bonds.
The alkene reacts with the metal catalyst, breaking the double bond, forming two weak metal-carbon bonds.
The metal catalyst causes the hydrogen atoms to bind with the carbon atoms, forming an alkane
![<ul><li><p>Unsaturated to saturated (alkanes)</p></li><li><p>Making margarine</p></li><li><p>metal catalyst needed [nickel, palladium, platinum or rhodium.]</p><ul><li><p>The hydrogen reacts with the metal, breaking the H2 bond, forming two weak metal-hydrogen bonds.</p></li><li><p>The alkene reacts with the metal catalyst, breaking the double bond, forming two weak metal-carbon bonds.</p></li><li><p>The metal catalyst causes the hydrogen atoms to bind with the carbon atoms, forming an alkane</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/81522671-31ba-4bc1-8155-e3522cbe4a8b.png)
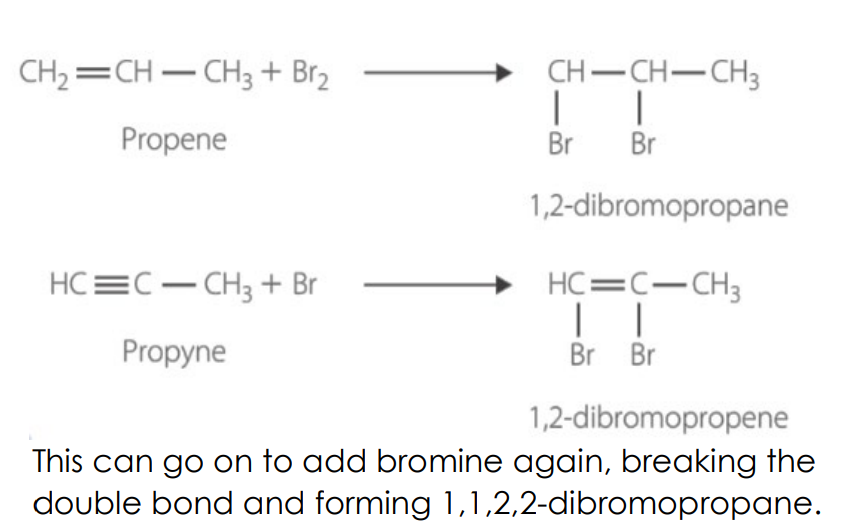
Halogenation → addition of halogens
unsaturated to dihalo-alkane
Alkynes → dihalo-alkene & → tetrahalo-alkane
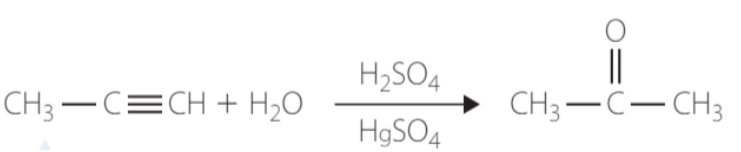
hydration → addition of water
H and OH added
Markovnikoff’s rule
Alkene catalyst → Dilute Sulfuric acid
Alkyne catalyst → mercury(II) compounds and sulfuric acid
Addition of water to an alkyne will produce a ketone
addition of hydrogen halides
H & halogen added (& again for alkynes)
Markovnikoff’s rule
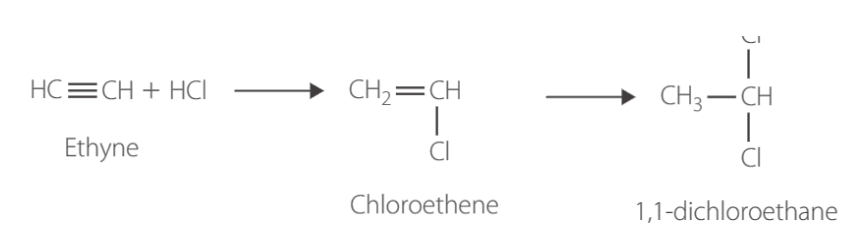
markovnikoff’s rule
applies to Hydration & hydrohalogenation.
Hydrogen atom will more likely attach to a carbon with the greater number of hydrogens already attached. Forming a major and minor product.
Major → H goes to C with most Hs
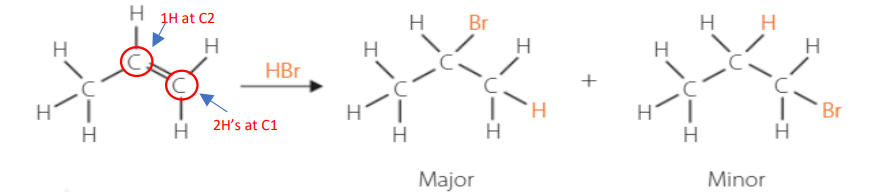
substitution of hydrocarbons
• Saturated (single) hydrocarbons have no bonds to “break & add” → substitute
halogen substitution
Energy source needed: UV light (NOT a catalyst)
Hydrogens are substituted with halogens
If there is an excess in halogens in the reaction vessel, all hydrogens will slowly be replaced until none remain
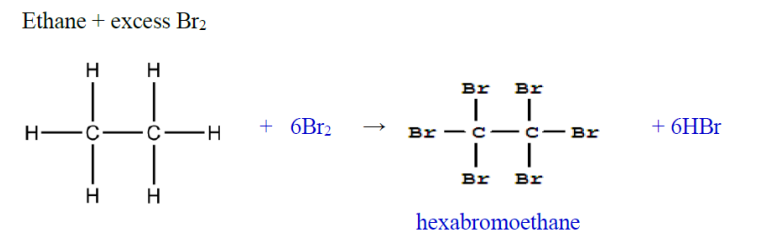
testing for saturated vs unsaturated hydrocarbons
Bromine water test
Alkane → very slow (remains brown)
Alkene or alkyne → rapidly turns colourless
combustion of alcohols
Complete combustion of alcohols have the same general reaction as alkanes.
Incomplete combustion is different and results in various products depending on the amount of oxygen present. Including carbon monoxide and soot (C)
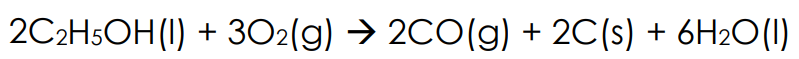
Enthalpy of combustion of alcohols
measured using calorimetry and must be done under a few assumptions;
1. The products of combustion are only carbon dioxide gas and liquid water. (enthalpy change when water changes states)
2. The value for ∆H must be negative as all combustion reactions are exothermic
Uses q=mc∆t and ∆H= -q/n
where m is the mass of the water and n is the moles of fuel
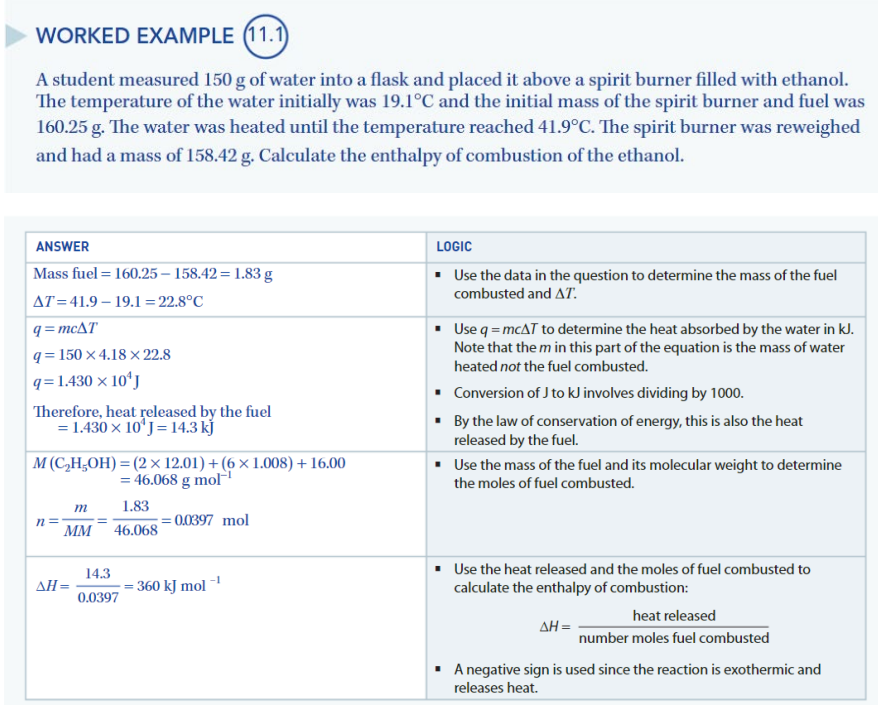
dehydration of alcohols
Dehydration reactions are the loss of water from a compound.
Dehydration of an alcohol requires an appropriate acid catalyst and results in the formation of an alkene.
Some alcohols can be passed over an aluminium oxide catalyst in gas form to achieve the same result
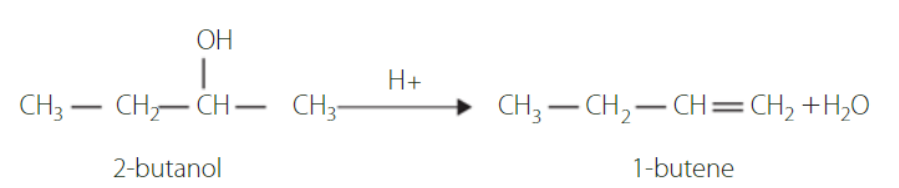
hyrdrohalogenation of alcohol
Substitution reaction
When in the presence of hydrogen halides, such as hydrogen chloride or hydrogen bromide, the –OH group is substituted with a halogen.
The general equation is; H-X + R-OH → R-X + H2O
The speed of these reaction depends on the type of alcohol.
Tertiary are very reactive, secondary are less reactive and primary are not very reactive
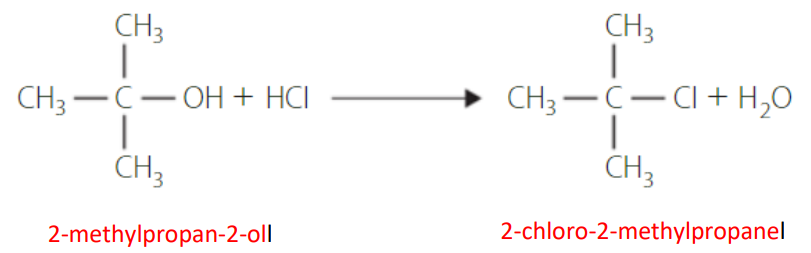
oxidation of primary alcohols
Primary alcohols have two stages, oxidation to an aldehyde and then into a carboxylic acid.
Little to no aldehyde will remain in the final product. If the aldehyde is required, then simple distillation can be used to acquire it.
Both stages occur with the same reactants and conditions
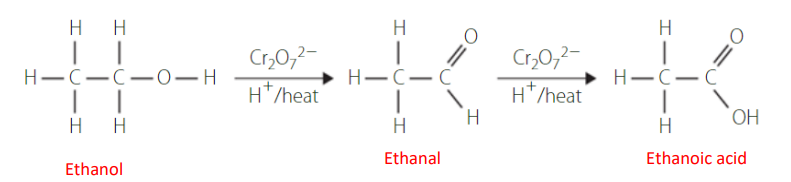
oxidation of secondary alcohols
Secondary alcohols are oxidised to form ketones and are unable to be further oxidised
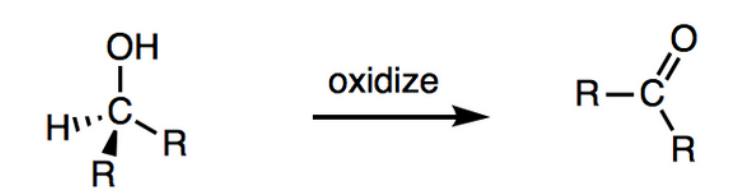
methods for production of alcohols
hydration of an alkene
substitution reaction of a halogenated compound where a halogen is replaced by a hydroxyl group
fermentation of simple sugars
fermentation of simple sugars
converting simple sugars into ethanol in an anaerobic environment.
Many enzymes in microorganisms can do this, the most common being yeast.
There are multiple conditions that are important to enzymes in the fermentation of alcohol, some being;
Temperature
pH
No oxygen
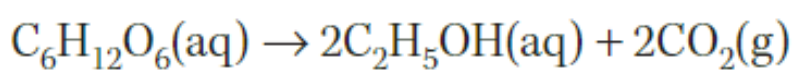
fossil fuels
fossils fuels are composed of organic matter that has decayed for millions of years and is non-renewable.
The types of fuels produced are coal, crude oil and natural gas but since they are not “recent” organic sources they are not considered biofuels.
This fuel produces large amount of energy but is currently the leading cause to environmental issues, like the enhanced greenhouse effect.
biofuels
Biofuels are produced from renewable resources such as crops. This is due to most biofuels being made from leftover organic matter from crops, algae or animal wastes.
The two main biofuels are ethanol and biodiesel, however, in Australia ethanol is only used as a fuel additive for petroleum (~10%)
Ethanol has less energy output than petrol but a higher octane rating (compression before ignition) and its net release of CO2 is considerably less than that of E10 (10% ethanol: 90% petroleum).
Biodiesel is produced from fatty acids from any organic source. The ester (biodiesel) is simply separated from a triglyceride via transesterification.
The resulting biodiesel is only slightly less energetic but can only run in specific biodiesel engines, or in unmodified diesel engines with =< 20% biodiesel to diesel mix.
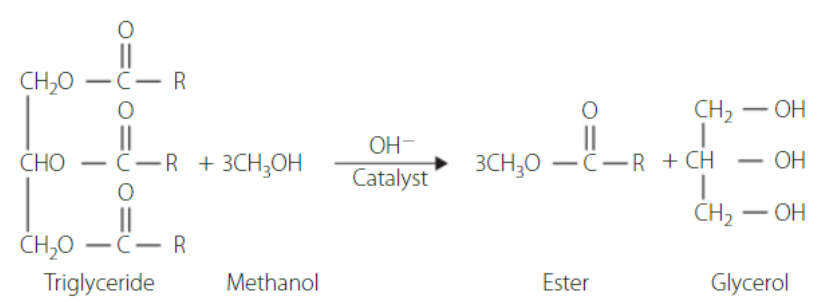
conditions for preparing esters
Rate of reactions for esters are generally slow and can be increased with the introduction of an acid catalyst and heating.
When heated, it must be done using reflux, which is simply the cooling of vapours to prevent product loss via evaporation. This is often done at 60-80℃
When refluxing, it must be completed with care due to the presence or organic substances in vapour form potentially getting near naked flames (Bunsen burners). Use heating mantles where possible.
To increase the yield of ester, one of the reagents can be in excess, pushing the reaction to the right (Le Chatelier’s principle).
purifying an ester
Washing with water removes soluble substances. Two layers, will form, aqueous(soluble) and organic(insoluble). Adding 1-2 drops of water can help determine which layer is which. Drain out the bottom layer and keep the organic layer.
Sodium carbonate is added to remove excess carboxylic acid. This causes it to become soluble as a carboxylate ion. Carbon dioxide is formed and so the separatory funnel must be ‘vented’. Water is added to dissolve any soluble impurities. The organic layer is kept.
Distillation will separate the organic components by boiling point. Simply keep the distillate that is received at the esters boiling point
organic acids
The most common organic acids are carboxylic acids.
There are simple and complex organic acids. Simple ones are straight chain carboxylic acids e.g. ethanoic acid, butanoic acid.
Some complex organic acids include; citric acid, fumaric acid, succinic acid
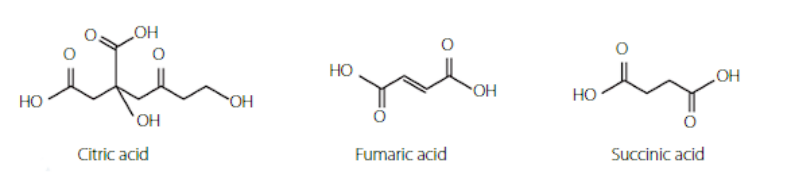
organic bases
Organic bases usually involve nitrogen. The most common organic bases are the nitrogenous bases found in DNA, adenine, cytosine, guanine and thymine.
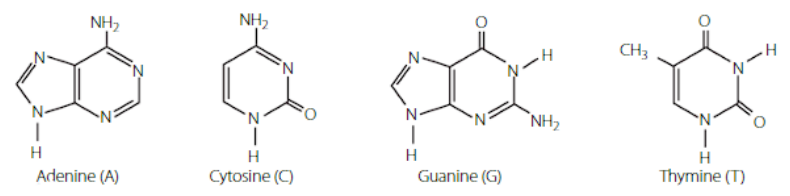
properties of organic acids and bases
Organic acids and bases act in a similar manner to their inorganic counterparts but are mostly weak.
Organic acids and bases are unable to reach very low or very high pH’s.
Organic acids can perform all reactions that weak inorganic acids can, such as reaction with metals and metal carbonates.
Organic bases can react with acids in the same way as inorganic bases; however, nitrogenous bases will form a protonated amine. This group is simply the conjugate acid of the amine and due to a charge, changes its properties.
soaps and detergents
cleaning due to their ability to remove dirt, oil and stains.
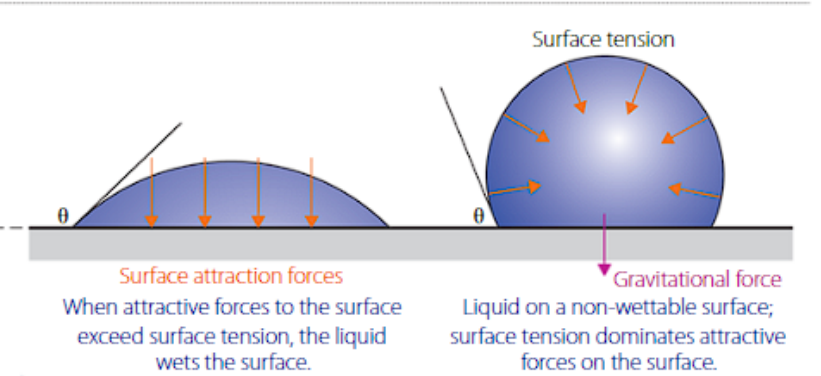
act as a surfactant, reducing surface tension of the liquid its dissolved in. This occurs due to decreased presence of hydrogen bonding.
When surface tension is lowered, the liquids' ability to ‘wet’ a surface increases. This is due to the inability to form a droplet. This property is known as wettability and is measured as an angle (θ).
They are also able to act as an emulsifier which can cause two immiscible liquids to become miscible in one another. When this occurs, the solution is known as an emulsion.
soap structure
relatively simple, with a long hydrophobic hydrocarbon tail and an ionic head that is hydrophilic (often carboxylate).

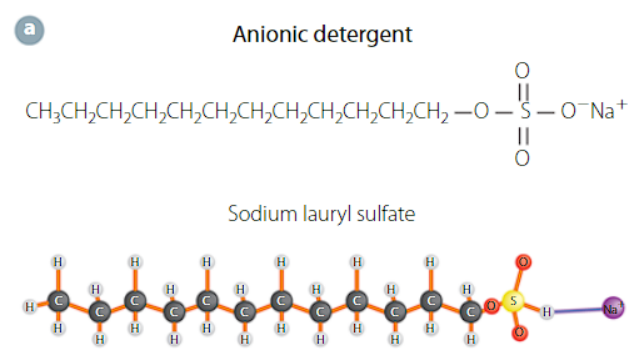
anionic detergent
the head group is anionic with a hydrocarbon tail
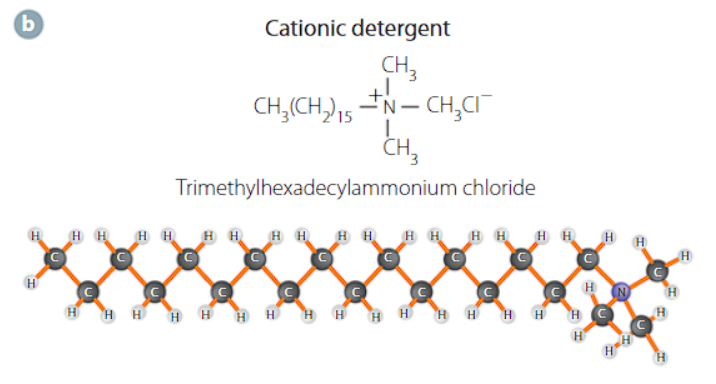
cationic detergent
the head group is cationic with a hydrocarbon tail
non-ionic detergent
the head group is polar with a hydrocarbon tail
characteristics of anionic detergents
create good lather
have a negative charge
harsh action (so not suitable for use as personal cleaners)
cheap
characteristics of cationic detergent
bond very strongly to negatively charged surfaces (reducing static friction and tangly)
biocidal (kills bacteria)
expensive
characteristics of non-ionic detergents
low lather formation (prevents foam build-up in dishwashers)
expensive
actions of soaps and detergents
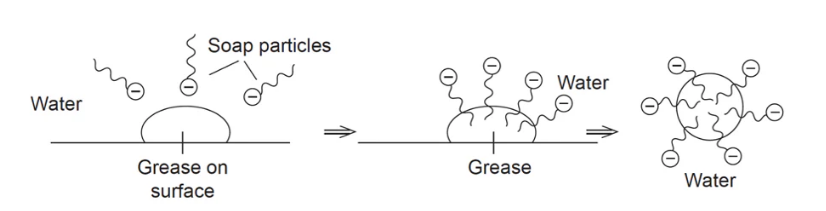
Soaps and detergents are effective due to their polar and non-polar components.
They surrounds dirt, or oil/grease, with the polar head of the outside and the non-polar tail attached to the dirt or oil/grease.
When the solution is agitated it causes the soap/detergent to pull the dirt or oil/grease off the surface.
Once fully surrounded, a structure known as a micelle forms.
Due to the negative charge outside of micelles, they do not merge and therefore remain small enough to stay as a suspension and not reattached to the surface/s
saponification
the chemical reaction in which fatty esters are hydrolysed in alkaline solution to produce soap
triglyceride (fat/oil) + strong base (NaOH or KOH) → glycerol + fatty acid salts (soap)
polymer
long chain molecules composed of repeating units called monomers
polyethylene (PE)
ethelyne monomers joined together in long chains by addition polymerisation
properties of polyethylene
High Density Polyethylene
• Linear polymer chains
• Rigid and tough due to strong dispersion forces
Low Density Polyethylene
• Many branching side chains
• Soft and flexible due to the weak dispersion forces
uses of polyethylene
High Density Polyethylene
• bottle caps for milk and carbonated drinks
• plastic toys
• agricultural pipes
• petrol tanks
Low Density Polyethylene
• plastic bags
• squeeze bottles
• agricultural pipes
• petrol tanks
polyvinyl chloride (PVC)
chloroethylene (commonly known as vinyl chloride) monomers joined together in long chains by addition polymerisation
properties of polyvinyl chloride
• Linear polymer chains
• Stiff and inflexible due to large chlorine atoms
• if plasticisers are added flexibility, softness, elongation and low temperature flexibility due to decreased dispersion forces
• maintains its properties for a long time and is resistant to atmospheric oxidation due to chlorine
uses of polyvinyl chloride
Rigid PVC
• agricultural drainage pipes
• window and door frames
Flexible PVC (plasticisers added)
• coatings for electrical wires
• shoe soles
polystyrene (PS)
phenylethylene/styrene monomers joined together in long chains by addition polymerisation
properties of polystyrene
• Clear with a high refractive index
• high stiffness due to large phenol groups
• good electrical insulator
• expanded polystyrene foam is a good thermal insulator
uses of polystyrene
Polystyrene (PS)
• car battery cases
• plastic cutlery
• handles for tools
• heat-pressed packaging
• clear containers such as plastic cups and packaging for DVDs and CDs
Expanded Polystyrene (EPS)
• building insulation in house walls and rooves
• packing materials
polytetrafluoroethylene (PTFE)
tetrafluoroethene monomers joined together in long chains by addition polymerisation
properties of polytetrafluoroethylene
• unbranched chains that form tightly packed helical structures due to the strong bonds
• inert
• low friction surface
• water insoluble
• high melting point
• high thermal stability
• high electrical resistance
uses of polytetrafluoroethylene
• coating for non-stick frypans
• industrial coatings (e.g gears and bearings)
• coating to prevent the corrosion of steal
• insulator in electrical tables
condensation polymer
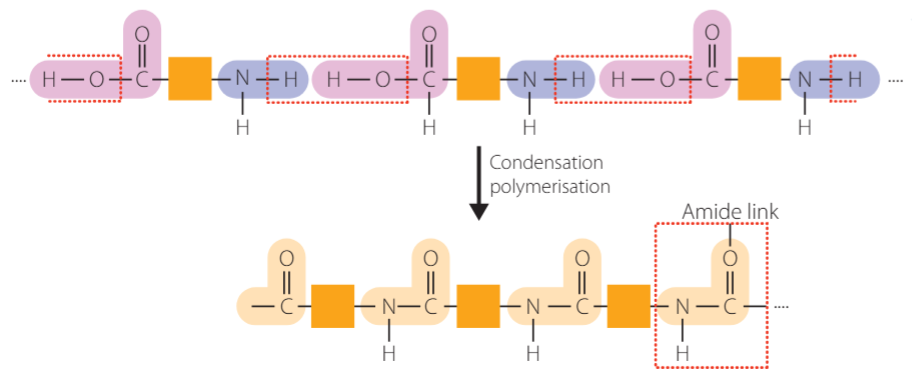
polyamides
condensation polymers held together by amide links (also found in peptides known as peptide links) e.g. natural → peptides, synthetic → nylon
properties of nylon
• high tensile strength
• high rigidity
• absorbs moisture
• elastic
• can be drawn into fibres
nylon uses
• production of textiles such as clothing, carpets, drapes and bedding
• production of machine parts and pipes
• seat belts
• ropes and nets
• sleeping bags and tents