FPS10: Factors affecting SN1/SN2/E1/E2
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
SN1 products
racemic mixture + LG-
SN2 products
Walden inverted product + LG-
E1 product
typically forms a more stable E-alkene (but can be Z-)
E2 product
stereospecific alkene depending on starting diastereomer (only E- or only Z-)
Factors affecting SN1 vs. SN2 pathway
1. nature of carbocation
2. steric effects
3. nature of Nu
4. leaving group
5. solvent effects
carbocation stability order
tertiary > secondary > primary
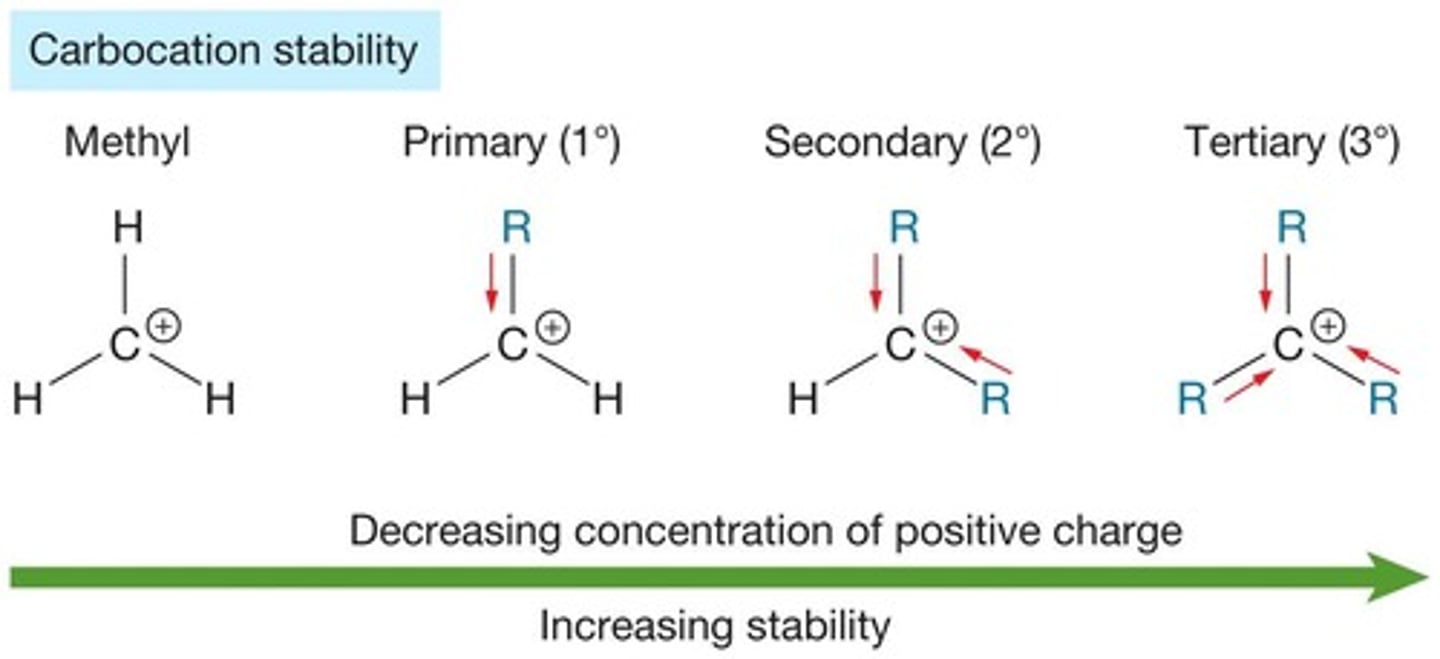
tertiary carbocation
carbocation w three carbon atoms directly bonded to the positively charged carbon
secondary carbocation
carbocation w two carbon atoms directly bonded to the positively charged carbon
primary carbocation
carbocation w one carbon atom directly bonded to the positively charged carbon
why is the tertiary carbocation the most stable?
due to hyperconjugation
hyperconjugation
stabilisation of carbocation by overlap between sigma bonds and an empty p orbital, allowing e- density donation into positively charged carbon i.e. sigma bond e- sharing e- density into positive C
resonance
stabilisation of carbocation when e- density is delocalised over multiple atoms through pi-bonds or lps, rather than being localised on 1 atom i.e. sharing + charge across molecule
the more stable the carbocation...
...the more likely it'll proceed via. the SN1 RXN mechanism
primary carbocations will never...
...proceed via. SN1, only SN2
SN2 steric implication
SN2 reqs. a backside attack so bulky substituents hinder Nu approach
substrate order for SN2
primary substrate > secondary substrate > tertiary substrate
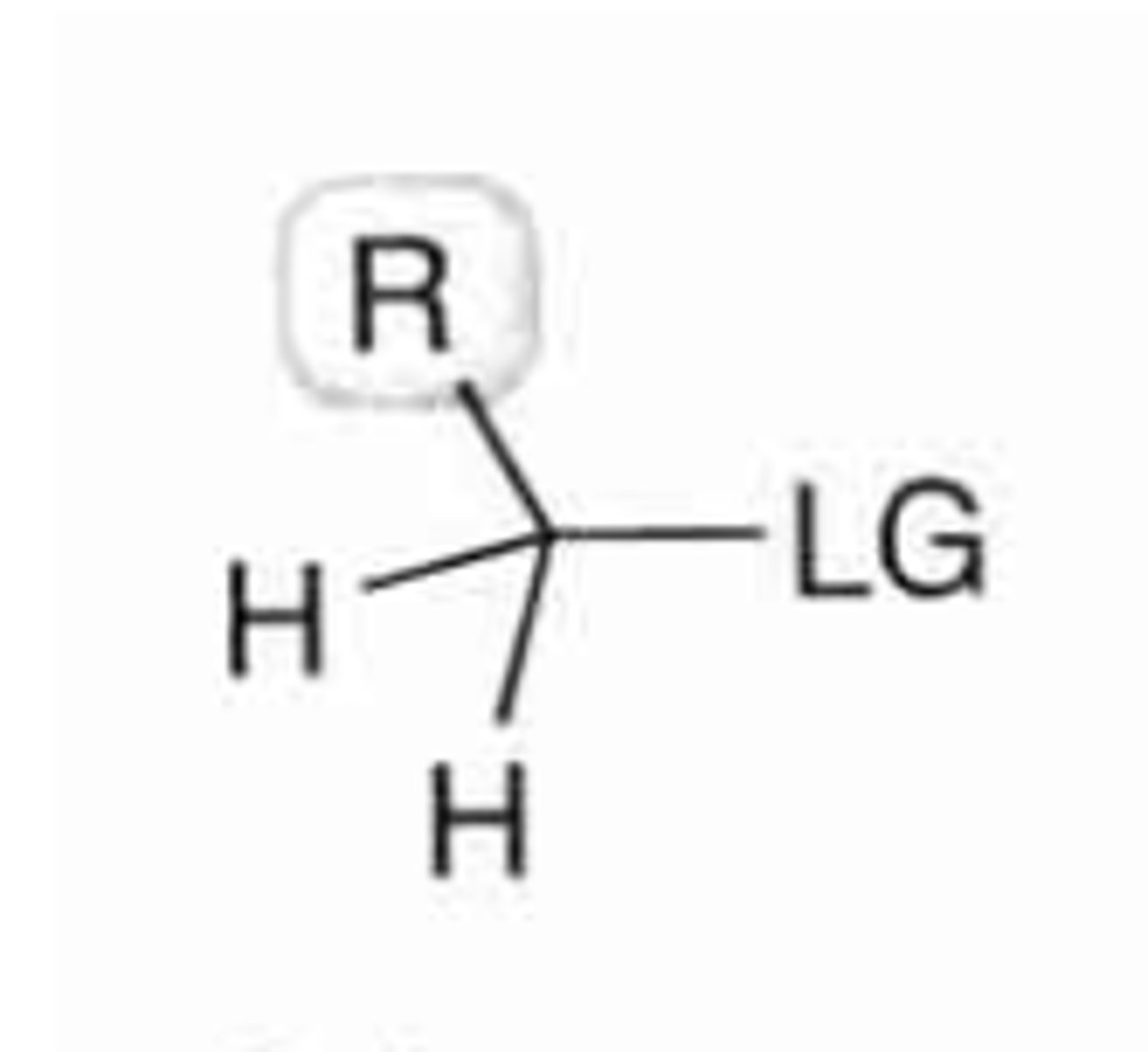
primary substrate
2 H's and 1 C bound to alpha-carbon
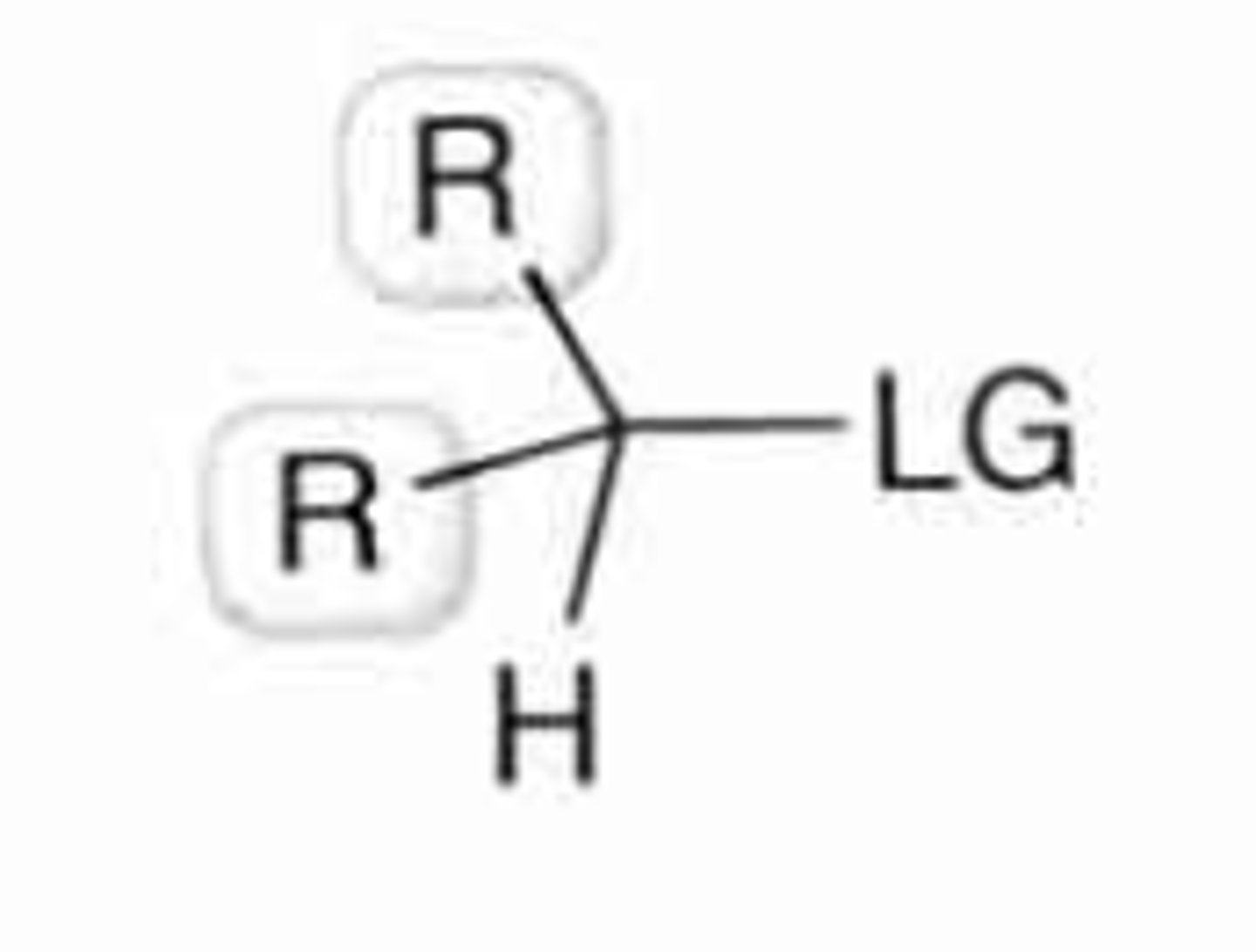
secondary substrate
1 H bound and 2 Cs to alpha-carbon
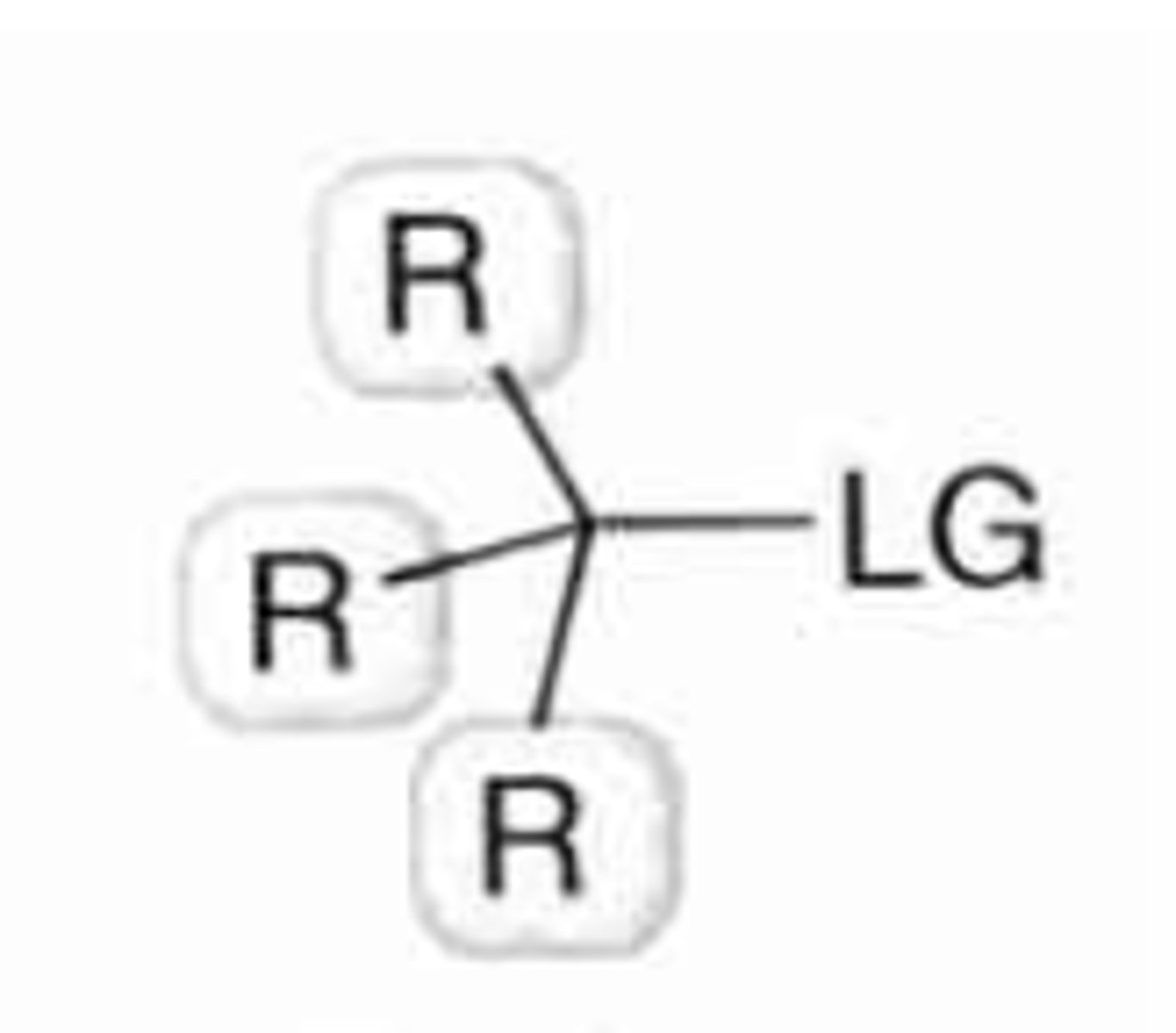
tertiary substrate
no H's and 3 Cs bound to alpha-carbon (bulky substituents instead)
alpha-carbon
carbon bound to leaving group
why do SN2 RXNs favour primary substrates?
as they're not v. bulky since they're bound to 2 H's. they therefore don't hinder Nu backside attack
the nature of the nucleophile is only...
...only important for SN2 bc their rate depends on the Nu, unlike SN1 where it's only dependent on substrate!
what do stronger Nu's do?
increase SN2 rate
what makes a stronger Nu?
1. basicity
2. negative charge
3. larger atom size
how does a larger atom make a stronger Nu?
bc e- more shielded from nucleus so weaker attractions and more e- available to react
what does a stronger Nu do?
pushes RXN to SN2
what does a weaker Nu do?
pushes RXN to SN1
main concept to get a stronger Nu?
e- availability. the more available, the better the Nu!
what makes a good LG?
1. forms stable anions
2. have weak C-LG bonds
3. higher EN so e- in bond attracted to LG so it can leave w them
4. well solvated anion
which RXN pathway do strong LGs favour
both but is essential for SN1 bc can't proceed to step 2 w/o LG departure
the departure of the LG is the...
...rate-determining step in both SN1 and SN2
which RXN pathway do polar protic solvents favour?
SN1 bc they favour formation of carbocations (which are only formed in SN1!)
which RXN pathway do polar aprotic solvents favour?
SN2 as they dissolve Nu's w/o reducing nucleophilicity and don't overly solvate Nu's, therefore leaving them more "free" and reactive
changing solvents can...
...switch SN1 and SN2 pathways
polar protic solvent
solvent containing O-H or N-H bonds that can donate a proton, strongly solvating ions through H-bonding (stabilises Nu but hinders ability to attack substrate. also favours carbocation formation for SN1)
polar aprotic solvent
solvent that lacks O-H and N-H bonds and cannot donate a proton, leaving Nu's relatively unsolvated, so more "free" and more reactive
what are E1 RXNs favoured by?
[similar to SN1]
1. favoured by stable carbocations (tertiary)
2. favoured by polar, ionising solvents e.g. polar protic
what are E2 RXNs favoured by?
[similar to SN2]
1. favoured by high base concentrations
2. favoured by strong base concentrations
[diff to SN2]
1. less sensitive to steric hindrance bc the base isn't attacking a C bound to multiple substituents like it is in SN2, it's attacking a more accessible H!
all Nu's can act as...
...bases and vice versa
what type of substrates/bases favour elimination?
strong, bulky ones
what do polar protic solvents do to Nu's?
solvate them, stabilising them but reducing nucleophilicity