Soil exam #3
1/105
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
106 Terms
pH
A measure of acidity or alkalinity of soils and other materials.
Macro-organisms
Soil organisms greater than 2 mm in size, including worms and termites.
Meso-organisms
Soil organisms ranging from 0.1 to 2 mm in size, such as springtails and mites.
Micro-organisms
Soil organisms less than 0.1 mm in size, including tardigrades, nematodes, fungi, bacteria, and archaea.
Chemoheterotrophs
Organisms that obtain energy from organic compounds carbon sources, and engage in biochemical oxidization.
Photoautotrophs
Organisms that use solar radiation to convert carbon dioxide into organic carbon. Typically includes plant ROOTS, algae, cyanobacteria.
Primary consumers
Herbivores(plants live), detrivores(dead plants and microbes on them) and saprotrophs (microorganisms that consume detritus corpses and feces
Detrivores
Organisms that feed on the remains of dead plants and microbes.
Saprotrophs
Microorganisms that consume detritus, corpses, and feces.
Secondary consumers
Carnivores that eat other animals, such as microbivorous that feed on microbes.
Soil organic matter (SOM)
Contains elements in living biomass, primarily carbon, nitrogen, and other nutrients. (58% carbon by mass, 1-6% nitrogen)
Turnover rate
The proportion of a pool that leaves during a given period of time. (turnover rate = output/pool)
Residence time
The average length of time that a molecule remains in a specific pool. (Residence time= pool/output)
Carbon cycle
The ongoing process of carbon movement between land, oceans, and the atmosphere.
Humification
The process by which organic matter decomposes into stable organic substances in soil.
Mineralization
The process by which organic nitrogen is converted into plant-available forms, such as NH4+.
Nitrification
The process by which ammonium (NH4+) is oxidized to nitrate (NO3-) by nitrifying bacteria.
Denitrification
The process by which nitrate is converted into gaseous nitrogen forms, reducing nitrogen in the soil.
Nitrogen fixation
The conversion of atmospheric nitrogen (N2) into organic compounds by certain bacteria.
Haber-Bosch process
An industrial process for synthesizing ammonia from nitrogen and hydrogen, significant for agriculture.
Potassium (K)
An essential nutrient for plant growth, involved in various cellular processes.
Eutrophication
The process by which excessive nutrients, primarily nitrogen and phosphorus, enter aquatic systems, leading to oxygen depletion.
Legacy P
Phosphorus that accumulates in soil beyond plant needs, which can slowly release into waterways.
Organic N inputs
Nitrogen inputs from organic sources, considered less mobile than mineral nitrogen inputs.
Volatilization
The process by which ammonium (NH4+) loses a hydrogen to become ammonia (NH3) gas.
N balance
The difference between nitrogen inputs and outputs in a given system, indicating nutrient management effectiveness.
Mycorrhizal associations
Symbiotic relationships between fungi and plant roots that enhance phosphorus uptake.
N leaching
The process by which nitrogen from the soil is moved away through soil layers , typically transported through rainfall and groundwater.
Carbon outputs
The loss of carbon from soil pools due to processes like respiration, harvesting, and erosion.
Chemoautotropphs
Use carbon dioxide and carbonate from INORGANIC sources as energy source. Ammonia oxidizers and sulfur oxidizers.
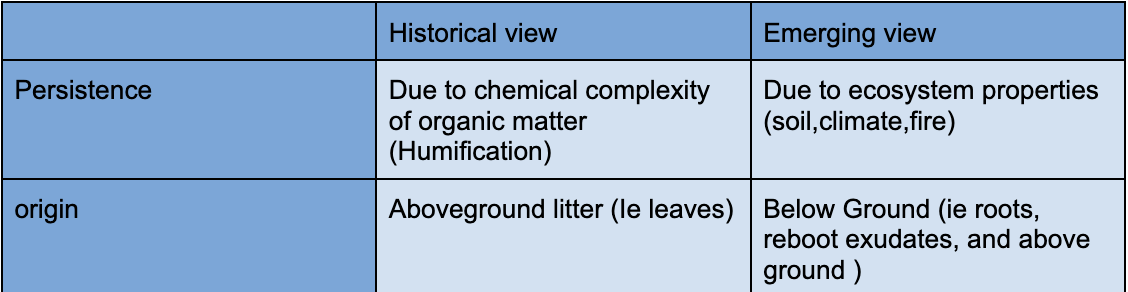
Why is SOM persistent
Carbon is deposited by plant matter, some gets immedately decomposed, some lasts for hundreds of years
Evolving views of SOM
Fast vs slow cycling pools
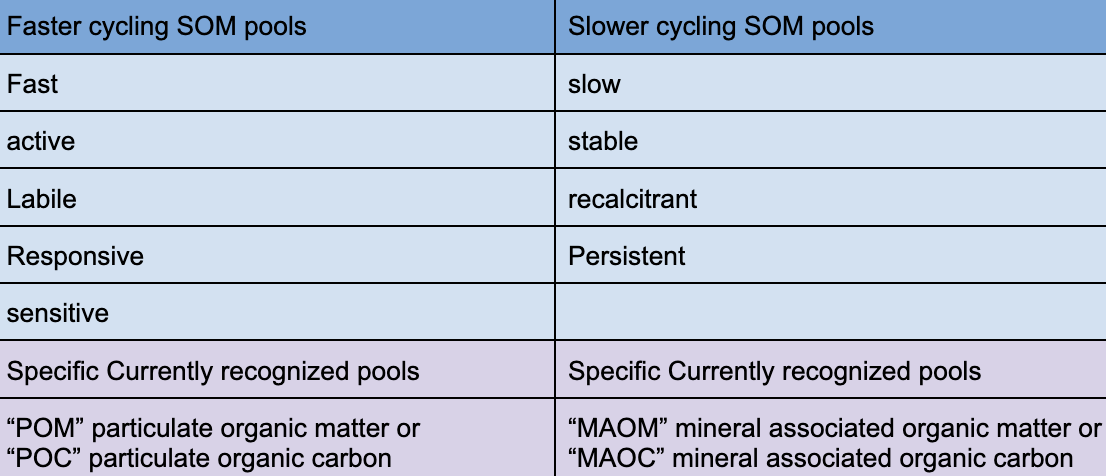
faster cycling SOM pools
POM and POC
Slow cycling
MAOM and MAOC
Formation of Particulate organic matter (POM)
A root or shoot enters soil (plant death or turnover) → +CO2 expelled → decomposer activity reduces size and mass of root, and alters chemical composition → CO2 expelled, remaining is >2mm> → Remaining fragments of root become POM
Formation of MAOM (mineral associated organic matter)
A root deposits carbon such as through exudation → plant carbon is directly adsorbed to minerals or processed by microbes → organic matter associated with minerals become MAOM
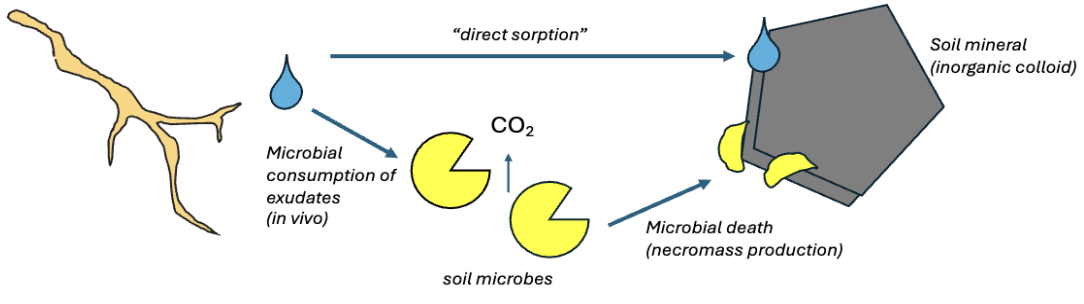
carbon inputs
Aboveground litter, animal waste, organic ammendements, roots, root exudation
Carbon outputs
Respired C (CO2) Harvest C (agricultural) Erosion, Leaching of dissolved organic C4
Protection mechanisms of SOM
Limit microbial access or activity in order to preserve the som
Protection of SOM for longer residence time are occluded in
Aggregates
SOM absorbed into —— surface is less vulnerable to microbial decomposition than a counterpark moelcule that is in a soil solution
Mineral surfaces, AKA (MAOM
Low O2 does what to microbial activity
induces anaerobic conditions, slowing down activity (happens when wet)
Low temp limits microbial activity
Reduces rates of ability to function
Low pH limits microbial function
More acidic solutions preserve for longer (like pickles)
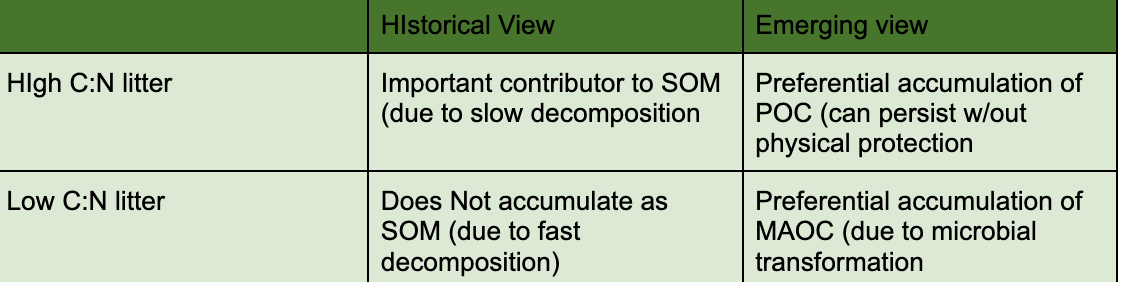
high C:N as a limitation of microbial activity
Ratio of carbon atoms per one nitrogen atom, as it gets higher the C number gets it corresponds to the higher difficulty in decomposing organic material
Historical view vs new view of litter inputs
Textures role in SOM
Total mass of silt+clay but also its activity increaase MAOC
Arid soils and Carbon correlation
Arid soils dont have water tp supply plant matter to supply carbon to the soil, no output if no input
Soil heath =
SOC
SOC is gained or lost under conversion to agriculture and SOC regeneration
Lost,
Why might not till plots have greater difference between SOC concentration between different parts of topsoil?
Tilled system its being incorporated into the soil, no till the plant matter status on surface. No tilled surface leaves plant matter on surface horizons
ways of Measuring a SOC pool
Concentration - mass SOC /mass soil
2. Stock = mass SOC / area and depth of soil
Why does bulk density affect measurement of SOC sock
Sampling two soils of the same depth but different bulk densitys will capture different masses of soil. Denser soil will have greater stock in top 30cm even if concentration is the same.
SOM fractionation
Causing disruption to a soil sample to measure the SOC and POC by separating the soil into different fractions based on chemical or physical processes
Phtotosynthesis -RuBisCo - requires nitrogen
An enzyme that catalyzese the conversion of CO3 to organic carbon
Nitrogen limits plant growth when there isnt enough
more nitrogen, more growth, limitation stronger in temperate soils
how to plants acces plant avaible nitrogen (usually in mieneral) form
Plants get nitrogen from soil via root uptake
Nitrogen cycle
The process through which nitrogen is converted between its various chemical forms, moving from the atmosphere into the soil and living organisms before returning to the atmosphere.
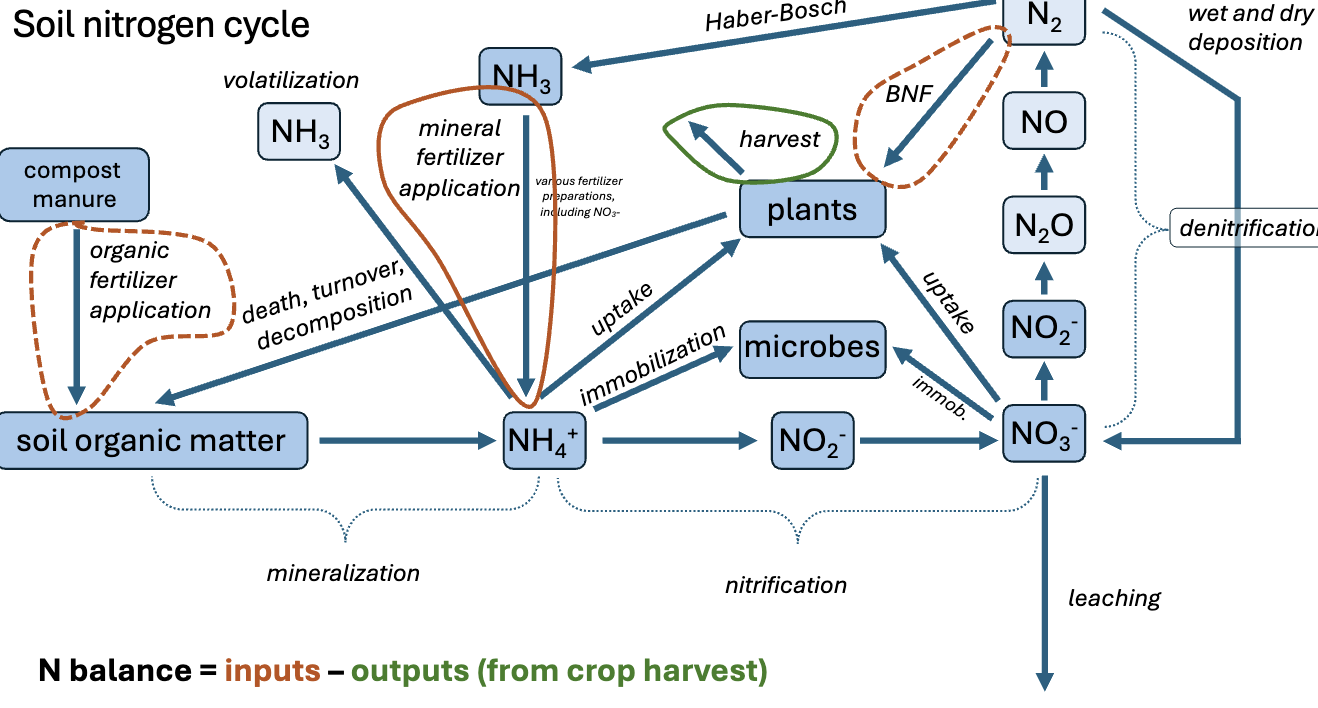
Two forms on N in soil
Ammonium (NH4) reduces electron rich N,Nitrate (NO3) oxidized or electron poor N species
Nitrification
NH4 will be oxidized to N03 for energy by microbe known as nitrifies (CHEMOAUTROTROPHS) using O2 as a terminal electron acceptor
Immobilization: microbial uptake of mineral N
IMportant for controlling N availablity to plants and preventing nitrogen loss by incorporating it into their biomass. This process involves the conversion of nitrogen from inorganic forms into organic forms by microorganisms, ultimately affecting the nitrogen balance in the ecosystem.
NH4 fication by soil colloids
adsorptiob of ammonium ions by the mineral or organic portion of the soil in a manner that they are raltively unexchangable by usual methods of cation exchange which helps retain cations.
Colloids can HIDE NH4 in this way
N LEACHING LOSS
Transport o fdissolved N out of soils and into coastal water - predominately NO3 due to its inability to be held in cation exchange but may also incldue NH4
Occurs when mineral N pools in soil > plant uptake + precipitation > evap
eutrophication
What is a highly oxidized species of N good for
nitrates are most accessable and usable form of nitrogen plant growth and industrial processes,
Denitrification
NO3- ions are converted gaseous forms of nitrogen by denitrifies. happens in the ABSENCE OF OXYGEN
Biological Nitrogen fixation (BNF)
The pathway for N2 to enter terrestrial cycle, via nitrogen fixing of bacgteria (rhizobia) and host legume. Impreded by low pH, low P Ca and K avaiblity
Haber - Bosch process
Converts atmospheric N2 to ammonia NH3 using hydrogen gas under high heat and pressure. Critical source of N in fertilizers
Nitrogen is the most abundant gas in the atmosphere in the primary form of
N2
Why is nitrogen an important element for life
because it is essential for the creation of protiens
The number of covalent bonds that connect two N atoms in a molecule of N2
Three
Is Oxygen the only terminal electron acceptor used my earthly life forms
NO
is Phosphorous as a gas; analogose for N fixation, P fixation by plant microbe symbioses, is essential for P cycling
false is not a gas but an essential nutrient for plants, involved in energy transfer and photosynthesis.
A cover crop by growing during times that woudl otherwise fallow
Can reduce nitrate leaching, can reduce rates of soil erosion, and promote BNF
Plant avaible nitrogen in soil refers to
Mineral N as both cation NH4 and anion NO3
Is The majority of soil N is held in plant avaible forms
False
Nitrification refers to
The oxidization of ammonium to nitrate
Processes in soil nitrogen transformations care carried out primatily by heterotrophic microbes include
SOM mineralization and denitrification
Leaching of mobile N from agricultural fields
Contributes to eutrophication of riverine and coastal waters
Denitrification
Is a formant microbial transformation of N when soils are wet (and therefore anaerobic)
Denitrification
Returns N to the terrestrial N cycle to the atmosphere
Pathways through which atmospheric N2 can enter soil and plant systems include
Biological nitrogen fixation adn haber bosch process
Crop responses to mineral N fertilizer input
Plateu once N becomes non- limiting
Sidedress N applications
Align timing of N application with timing of plant N uptake
The two pools that make up most soil organic matter currenlty recognized as
Particlate organic matter and mineral-associated organic matter
As a part of the global carbon cycle, soil organic matetr is imporant becaue
It comprises more carbon than what is held in the atmosphere and terrestrial vegetation combines
best descrption of pools and turnover of organic carbon
smalelr faster cycling pools and larger slower cycling pools
Mineral associated organ carbon
Is more persistent in soil than particulate organic carbon (POC)
Particulate organic carbon
Various forces can limit microbial respiration and ability to encounter organic C compounds in soil
The pool size of SOC is determined by
the historical balance of C inputs and C outputs
C:N ratio of litter
corresponds to its decomposition rate, with lower C:N litters decomposing more rapidly
n order to calculate stock of SOC (Mg, C/hA youll need)
concentration of SOC, bulk density and sampling depth
is fractionating SOC into POC and MAOC is the only approach currently or historically used to study SOC dynamics
No, methods include physical chemical and biological techniques
using practices that protect or increase SOC stoks in soil can aid in
climate change mitigation, improving soil health and enhancing agricultural productivity.
Phosphorous is a critical element of life because
It is part of nuceleic acids and lipids
does P in soil exists in organic and inorganic forms
yes
The phosphorus cycle differs from nitrogen cycle becuase
P cycle doesnt have a gaseous pool
If you add inorganic phsophorus fertilizer to soil you might expect
A significant proportion is fixed into inorganic forms, depending on Fe and Al oxide avaiblaity
inputs of phosphorous to soil with no human altercation include
dust