Chem. S1 Ch. 1 & 2
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
60 Terms
. ___ is a logical approach for solving problems by observing and collecting data, formulating hypotheses, testing hypotheses, and formulating theories that are supported by data.
scientific method
___ in science is more than a physical object; it is often an explanation of how phenomena occur and how data or events are related.
model
___ is a testable statement that is the basis for making a prediction.
hypothesis
___ is a broad generalization that explains a body of facts or phenomena.
theory
58 km = _____ m
58 000
37 982 mm = ___ km
0.037 982
1.73 m = ___ cm
173
0.45 km = ___ cm
45 000
Find the density of a material given that a 5.03 g sample occupies 3.24 ml.
1.55 g/ml
What is the mass of a sample of material as a volume of 55.1 cm3 and the density of 6.72 g/cm3?
370 g
A sample of a substance has a density of 0.824 g/ml has a mass of 0.451 g. Calculate the volume of the sample.
.547 ml
Compare accuracy and precision
Accuracy refers to the closeness of measurements to a correct or accepted value, precision refers to the closeness of a set of number to one another.
A handbook gives the density of calcium as 1.54 g/cm3. Based on the lab measurements, what is the percentage error of a density calculation from 1.25 g/cm3?
-18.8%
How many significant figures on each of the following measurements
B 6 000 g
1
How many significant figures on each of the following measurements
C 1.000 30 km
6
Complete the following math problem and give the final rounded, correct significant figures answer.
4.567 cm + 2.35 cm + 83.23 cm = ?
90.15
Complete the following math problem and give the final rounded, correct significant figures answer.
4.567 cm - 2.3539 cm = ?
2.213
Complete the following math problem and give the final rounded, correct significant figures answer.
84.5967 cm ² / 7.85 cm = ?
10.8
Complete the following math problem and give the final rounded, correct significant figures answer.
49.67 cm x 12.3415 cm x 13.53 cm = ?
Round this number to 3 significant figures and put it in scientific notation.
739 670 000 000 000 000
7.40x10^17
Round this number to 4 significant figures and put in scientific notation.
0.000 000 000 000 348 298 932
3.483x10^-13
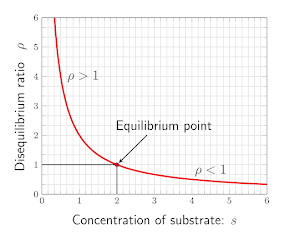
From this graph, what kind of relationship do these variables share?
inversely proportional
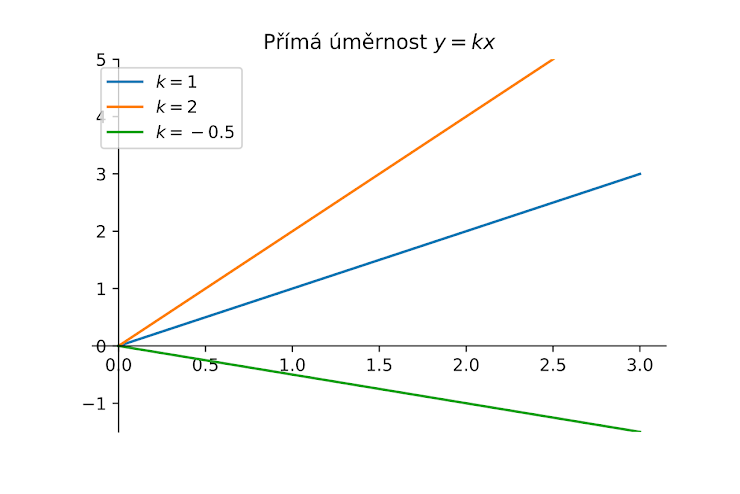
From this graph, what kind of relationship do these variables share?
direct proportion
How many significant figures are in this number? 0.000 39
2
2
How many significant figures are in this number? 510 00
How many significant figures are in this number? 5100
4 sig. figs; 5100
How many significant figures are in this number? 10.00
4
Define Chemistry.
the study of the composition, structure, and properties of matter, the processes that matter undergoes, and the energy changes that accompany these processes.
Give 3 of the 6 branches of chemistry
organic, inorganic, physical, analytic, biochem, theoretical
Define chemical
A ___ is any substance that has a definite composition.
If someone is trying to solve the problem of too much carbon dioxide in the atmosphere, what kind of research are the performing?
applied
technological
If someone if using science to improve the speed of computers, what kind of research are they performing?
If someone is studying motor proteins in the cell, just to learn more about motor proteins, what kind of research are they performing?
basic
mass
___ is a measure of the amount of matter.
___ has mass and takes up space.
matter
atom
___ is the smallest unit of an element that maintains the chemical identity of that element.
___ is a pure substance that cannot be broken down into simpler substances and is made of only 1 type of atom.
element
compound
___ is a substance that can be broken down into simple substance.
If the property is extensive, check the box, if the property is intensive, do not check the box.
volume, mass
color, mass, texture
If the property is physical, check the box, if the property is chemical, do not check the box.
A solid has
definite size and definite shape
definite size and indefinite shape
a liquid has
A gas has
indefinite size and indefinite shape
melting is
solid to liquid
liquid to solid
freezing is
liquid to gas
evaporation is
solid to gas
sublimation is
gas to liquid
condensation is
oxygen and hydrogen
In the following reaction, what are the reactants? oxygen + hydrogen ➡ water
water
water In the following reaction, what are the products? oxygen + hydrogen ➡ water
true
T or F: The law of conservation of matter states that matter can not be created or destroyed, but it can change form.
false
T or F: The law of conservation of energy states that matter can be created or destroyed, but it can not change form.
pure substance, element
a gold coin (24K) is (pick 2)
mixture, homogeneous
salt water is (pick 2)
chicken noodle soup is (pick 2)
mixture, heterogeneous
groups or families
On the periodic table, vertical columns are called?
On the periodic table, horizontal rows are called?
periods
all the above
Which is true for metals
none of the above
Which is true for nonmetals
On the periodic table, what is the name of group 18 elements?
noble gases