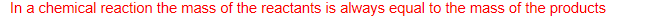Y7 Chemical Reactions and Limestone
0.0(0)
Card Sorting
1/16
Earn XP
Description and Tags
Topic 3
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
1
New cards
What is a fuel?
A material that burns to transfer useful energy.
2
New cards
What is combustion?
A chemical reaction in which a substance reacts quickly with oxygen and gives out heat and light.
3
New cards
What is oxidation?
A chemical reaction in which substances react with oxygen to form oxides.
4
New cards
What are the products of combustion?
Carbon dioxide and water.
5
New cards
What is a decomposition reaction?
A chemical reaction in which a compound breaks down to form simpler compounds and/or elements.
6
New cards
What is thermal decomposition?
A chemical reaction where a compound breaks down when heated.
7
New cards
What is the clue to look for to tell if a decomposition reaction is taking place?
There will be one reactant and many products.
8
New cards
What is the chemical formula for limestone?
CaCO3.
9
New cards
What is the chemical formula for calcium oxide?
CaO.
10
New cards
What is the chemical formula for calcium hydroxide?
Ca(OH)2.
11
New cards
How do you turn calcium carbonate into calcium oxide?
Heat it strongly.
12
New cards
How do you turn calcium oxide into calcium hydroxide?
Add water.
13
New cards
How do you turn calcium hydroxide into calcium carbonate?
Bubble carbon dioxide through it.
14
New cards
What is the word equation for the thermal decomposition of calcium carbonate?
Calcium carbonate → Calcium oxide + carbon dioxide.
15
New cards
what is an endothermic reaction?
A reaction that takes in heat (it gets colder/the temperature goes down)
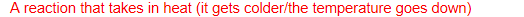
16
New cards
what is an exothermic reaction?
A reaction that gives out heat (it gets warmer/the temperature goes up)

17
New cards
What is the law of conservation of mass?

In a chemical reaction the mass of the reactants is always equal to the mass of the products