chem 2211L review
5.0(1)
Card Sorting
1/145
Earn XP
Description and Tags
Last updated 11:03 PM on 11/16/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
146 Terms
1
New cards
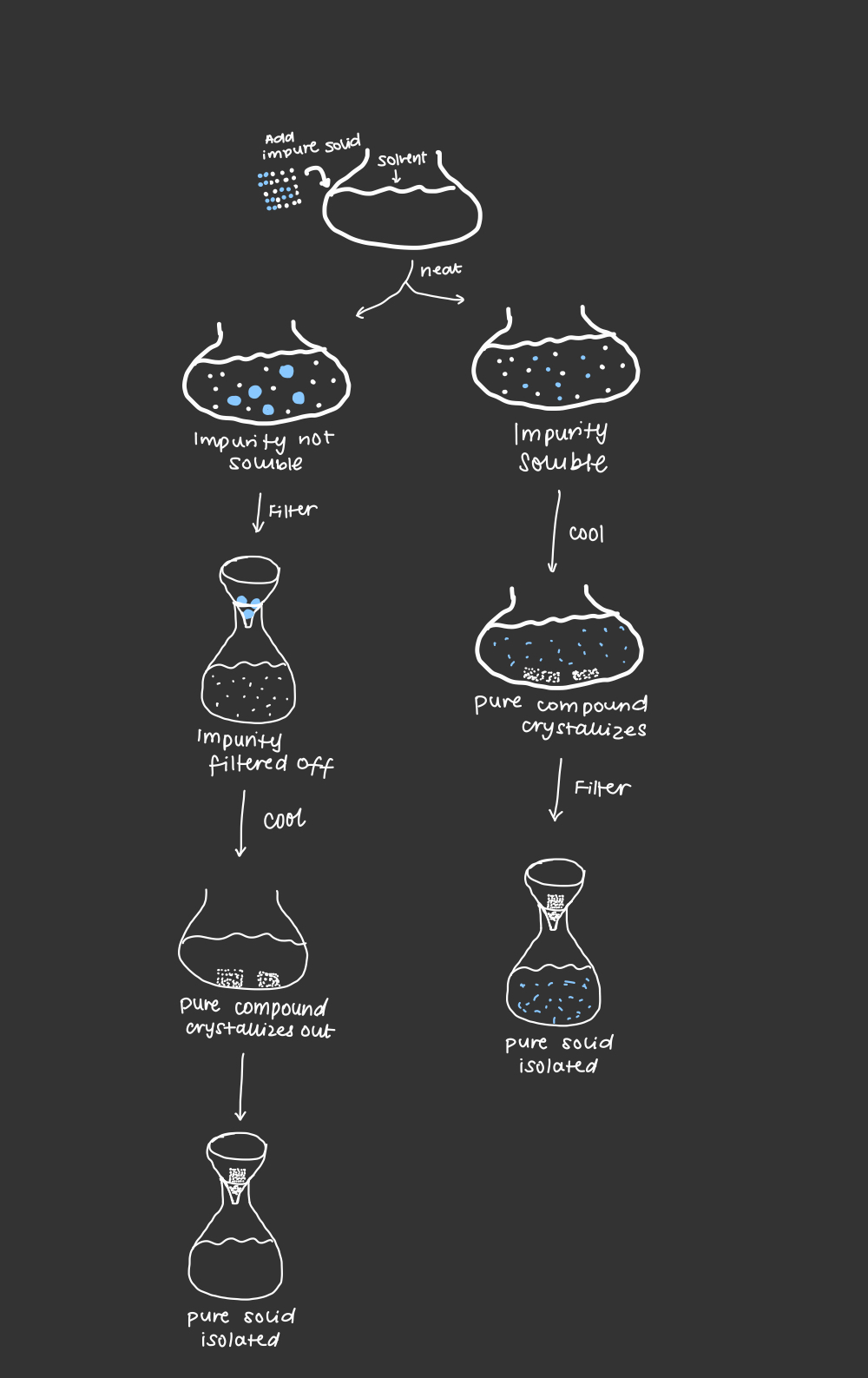
5 steps for recrystallization
1. choose the appropriate solvent
2. dissolve impure compound in hot solvent
3. remove the insoluble impurities from hot solvent via suction filtration
4. cool to crystallize/precipitate the pure compound from the solution
5. isolate the pure compound via suction filtration
2. dissolve impure compound in hot solvent
3. remove the insoluble impurities from hot solvent via suction filtration
4. cool to crystallize/precipitate the pure compound from the solution
5. isolate the pure compound via suction filtration
2
New cards
recrystallization graphic
3
New cards
step 1: how to choose a solvent for recrystallization
--> compound has to be fully soluble at elevated temperature and not room temperature
--> impurities should not be soluble either at elevated temperatures or room temperature
--> chemically inert with the compound and impurities
--> suitably volatile so it can be removed easily once pure crystals have been isolated
--> impurities should not be soluble either at elevated temperatures or room temperature
--> chemically inert with the compound and impurities
--> suitably volatile so it can be removed easily once pure crystals have been isolated
4
New cards
why should the compound not dissolve in the solvent at room temperature (recrystallization)
dissolution is necessary so that the crystalline lattice can be broken down and purified. if the compound is soluble at room temperature, it will be difficult to retrieve the product from the solution
5
New cards
why must the solvent be chemically inert (recrystallization)
if the solvent reacts with the compound or impurities, a chemical transformation will occur rather than a purely physical dissolution/recrystallization
6
New cards
why must the solvent be suitably volatile (recrystallization)
--> if the solvent is not volatile enough, it will linger in the sample and affect the percent yield and melting-point determination
--> if the solvent is too volatile, it will evaporate before the compound is fully dissolved in the first place
--> if the solvent is too volatile, it will evaporate before the compound is fully dissolved in the first place
7
New cards
examples of a bad solvent for recrystallization
the compounds have similar solubilities at both temperatures, or the compounds will dissolve at room temperature, or the solubilities don't increase with temperature
8
New cards
step 2: dissolving the impure solid for recrystallization
--> hot solvent is added to the solid to dissolve it
--> only use sufficient amount of solvent
--> only use sufficient amount of solvent
9
New cards
What are the consequences of using too much solvent for recrystallization
if you use too much solvent, it will be far more difficult to retrieve the pure solid at the end. you will have to wait more time for all of the solvent to evaporate
10
New cards
step 3: separation of impurities via hot filtration
--> place a funnel and filter paper on top of erlenmeyer flask
--> add few milliliters of solvent with boiling chips and place on hotplate
--> cover with watch glass
--> once vapors of solvent permeated through filter, remove watch glass and filter the hot solution
-->pour small amount of boiling solvent to dissolve any solid that may have crystalized during filtration
--> add few milliliters of solvent with boiling chips and place on hotplate
--> cover with watch glass
--> once vapors of solvent permeated through filter, remove watch glass and filter the hot solution
-->pour small amount of boiling solvent to dissolve any solid that may have crystalized during filtration
11
New cards
Step 4: cooling the solution and crystallizing the solid
--> crystals will begin to form as flask reaches room temperature
--> ice bath
--> ice bath
12
New cards
how to induce crystallization
--> scratch the inner wall of the flask with a stirring rod
--> introducing a seed crystal that matches the compund to the solution
--> introducing a seed crystal that matches the compund to the solution
13
New cards
why are the crystals not precipitating out of the solution
too much solvent during dissolution.. heat more more time to evaporate the solvent.
14
New cards
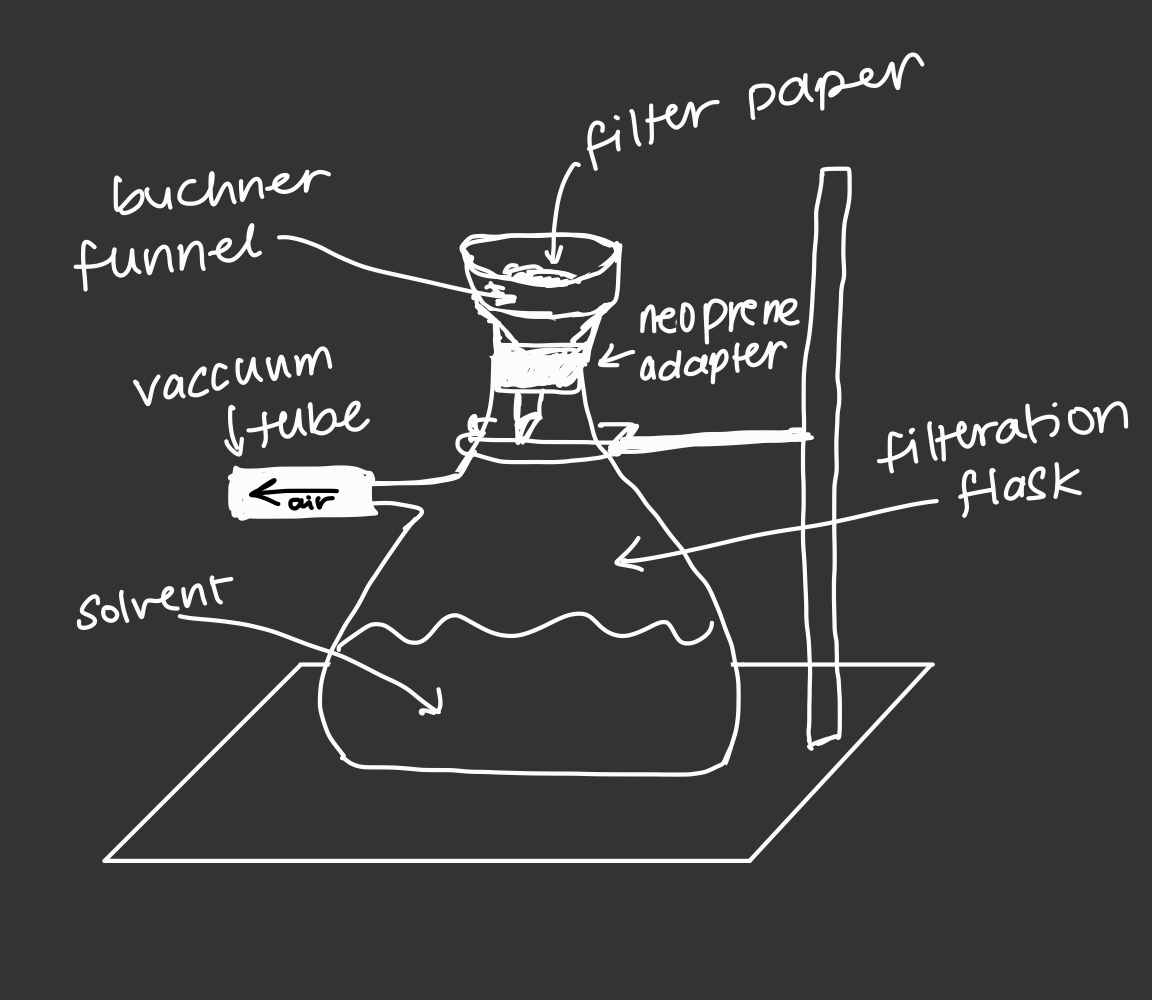
step 5: isolating the pure compound via suction filtration
--> suction filtration for several minutes
-->remove filter paper from the funnel and place it on a watch glass and scrape off the crystals from the filter paper
--> weigh the product
-->remove filter paper from the funnel and place it on a watch glass and scrape off the crystals from the filter paper
--> weigh the product
15
New cards
suction filtration graphic
16
New cards
Melting point range of a chemical substance
The temperature at which the sample first shows signs of melting and the temperature at which the sample becomes fully liquified
17
New cards
what effect do impurities have on melting point range of a substance
impurities broaden and lower (depress) the range
18
New cards
eutectic point of the system
as the mole percentage of compound A continues to increase, a minimum melting point value for the two-component mixture is attained. at the specific composition of mixture A:B both compounds will melt simultaneously.
19
New cards
why do you need a dry sample?
--> difficult to load the sample into the capillary tube
--> the solvent acts as an impurity and lower and broaden the range
--> the solvent acts as an impurity and lower and broaden the range
20
New cards
how to prepare a melting point sample
--> make sure sample is dry
--> crush a small portion into a fine powder on you watch glass
--> press the open end of the capillary tube into the crystals so that a small portion of the sample is forced into the tube
--> settle the sample into the tube and use a condenser to drop the tube and pack the sample into the closed end of the capillary
--> crush a small portion into a fine powder on you watch glass
--> press the open end of the capillary tube into the crystals so that a small portion of the sample is forced into the tube
--> settle the sample into the tube and use a condenser to drop the tube and pack the sample into the closed end of the capillary
21
New cards
what is a capillary tube?
thin, glass tube with an opening at one end
22
New cards
Mel-Temp apparatus
consists of heating block, thermometer well, a capillary holder, a temperature/voltage control, a light source and an observation window.
23
New cards
how to set the temperature for Mel-Temp
first attempt - a higher setting and a heating rate of 6-8ºC per minute.
second attempt - heat the sample quickly within 20ºC of original melting point range and lower the heat rate to 1-2ºC per minute
second attempt - heat the sample quickly within 20ºC of original melting point range and lower the heat rate to 1-2ºC per minute
24
New cards
sources of error for melting point determination
--> sample is not dry
--> heat too quickly
--> use a new sample on a hot apparatus, it most likely melt immediately
--> heat too quickly
--> use a new sample on a hot apparatus, it most likely melt immediately
25
New cards
50:50 mixture method for compound determination steps
--> choose two most likely compounds that resemble the melting points (same or higher)
--> prepare two samples - the first has 50:50 of unknown sample and first likely compound and second has 50:50 of the unknown sample and second likely compound
--> prepare two samples - the first has 50:50 of unknown sample and first likely compound and second has 50:50 of the unknown sample and second likely compound
26
New cards
how to determine which compound from 50:50 mixture method
there will be no noticeable change from the melting point of the mixture and the previously observed melting point range of just the unknown sample.
27
New cards
techniques used in recrystallization experiment
recrystallization, melting point determination, gravity filtration, suction filtration
28
New cards
calculate the volume necessary to recrystallize a certain amount of solid
use the ratios given and set up equation to solve for the unknown value
29
New cards
techniques used for distillation experiment
simple distillation, fractional distillation, and boiling point determination
30
New cards
what are the two types of distillation
simple and fractional
31
New cards
what is the purpose of distillation
--> separate/ isolate two volatile and miscible liquids
--> isolate a volatile liquid from a nonvolatile, but soluble solid
--> isolate a volatile liquid from a nonvolatile, but soluble solid
32
New cards
what is the distillation separation based on
boiling point differences
33
New cards
dalton's law of partial pressures
used to define the composition of the solution vapor for the mixture of miscible liquids
Ptot = Pa + Pb + Pc +...
Ptot = Pa + Pb + Pc +...
34
New cards
Raoult's Law of partial pressures
partial vapor pressure of each component is equal to the vapor pressure of the pure component multiplied by its mole fraction within the mixture
Pa = (Pºa)(mol fraction)
Pa = (Pºa)(mol fraction)
35
New cards
Combined law
Ptot = Pºa + Pºb + Pºc +...
helps to understand how the two primary methods of distillation are used to purify mixtures containing liquids of varying boiling points
helps to understand how the two primary methods of distillation are used to purify mixtures containing liquids of varying boiling points
36
New cards
Simple distillation
when solution is heated, the temperature of the vapor rises to the boiling point temperature of the lower boiling point component. then the solution and vapor are in thermal equilibrium and the temperature remains constant. this plateau is good indicator of a successful separation
37
New cards
difference between simple and fractional distillation
--> simple used when the liquids have boiling point difference of 100ºC or more
--> fractional distillation is used when the liquids have boiling point difference less than 100ºC
--> fractional distillation is used when the liquids have boiling point difference less than 100ºC
38
New cards
fractional distillation
involves a series of concurrent simple distillations occurring within the same apparatus that is achieved by adding a fractionating column in between the distillation flask and still head
39
New cards
fractionating column
glass tube filled with tightly packed media such as steel wool or glass beads. as the vapor comes into contact with the packing material, it condenses and re-vaporizes slowly makes it way up the column (column increases surface area). with each condensation and vaporization, the vapor becomes more and more enriched in the lower boiling component of the mixture
40
New cards
fractionating column efficiency
measured in the number of theoretical plates that it can generate.
measured in column length and density of packing material
measured in column length and density of packing material
41
New cards
theoretical plate
the mini distillations that occur within the fractionating column. as the number of theoretical plates increase, the efficiency of the fractionating column increases
42
New cards
azeotrope
the vapor and liquid concentrations are the same. the two liquids form constant boiling points. separation via distillation would not work for these compounds.
43
New cards
what is the consequence of reversing the water hose inlet and outlet
less water would be present and the condenser would not be cooled enough and the vapor will remain as vapor and will not condense back to liquid state.
44
New cards
simple distillation apparatus
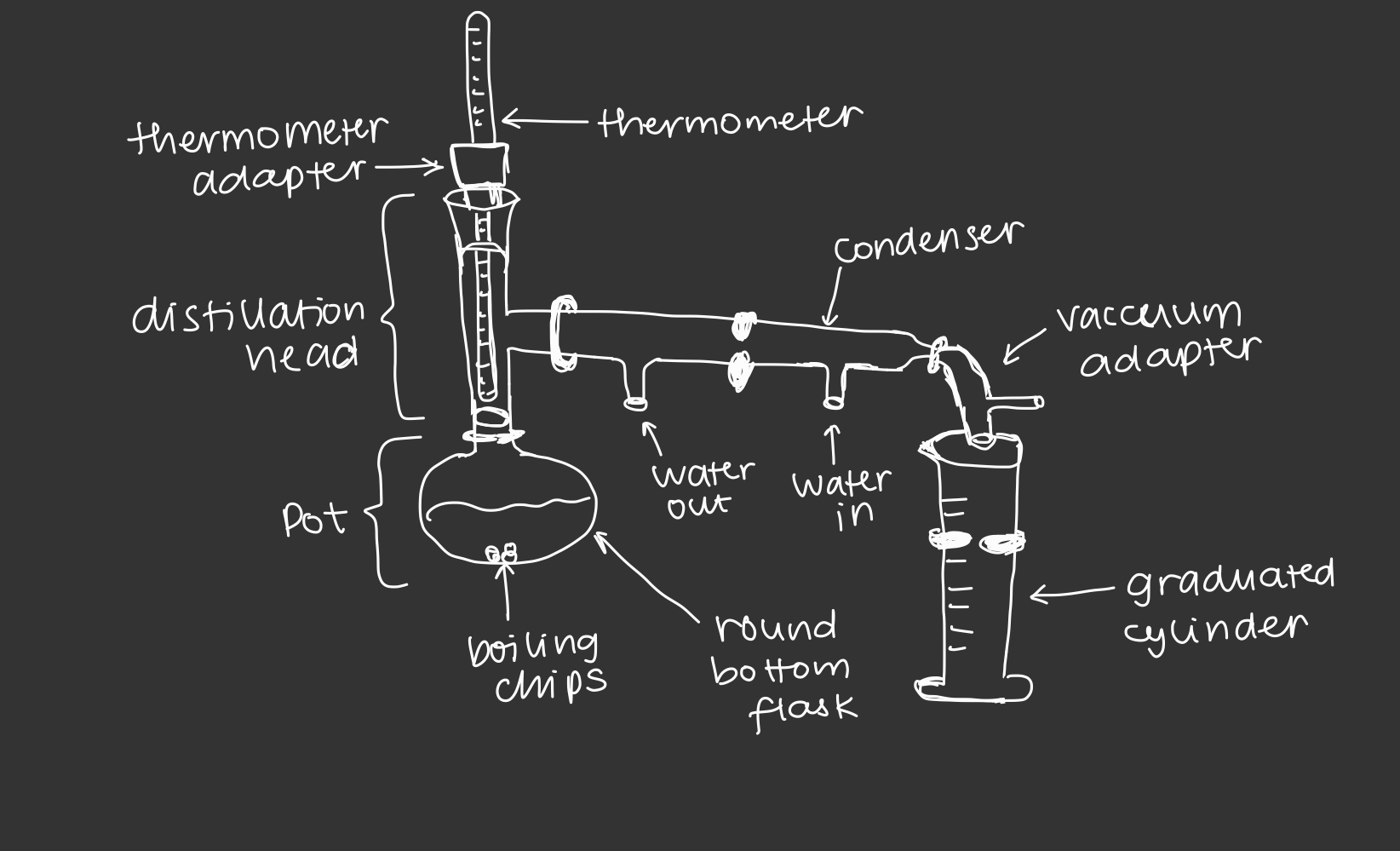
45
New cards
fractional distillation apparatus
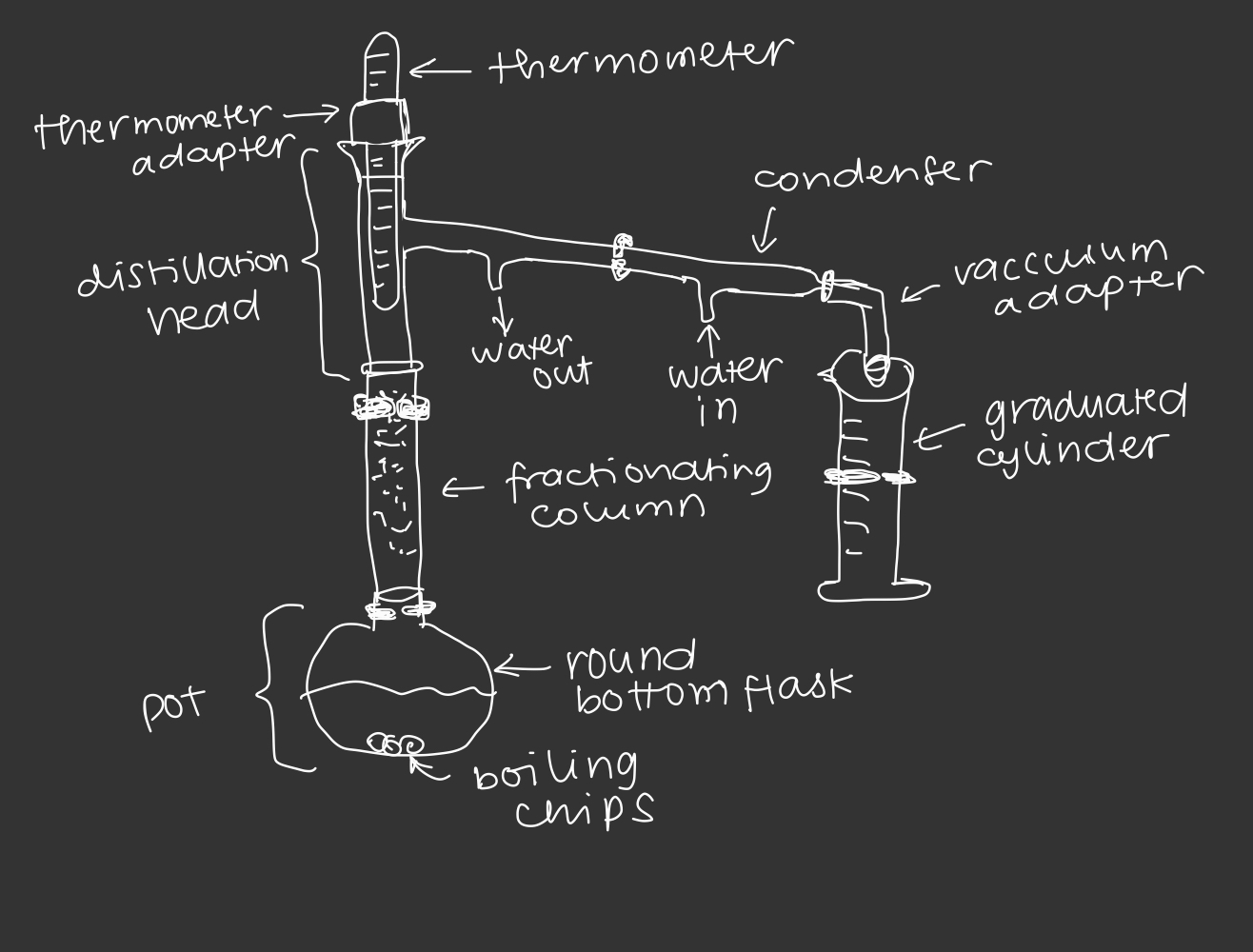
46
New cards
how to make sure fractional distillation is successful
the experiment must be run at a moderate pace using appropriate temperature settings. the boiling must be steady and slow to achieve the desired result
--> if the distillation is rushed, the individual mini-distillations in the fractionating column will decrease and the resulting separating will be poor
--> if the distillation is rushed, the individual mini-distillations in the fractionating column will decrease and the resulting separating will be poor
47
New cards
how to calculate the composition through the data graph
(mL of compound passed at boiling point/ mL of total solution) x 100 = % of compound in solution
48
New cards
techniques used in nutmeg extraction
solid/liquid extraction, gravity filtration, and simple distillation
49
New cards
Extraction
a physical process by which a single component is removed from a mixture of others
50
New cards
two types of extraction
solid/liquid and liquid/liquid
51
New cards
solid/liquid extraction
facilitates the removal of a pure substance from a permeable, composite solid. this separation is achieved based on a difference in solvent solubility between the desired compound and the bulk of the components comprising the complex solid
52
New cards
what was extracted from what in the solid/liquid extraction
trimyristin is extracted from nutmeg (20-25%)
53
New cards
gravity filtration in the nutmeg extraction
separates the Trimyristin from the residue of the nutmeg after reflux
54
New cards
simple distillation in nutmeg extraction
removes the solvent (methylene chloride) from the extracted product. the distillation was complete when the temperature reached the boiling point of methylene chlorine and plateaued
55
New cards
suction filtration in nutmeg extraction
isolates the pure crystals
56
New cards
reflux apparatus
heating mantle, round bottom flask, condenser (water inlet at the bottom)
57
New cards
reflux
process of boiling reactants wile continually cooling and condensing the vapor thereby returning it back to the boiling flask so there is no loss of solvent
58
New cards
reflux purpose in nutmeg extraction
heat mixtures for extended periods of time without solvent loss to extract compounds or carry out reactions
59
New cards
melting point determination in nutmeg extraction
melting point was used to characterize the recovered trimyristin product
60
New cards
solvent used in nutmeg extraction
methylene chloride, acetone
61
New cards
methylene chloride purpose in nutmeg extraction
used because it's nonpolar so it could dissolve the nonpolar trimyristin compound and not the other compounds in nutmeg
62
New cards
acetone purpose in nutmeg extraction
rinse/wash flask and crystals and wash away impurities
63
New cards
nutmeg extraction reaction
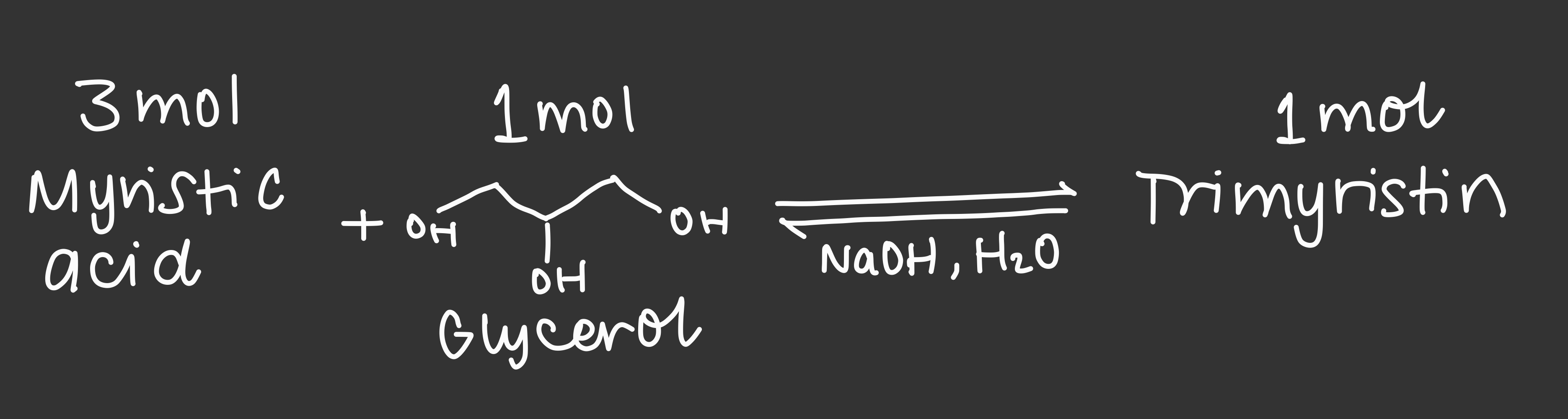
64
New cards
Thin layer chromatography
technique used to separate non volatile mixtures
65
New cards
What is thin layer chromatography used for
--> identifying individual components of a mixture
--> compound purity determination
--> compound/ multi-component mixture identification
--> monitoring the progress of a chemical reaction
--> compound purity determination
--> compound/ multi-component mixture identification
--> monitoring the progress of a chemical reaction
66
New cards
What is the separation based on for TLC
stationary phase and mobile phase. the more strongly a compound is absorbed on the stationary phase, the slower it moves through the system, separating the components of the mixture from each other
67
New cards
solid stationary phase
silica gel, alumina (polar)
68
New cards
mobile liquid phase
low BP organic solvent (ethyl acetate with 0.5% acetic acid)
69
New cards
how does the TLC plate work
as the solvent travels up the plate through the capillary action, it passes over the compounds absorbed on the solid. a partitioning sets up between the absorptivity of the compounds on the solid absorbent and their solubility in the solvent
70
New cards
what is the effect of polarity for TLC
--> polar compounds means strong interactions with polar stationary phase so they elute slower
--> the molecules that moved the most towards the top of the plate were the most nonpolar
--> like dissolves like
--> the molecules that moved the most towards the top of the plate were the most nonpolar
--> like dissolves like
71
New cards
choice of solvent for TLC experiment
--> the polarity of the solvent ultimately determines the distance from the origin that a compound move up the plate
--> solvent system is chosen that will move the desired component between 1/3 to 2/3 of the way up the plate
--> ethyl acetate containing 0.5% acetic acid was the solvent because it's non polar and the silica gel is polar
--> solvent system is chosen that will move the desired component between 1/3 to 2/3 of the way up the plate
--> ethyl acetate containing 0.5% acetic acid was the solvent because it's non polar and the silica gel is polar
72
New cards
calculating Rf value
--> Rf = distance traveled by solute/ distance traveled by solvent
--> Rf = 0 means the compound doesn't move from the original position (polar)
-->Rf = 1 means the compound traveled along with the solvent front because the compound is completely soluble in the solvent
--> if two substances have the same Rf values it doesnt mean they are same
--> Rf = 0 means the compound doesn't move from the original position (polar)
-->Rf = 1 means the compound traveled along with the solvent front because the compound is completely soluble in the solvent
--> if two substances have the same Rf values it doesnt mean they are same
73
New cards
what were the TLC plates visualized with
ultraviolet lamp (dark spots)
74
New cards
what was the solvent system used to dissolve the compounds in preparation for TLC spotting
1:1 methylene chloride: ethanol
75
New cards
what were the 4 ingredients used in the TLC experiment
ibuprofen, acetaminophen, caffeine, aspirin (acetylsalicylic acid)
76
New cards
what if you remove the TLC plate before the solvent reaches the top of the plate
all the compounds would be displaced, causing them to travel up the plate near the solvent front with no separation. the plate would continue developing and there would be no way to determine how far the spots traveled, so we could not calculate the Rf values.
77
New cards
what if the solvent was only allowed to travel halfway up the plate before it was removed (TLC experiment)
the distance traveled by the compounds would not be accurate. there isn't enough solvent to move the compounds fast enough for separation
78
New cards
what if you apply a too large of a spot to the TLC plate
result in one spot rather than two distinct spots. there is not enough time for the compounds to separate. Rf values cannot be accurately calculated
79
New cards
what if the origin line is too low on the TLC plate
the component of the mixture would dissolve into the developing solution instead of traveling up the TLC plate. there would be no spots after the plate because the sample got lost in the solvent
80
New cards
does TLC plate size make a difference in Rf values
Rf values are based on ratio so the plate size is not important
81
New cards
acid/base extraction experiment techniques
liqui/liquid extraction, separatory funnel, IR spectroscopy, 1H NMR spectroscopy, suction filtration
82
New cards
acid/base extraction
three component mixture containing an acidic, basic, and neutral component
83
New cards
what is the separation based on for acid/base extraction
liquid/liquid extraction --> relative solubilities in two different immiscible liquids
each compounds acid/base properties will be exploited to move the components between immiscible aqueous and organic layers
each compounds acid/base properties will be exploited to move the components between immiscible aqueous and organic layers
84
New cards
how do the acid/base reactions work
the reactions are simple proton transfers that lead to significant shifts in compound stability. the changes allow to the sequential isolation of each compound
85
New cards
ways of liquid/liquid extraction
--> an aqueous solvent-organic solvent
--> two organic solvents of differing densities/polarities
--> two organic solvents of differing densities/polarities
86
New cards
two types of liquid/liquid extraction
--> aqueous solvent-organic solvent
--> two organic solvents of different densities/polarities
--> two organic solvents of different densities/polarities
87
New cards
Separatory funnel in the liquid/liquid extraction
--> organic solvents are not miscible with water so they form two different layers when mixed in the separatory funnel
--> the denser liquid is the bottom layer
--> the denser liquid is the bottom layer
88
New cards
separatory funnel apparatus
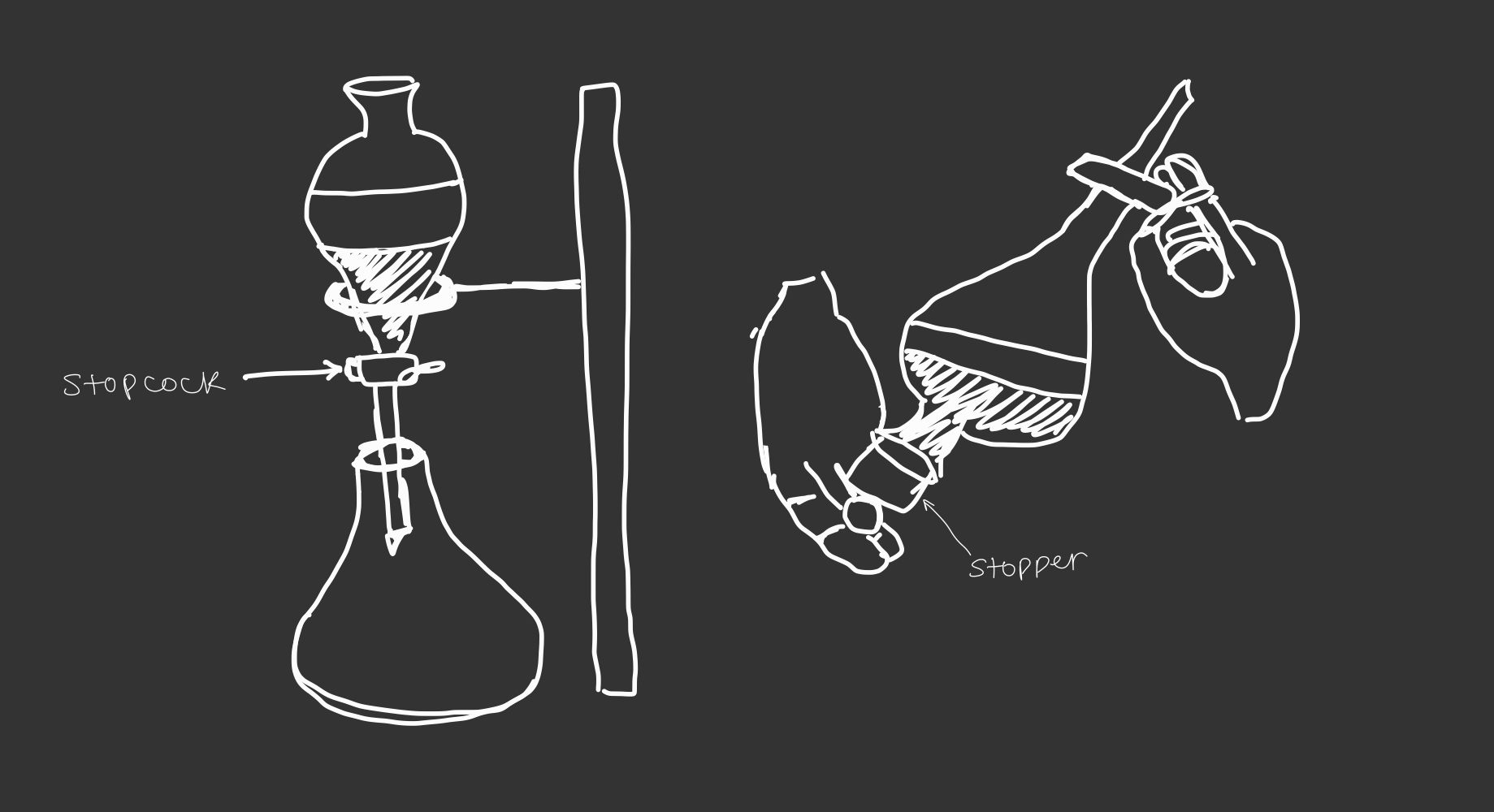
89
New cards
what was the solvent in the liquid/liquid extraction experiment
methylene chloride (organic, nonpolar)
90
New cards
characteristics of an extraction solvent
--> less polar than water
--> immiscible with water
--> more volatile than liquid component of the original solution
--> non-toxic
--> immiscible with water
--> more volatile than liquid component of the original solution
--> non-toxic
91
New cards
what solvent was used to isolate the basic component
2M HCl (acid)
--> 6M NaOH was used to neutralize the positively charged benzocaine
--> 6M NaOH was used to neutralize the positively charged benzocaine
92
New cards
what was used to isolate the acidic component
1M NaOH
--> 6M HCl was used to protonate the neutralized benzoic acid
--> 6M HCl was used to protonate the neutralized benzoic acid
93
New cards
what was used to isolate the organic component
water and brine
94
New cards
what happens to an amine in acidic conditions
protonated to form a water-soluble ammonium salt. the acidic compound remains unaffected
95
New cards
what happens to carboxylic acid in basic conditions
deprotonated to form a water soluble carboxylate salt. the basic component remains unaffected
96
New cards
which layer is on the top/bottom and based on what
--> aqueous on top - acidic and organic on bottom - basic
--> layer separation is based on differing densities
--> layer separation is based on differing densities
97
New cards
what are three components
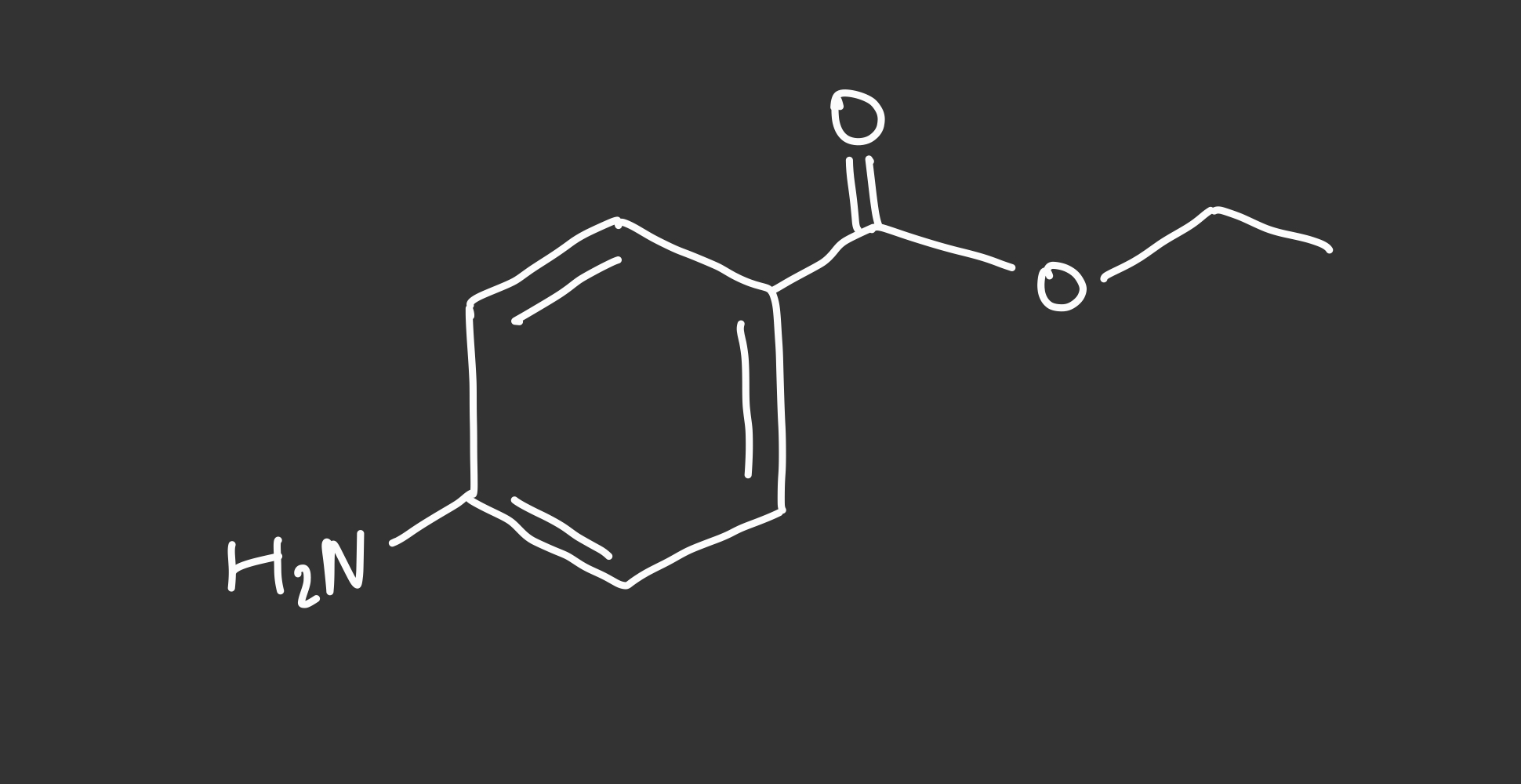
--> benzocaine: basic
--> benzoic acid: acidic
--> diphenylmethanol: neutral
--> benzoic acid: acidic
--> diphenylmethanol: neutral
98
New cards
benzoic acid structure
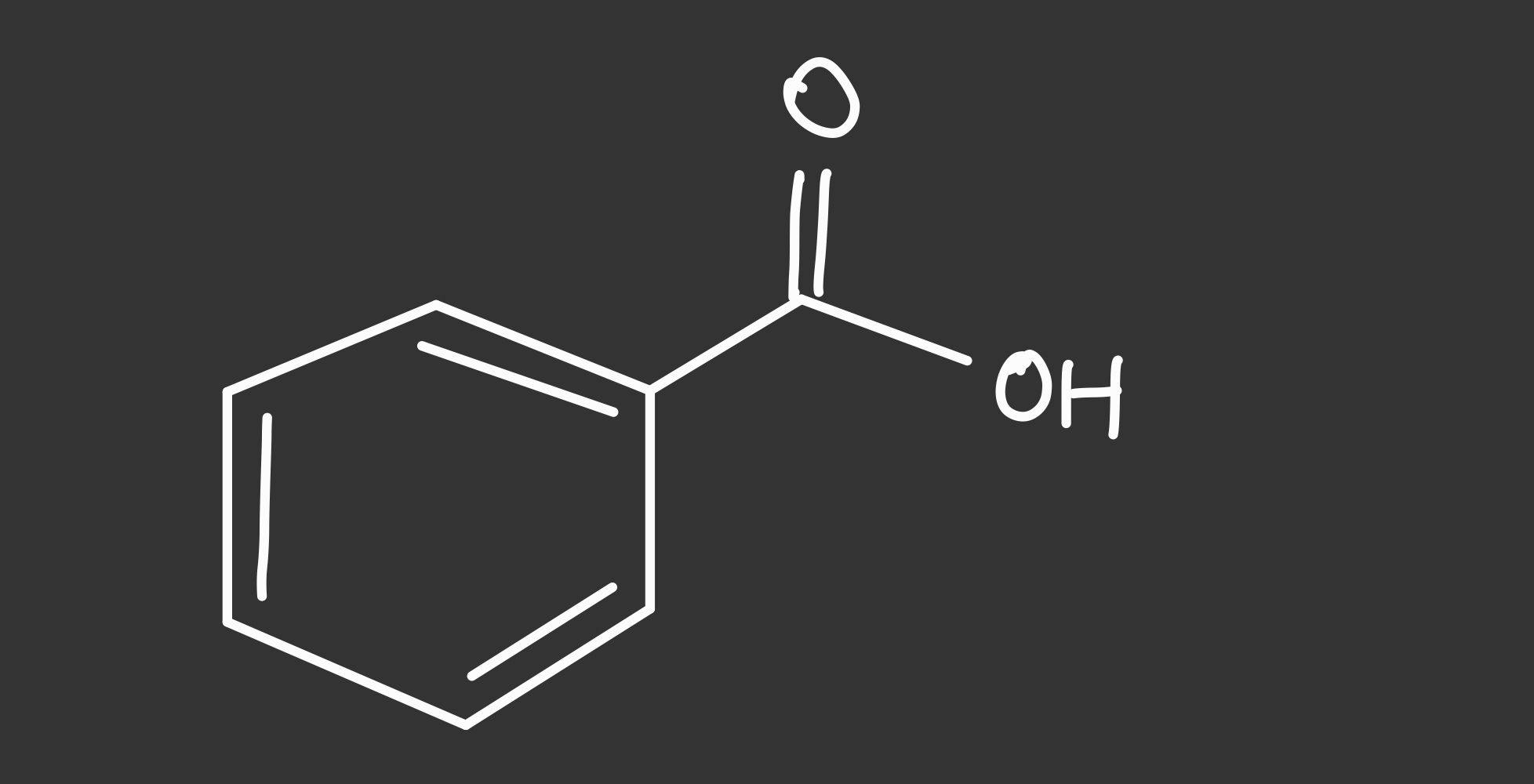
99
New cards
diphenylmethanol structure
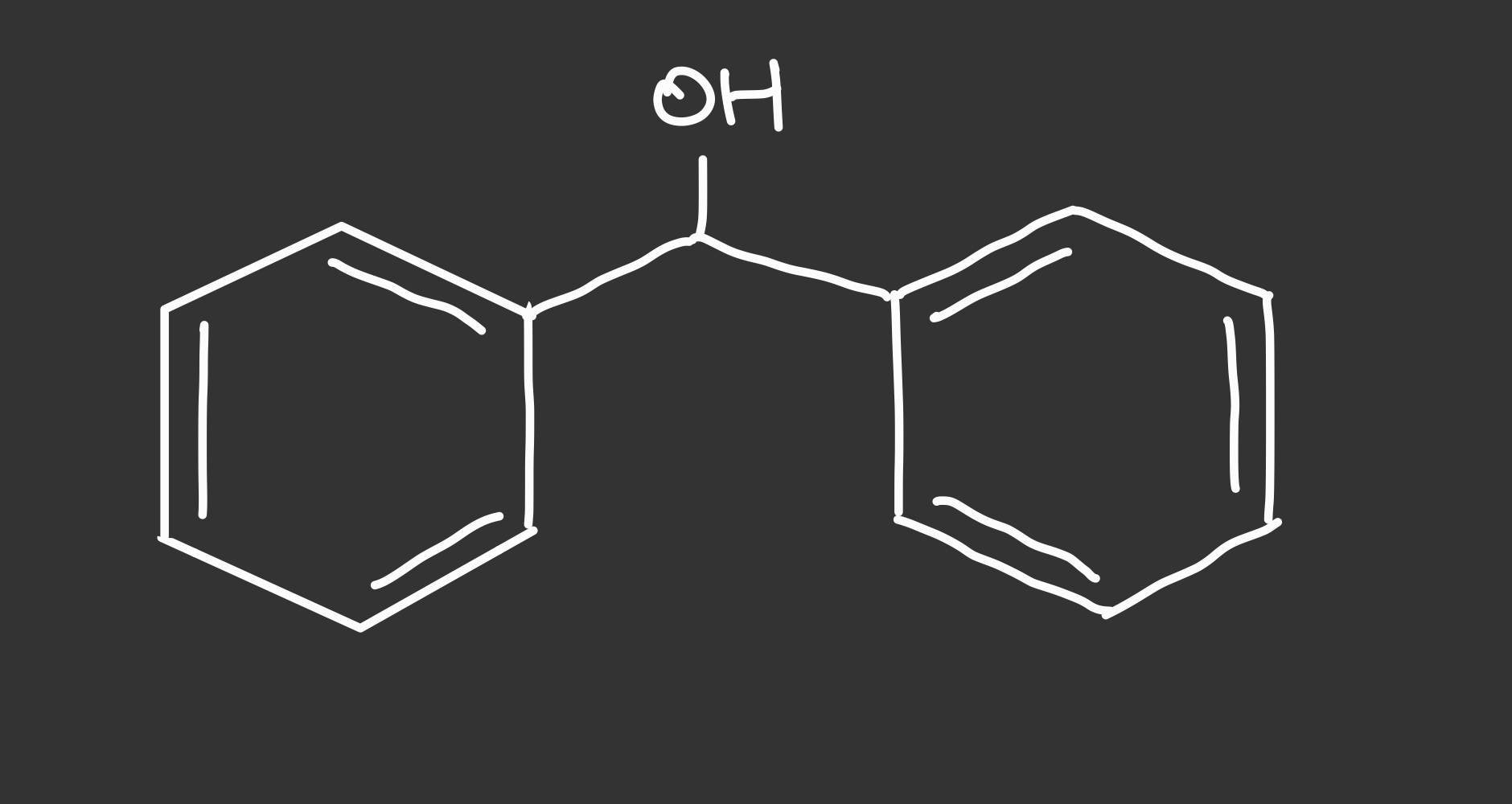
100
New cards
benzocaine structure