Q4: Boyle's Law
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
Boyle’s Law def.
P is indirectly proportional to V at constant T.
Boyle’s Law founder
Robert Boyle
Boyle’s Law Formula?
P1 V1 = P2 V2
Unit for Pressure
atm (atmosphere)
Unit for Volume
L (liter)
Unit for Temperature
K
two properties of gas are compared in Boyle’s Law?
P and V
Which property of gas remains constant in Boyle’s Law?
T
What will happen to the V of a gas if its P is increased at constant T?
decreases
What will happen to the P of a gas if its V is decreased at constant T?
increases
If the P of a gas is decreased by 2x the original, what will happen to its V at constant T?
increased by 2x
If the V of a gas is increased by 4x the original, what will happen to its P at constant T?
decreased by 4x
What kind of proportionality exists between P and V at constant T?
inverse/indirect
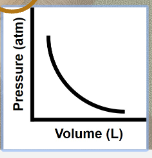
graph that depicts the relationship between P and V at constant T