Rates of reaction, rate constants and assigning units and orders
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
If [A] x 2 and rate=0 change what is the order?
0 order
If [A] x 2 and rate= x 2 what is the order?
1st order
If [A] x 2 and rate =x 4 what is the order?
2nd order
If rate=k [A] ^1 [B]² what is the total order?
1+2=3
What is the equation for rate?
Rate=K[A]^x [B]^y
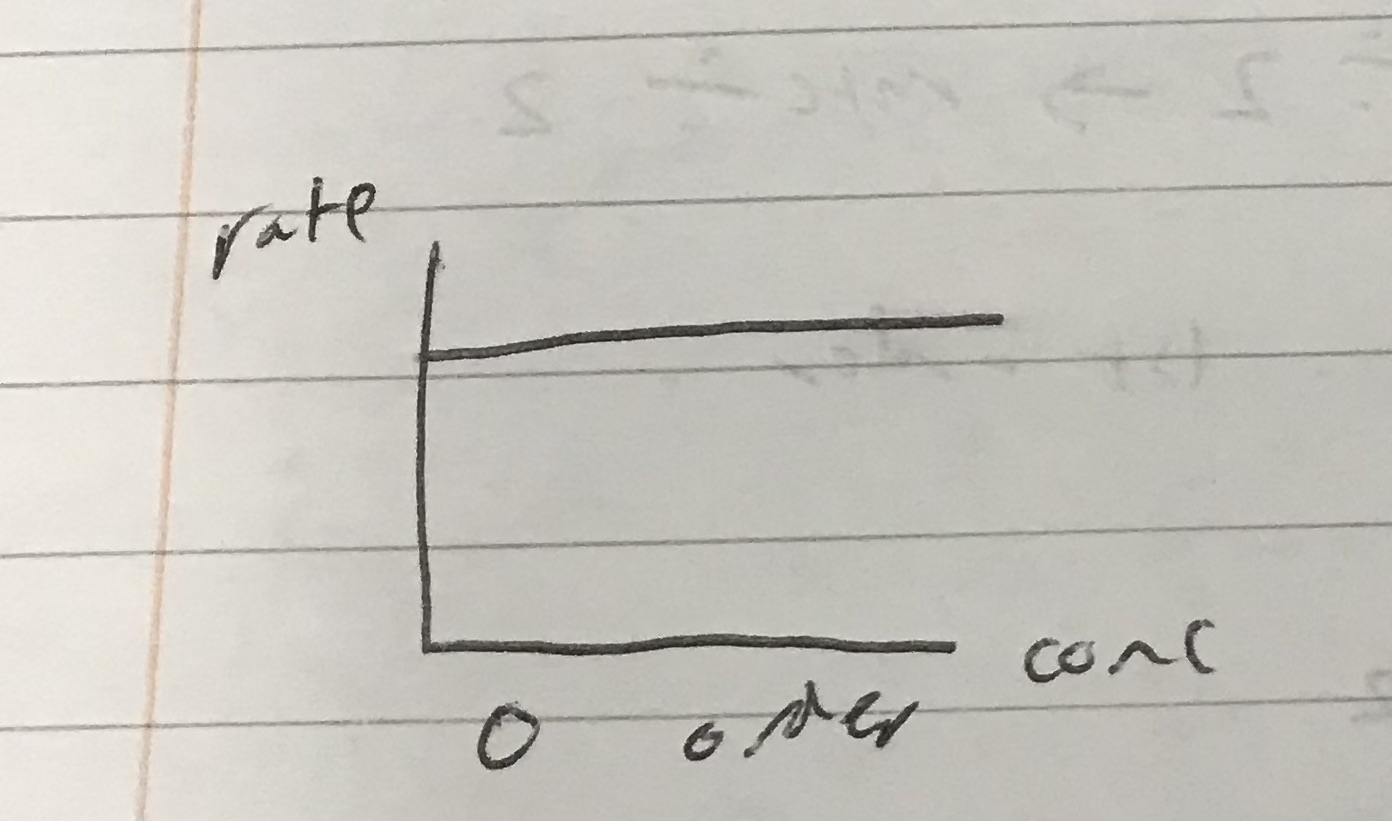
Identify which order this rate graph represents
Because there is no change in rate, so it is a straight line, because rate is constant.
0 order
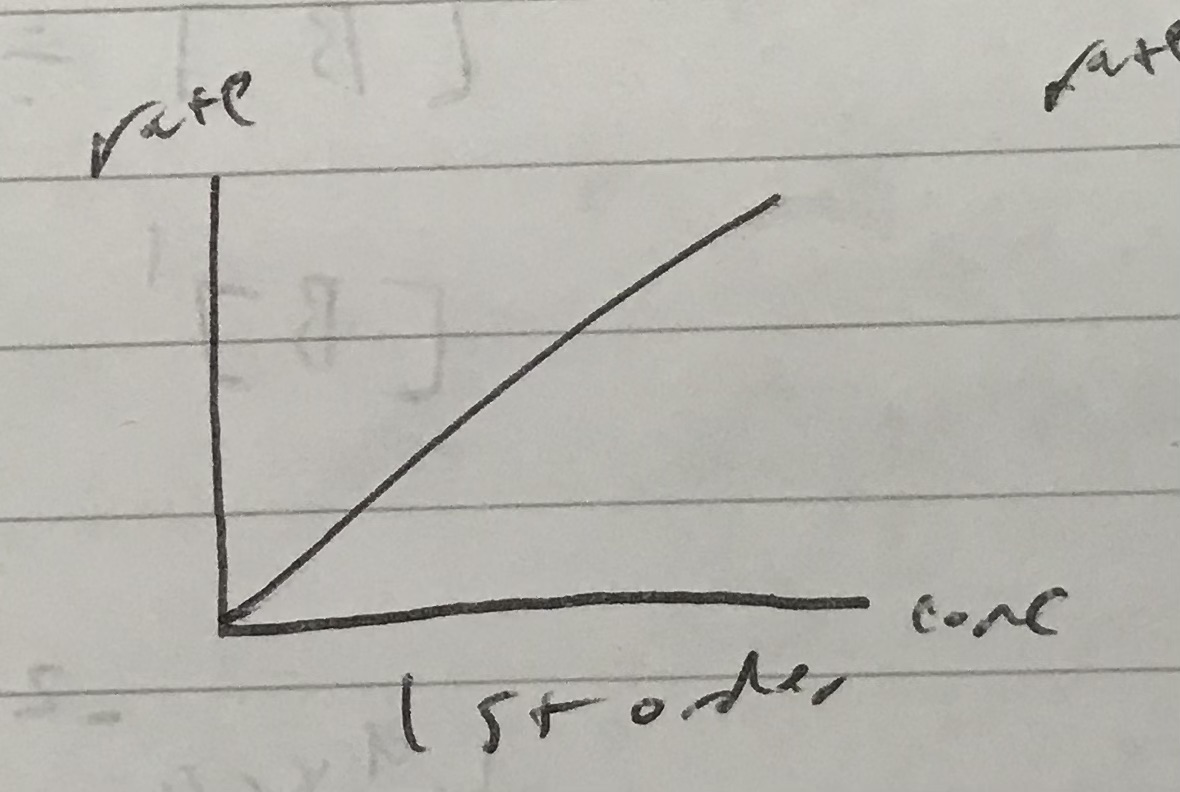
Identify which order this rate graph represents
1st order
Because the rate increases
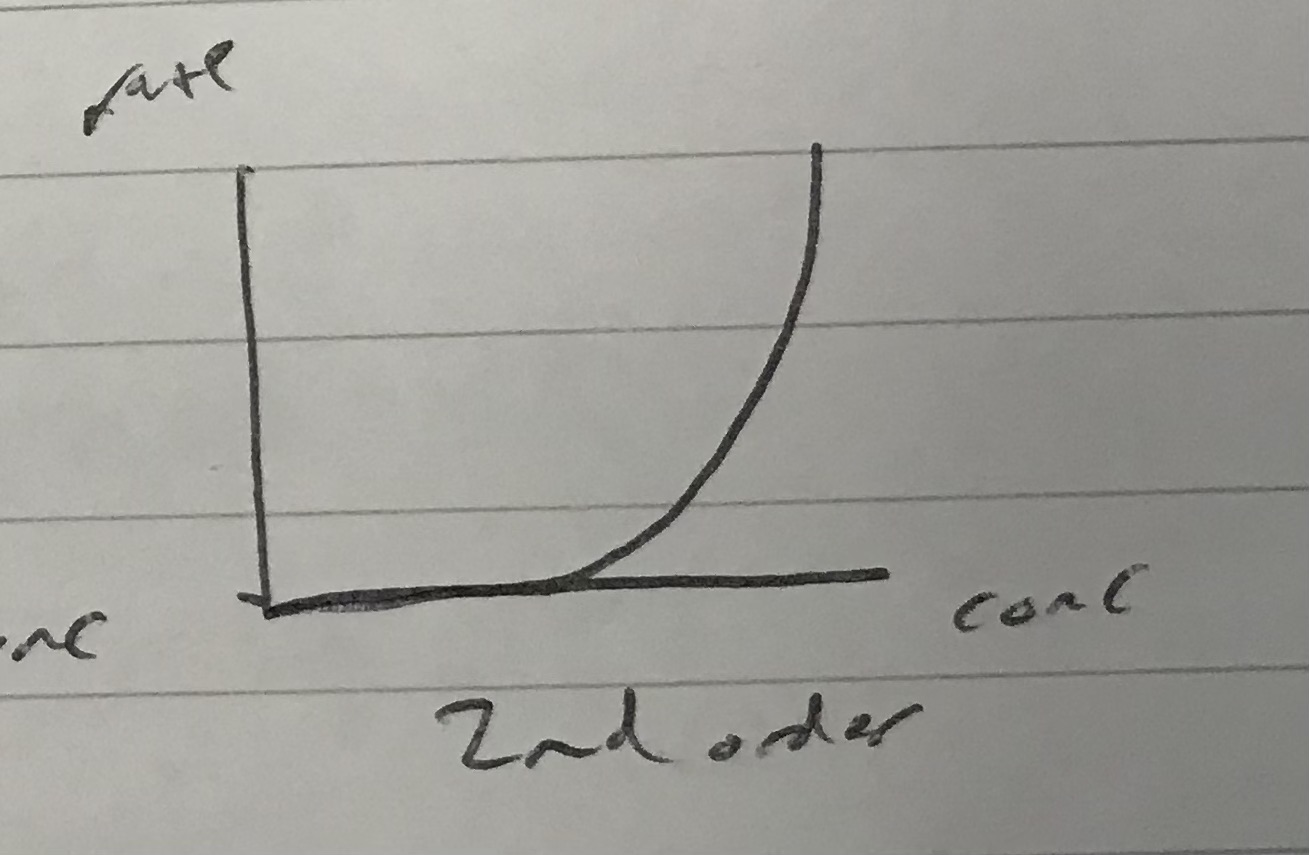
Identify which order this rate graph represents
2nd order
As the rate quadruples
What is the unit it for rate?
Mol dm^-3 s^-1
What is the unit for concentration?
Mol dm^-3
What order is is if [A] /2 and rate/2?
1st order
Because it would be the rate would have doubled if it had doubled
Explain why doubling the temperature has a greater effect than increasing the concentration?
Increase in energy of the molecules causing more molecules to have energy higher of equal to the activation energy.
Rate of successful collisions increases.
Which has a greater effect.
What is the unit for k when k=rate/[A]^1 [B]^1
mol^-1 dm³ s^-1
What is the units for k when k=rate/[A]^1 [B]²
mol^-2 dm^6 s^-1
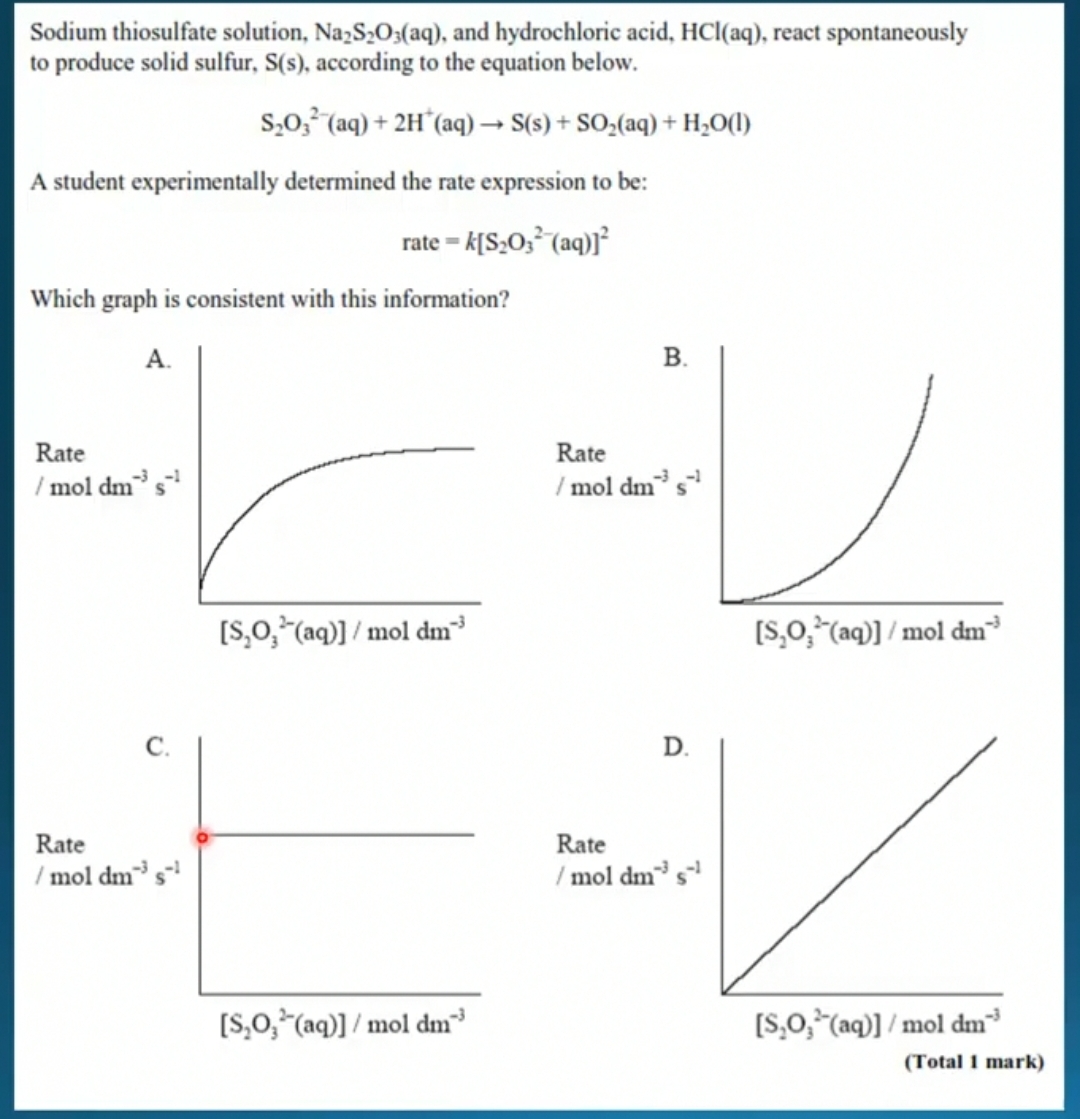
It is B because it is a second order reaction