A1.1 Water (SL)
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
DEFINE: Metabolism
The combination of all the chemical reactions taking place inside (and sometimes outside of*) a living organism.
EXPLAIN: The meaning of water as the “medium for life”
Metabolic processes all need a medium - a physical environment in which the chemistry of life can break down, assemble, and reassemble into the component of life.
That medium is water.
EXPLAIN: Why water is such a good solvent
Because of its (+/-) polar ends, water will interact with anything that also has a charge.
This is why many substances dissolve in water.
Abundant throughout the universe (it’s floating all throughout space right now),
Polarity causes it to dissolve more substances than any other liquid
OUTLINE: The evidence that life originated in water
All known life on Earth requires water to survive
The molecular building blocks of life are found in water
The fossil record shows evidence of ancient aquatic life
ESSENTIAL BUILDING BLOCKS OF LIFE
Amino acids, Nucleic acids, and Sugars.
Each of these are soluble in water.
THE FOSSIL RECORD
Evidence of Ancient Aquatic Life, Aquatic Organisms and Fossilized Stromatolites.
EXPLAIN: 3 thermal properties of water that are useful to living organisms
Water’s buoyancy and viscosity
Water’s high specific heat capacity
Water’s low thermal conductivity
Water’s unique density
BENEFITS OF BUOYANCY
Water's buoyancy allows aquatic organisms to stay afloat and move around more easily, and allows cellular components to do the same.
BENEFITS OF VISCOSITY
Water is a relatively low viscosity fluid. This permits objects - both on a cellular level and on macro level - to easily move through water.
BENEFITS OF HIGH SPECIFIC HEAT CAPACITY
This helps living organisms because it keeps the temperature of water relatively constant, protecting life from rapid, far-reaching temperature fluctuations.
BENEFITS OF LOW THERMAL CONDUCTIVITY
Water's relatively low thermal conductivity is essential for life because it helps to regulate temperature. Water can act as a heat sink (or heat reservoir), helping living organisms maintain a fairly constant internal temperature.
BENEFITS OF DENSITY
Because of hydrogen bonding, water’s solid form (ice) floats - this is actually unusual for most substances in their solid/liquid states.
This makes ice float on top of water - which insulates the water underneath the ice from the outside temperature.
If ice sank in water, there would be none of this insulation. Lakes/rivers would completely freeze up in the winter, killing most of the organisms inside of them.
2 EXAMPLES OF WATER SPECIES ADAPTATIONS
BLACK THROATED LOON
Diving ability: The black-throated loon is an expert diver, with dense bones that help counter the buoyancy of water.
Feathers: Are both waterproof and have air trapped inside them to help increase buoyancy without using energy.
Webbed feet: Provide propulsion and stability to swim with minimal resistance.
RINGED SEAL
Thick layer of blubber: A thick layer of blubber beneath its skin provides insulation and helps to maintain body temperature in cold water.
Streamlined body shape: The ringed seal’s streamlined body shape reduces drag and allows it to move through the water more efficiently.
Webbed feet: Provide propulsion and stability while swimming.
What is the Molecular Formula for Water?
H20
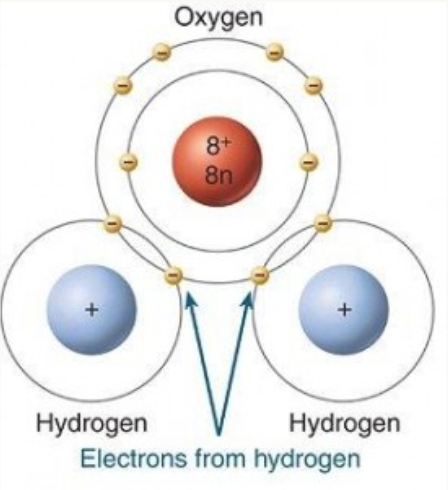
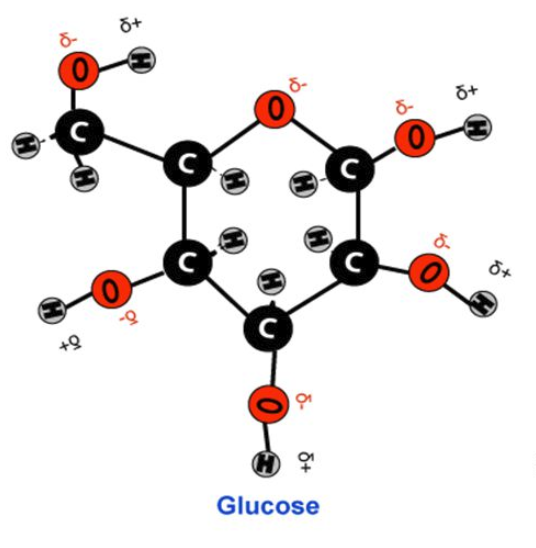
DESCRIBE: The cause and effect of the polar nature of water
In water, the uneven distribution of electrons around oxygen results in:
a negative polar charge around oxygen
a positive polar charge around the 2 hydrogens
The resulting “clouds” of (δ+) and (δ-) charges surrounding water are what make it a polar molecule.
δ is called “delta” in the greek alphabet and is used in chemistry to note a polar charge.
DESCRIBE: Hydrogen Bonds
Hydrogen bonds are a special type of bond that can exist between polar molecules.
Hydrogen bonds can exist between the hydrogen of one polar molecule and the oxygen or nitrogen of a neighboring polar molecule.
CONTRAST: Covalent and Hydrogen Bonds
A hydrogen bond can therefore be defined as a weak bond between two or more polar molecules.
Because water is polar, it forms hydrogen bonds.
Hydrogen bonds are significantly weaker than covalent bonds
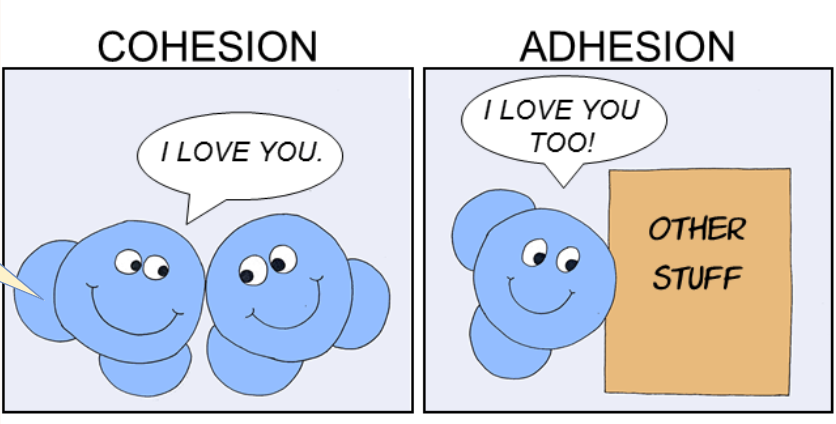
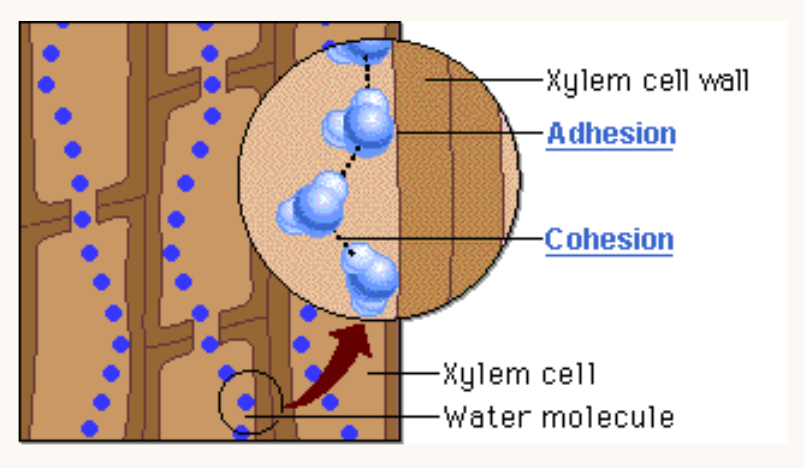
CONTRAST: Adhesion with Cohesion
Cohesion is water molecules sticking to fellow water molecules.
The water is “sticking” to the spider web by adhesion.
Adhesion is water molecules sticking to other stuff (e.g. xylem walls in plants).
The water droplets are in groups of many water molecules bound to each other by cohesion.
What is Xylem?
The xylem is a specialised tube that functions to facilitate the movement of water throughout vascular plants*.
It is for this reason that xylem tubes are narrow and come buncles up in groups called vascular bundles.
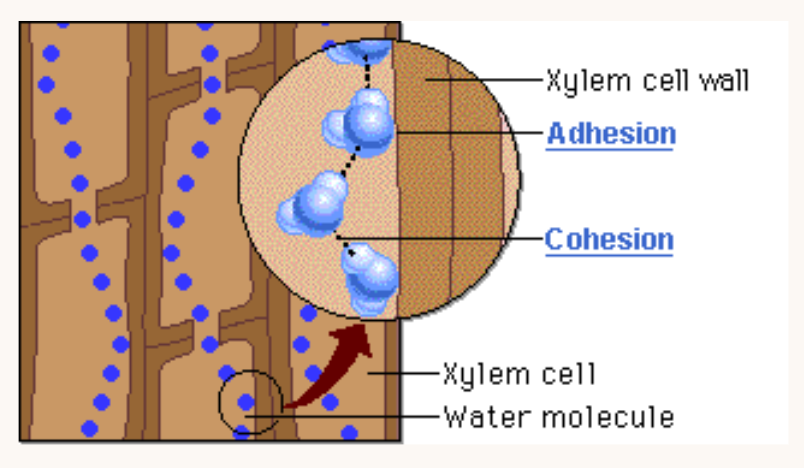
OUTLINE: An example of the cohesive and adhesive properties of water being of benefit to life
The xylem is a specialised tube that functions to facilitate the movement of water throughout vascular plants*.
Both adhesion and cohesion allow water to move up a xylem and against gravity.
The water is pulled through the xylem due to the adhesive attraction between water and the leaf cell walls, as well as the cohesion between neighboring water molecules*.
OUTLINE: An example of the cohesive property of water being of benefit to life
Water cohesion also creates an effect called surface tension, which is a force that holds the molecules of a liquid together at its surface.
Surface tension can be thought of as a “skin” that surrounds a cluster of water, such as droplets of water accumulating on a coin.
Animals such as the water strider (an insect) and the water spider (an arachnid) are able to stay on the surface of water.

DEFINE: Adhesion
Because water is polar, it will adhere to substances that are also polar (or charged, for example ionic substances).
DEFINE: Capillary Action
Capillary action is the ability of a liquid to flow in narrow spaces, such as small tubes called capillaries (e.g. xylem), due to the combined effects of adhesion and cohesion.

OUTLINE: An example of the adhesive property of water being of benefit to life.
tendency to stick to other polar surfaces, is crucial for life, particularly in plant survival; it allows water to cling to the walls of xylem tubes (capillary action), pulling water and dissolved minerals from roots up to the leaves against gravity, enabling photosynthesis and nutrient transport.
OUTLINE: The effects of capillary action in soil
Soil contains a complex network of tiny pores and channels, ranging in size from millimeters to micrometers, which create a capillary network.
Capillary action allows the water to move through the soil pores and channels.
This network allows water to move from wet to dry areas of soil, often against gravity.
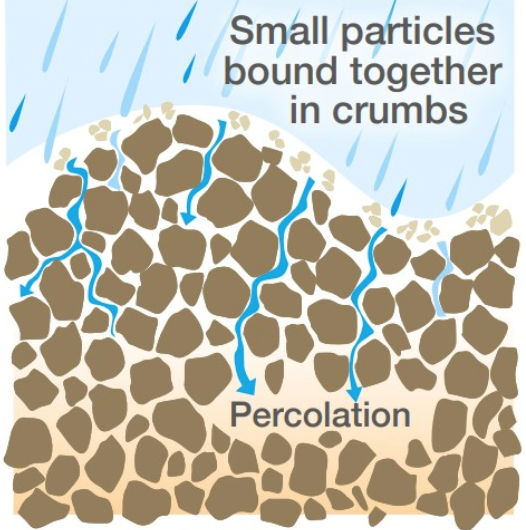
OUTLINE: How capillary action supplies supply plants with mineral nutrients
The water moving throughout soil carries dissolved minerals within it.
Therefore, capillary action also helps to distribute these nutrients to plants to as needed.
Among the most essential are nitrogen (N), phosphorus (P), and potassium (K).
OUTLINE: An example of the cohesive property of water being of benefit to life
Along with cohesion, adhesion is needed for water to move up from the roots of plants to the leaves. Water sticks to the sides of a tube called the xylem through adhesion.
STATE: Why polar and ionic molecules are hydrophilic
Substances that are attracted to water in this manner are called hydrophilic (“water-loving”).
Substances can also be hydrophobic (“water fearing”).
Technically speaking, hydrophobic substances aren’t really “water-fearing”, they just end up grouping together because any surrounding water molecules are attracted to each other.
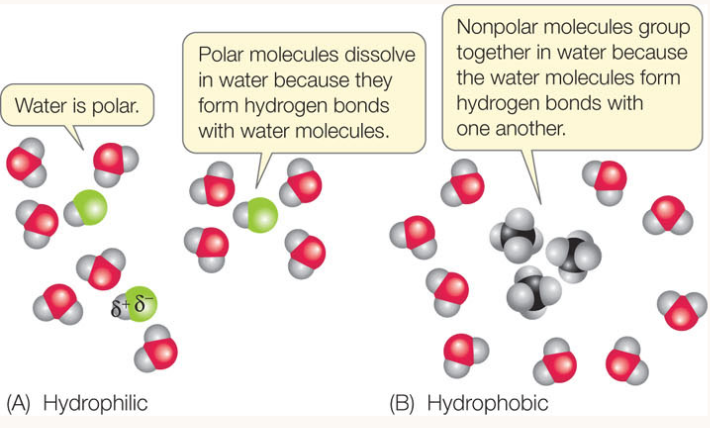
STATE: Why polar and ionic molecules are hydrophilic
Glucose (the basic fuel for almost all life) is is also hydrophilic (water-loving). It has many polar covalent bonds on its many O-H groups.
Hydrogen bonds form between these groups and water. This interaction is the reason glucose can dissolve in water.
OUTLINE: The mechanism of transport in the blood for hydrophilic molecules
Blood is primarily water.
Hydrophilic (“water-loving”), molecules can therefore dissolve and be directly transported in the blood. Examples include:
Glucose
Many amino acids (those with polar/ionic R groups)
Ions (Minerals such as Na+, K+, Cl-, etc)
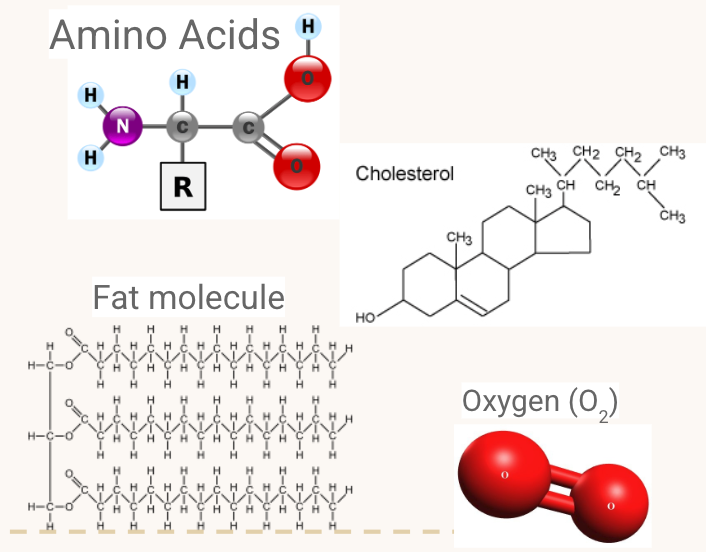
STATE: If the following molecules are hydrophobic or hydrophilic
Hydrophobic (“water-fearing”) substances are also essential to life. Some examples include:
Some amino acids - Again, depends on the side chain (the R group in the diagram)
Cholesterol - Primarily a non-polar hydrocarbon, despite a small O-H end
Fats - lipids, phospholipids, steroids
Oxygen gas (O2) - it’s symmetrical!
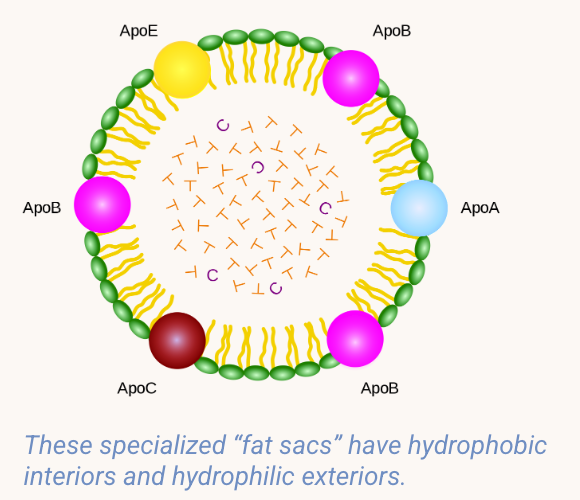
OUTLINE: The mechanism of transport in the blood for hydrophobic molecules
Hydrophobic (“water-fearing”) substances are also essential to life, but because they cannot dissolve in water, they have evolved unique methods of transport.
The following molecules must be transported by special lipo-protein packages:
cholesterol
fats
oxygen (must bind to hemoglobin in red blood cells)
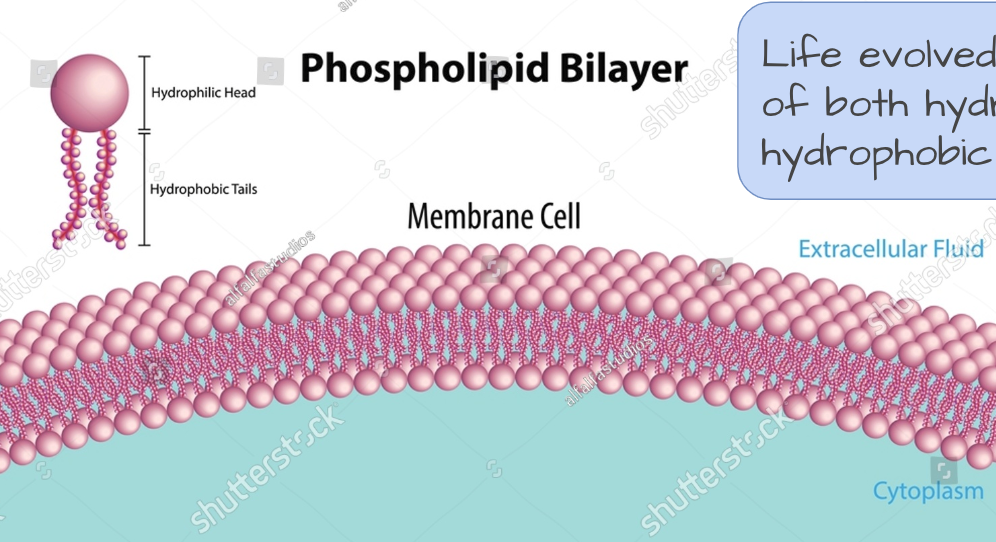
EXPLAIN: Why the functions of some molecules in cells depend on them being hydrophobic and insoluble
Life evolved to make use of both hydrophilic and hydrophobic molecules.
For example, phospholipids (in pink here) are part hydrophobic, part hydrophilic molecules that make up cell membranes. They form a barrier that separates the inside of the cell from the outside environment.
This barrier is critical for maintaining the internal environment of the cell and regulating what molecules can enter or leave.