CHE108 - States of Matter and Analytical Chemistry
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
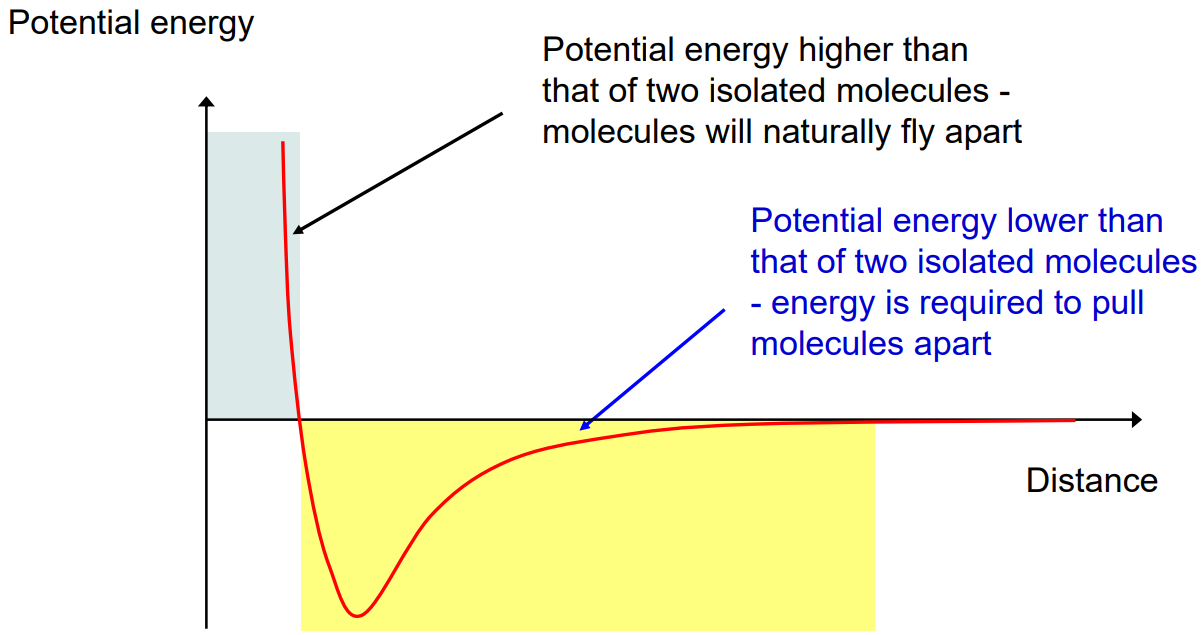
In this potential energy curve, what does the minimum point represent?
The most stable separation between two molecules, where attractive and repulsive forces balance.
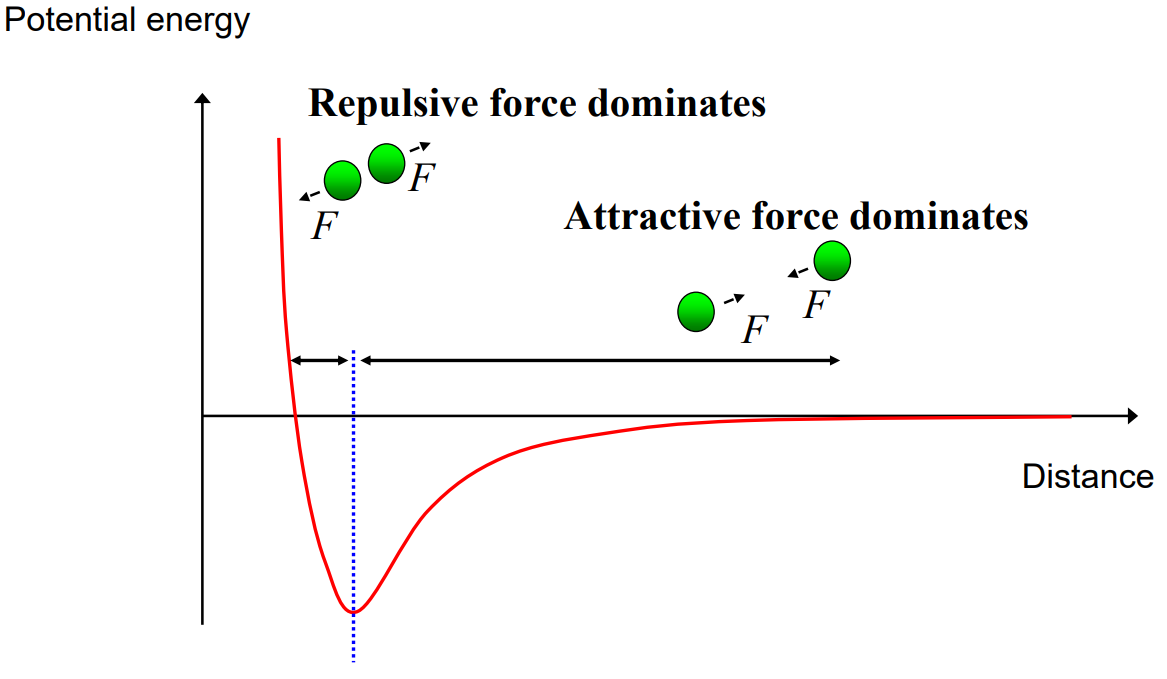
According to this diagram, when does repulsion dominate and when does attraction dominate?
Very short distance: Repulsion dominates (electron cloud overlap)
Longer distance: Attraction dominates (intermolecular forces pulling molecules together)
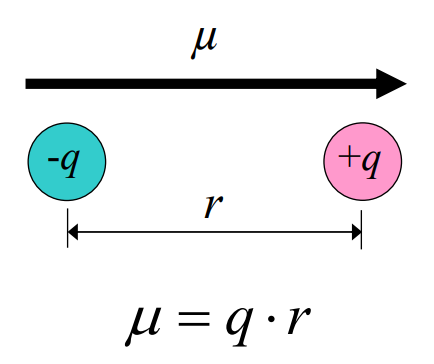
In the dipole diagram, what does the arrow for μ represent?
The dipole moment vector, pointing from negative charge (−q) to positive charge (+q), with magnitude μ = q × r.
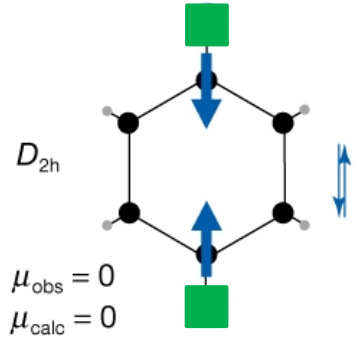
Why does the molecule labeled D₂h have μ = 0 in this diagram?
Because the bond dipoles cancel due to symmetry, giving no net dipole moment.
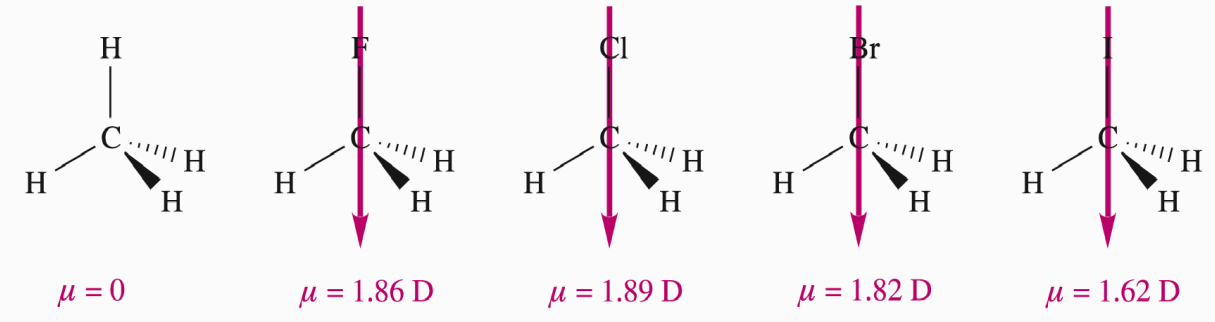
The diagram shows C–F, C–Cl, C–Br, C–I dipoles. Why doesn’t dipole moment strictly follow electronegativity?
Because bond length also affects μ — dipole moment depends on both charge difference and distance between charges.
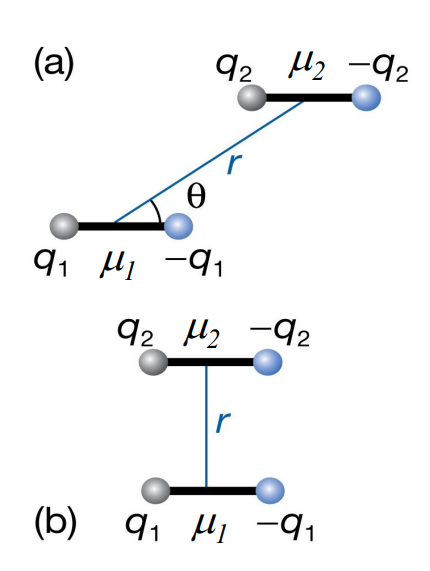
In this dipole–dipole diagram, what determines whether the interaction is attractive or repulsive?
The relative orientation (angle θ) and distance r between dipoles.
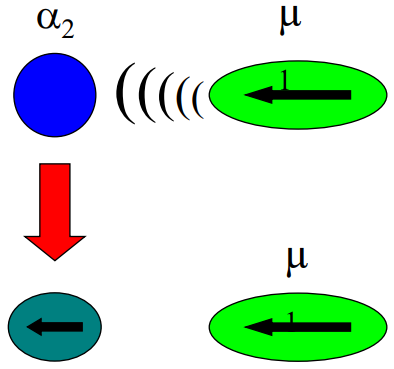
What process is shown when the blue nonpolar molecule distorts near a green polar molecule?
Dipole - Induced Dipole
A permanent dipole induces a temporary dipole in a nonpolar molecule. This attraction depends on polarizability (α).
How do dispersion forces arise?
Temporary electron fluctuations create a dipole in molecule A, which induces a dipole in molecule B, leading to attraction.
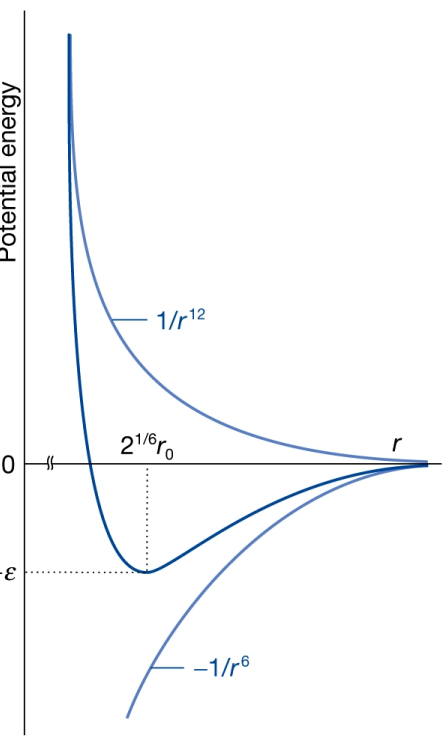
In this Lennard-Jones graph, what do the two curves represent?
−1/r⁶: Attractive van der Waals forces
+1/r¹²: Strong short-range repulsion
The minimum shows the equilibrium intermolecular distance.
Hydrogen bonding typically occurs in molecules containing which bonds?
N–H, O–H, or F–H bonds, where hydrogen is attached to a highly electronegative atom.
What are the three main types of van der Waals attractive interactions?
Dipole–dipole
Dipole-induced dipole
Dispersion
What type of van der Waals attractive force is strongest in small molecules?

How are the strength van der Waals attractive forces different in large molecules?
The “contact area” increases and dispersion forces become
very significant.