Equilibrium
0.0(0)
0.0(0)
Card Sorting
1/143
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
144 Terms
1
New cards
When is equilibrium reached?
When the concentration (or \[\]) of your reactants (R) and products (P) cease changing with time; when your RATES ARE EQUAL, NOT WHEN R amt. = P amt.
2
New cards
What type of system does equilibrium exclusively exist in?
A closed system (a system that can only exchange energy with the surroundings, not matter)
3
New cards
Does equilibrium end on its own?
No, as the system is always dynamic despite a perceived stop
4
New cards
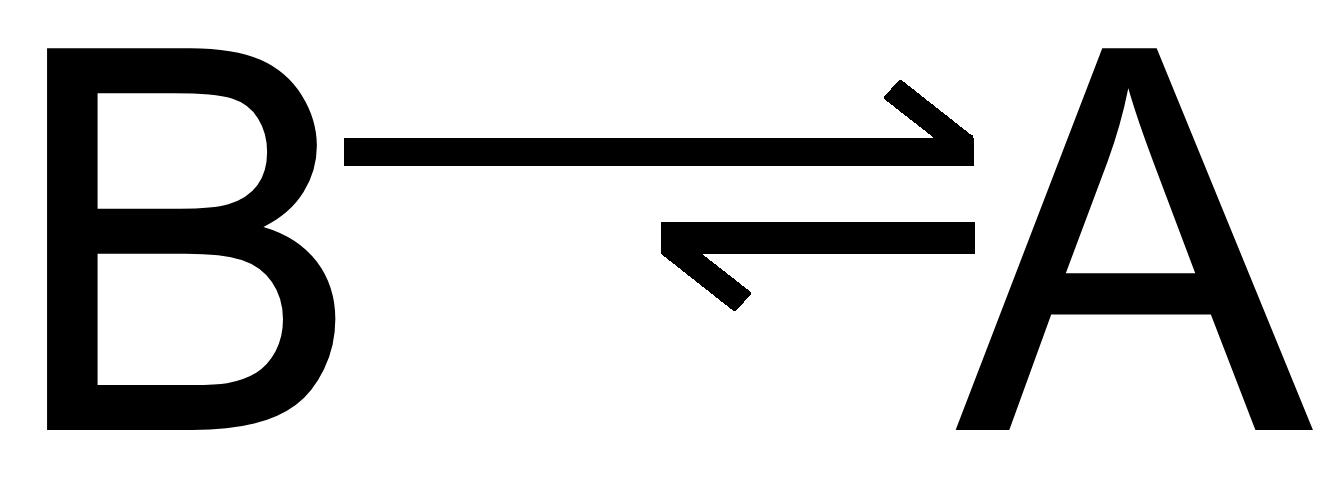
With the reaction A⇌B, can you start with either product? What happens when you start with B and decompose it into A? How does this change the rates?
1) You can start with either the R or P
2) If you begin with B, you’ll create more A disproportionate to the creation of B because there hasn’t been established enough A to start synthesizing into B, thus making the creation of A much faster than the creation of B (and vice versa if you began with A)
2) If you begin with B, you’ll create more A disproportionate to the creation of B because there hasn’t been established enough A to start synthesizing into B, thus making the creation of A much faster than the creation of B (and vice versa if you began with A)
5
New cards
What is the Law of Mass Action?
This expresses the relationship between the AMOUNTS of R and P in any reaction (RXN)
6
New cards
What is used to express the Law of Mass Action?
An equilibrium-constant expression (ECE) (should remain the same given a reaction, changing with temperature)
7
New cards
For the equilibrium system aA+bB⇌cC+dD, the Law of Mass Action says that an equilibrium-constant expression (K) is given by (define the ECE/K equation):
K=(((amt. C)^c)\*((amt. D)^d)))/(((amt. A)^a)\*((amt. B)^b)))
OR SIMPLY
K=P/R
OR SIMPLY
K=P/R
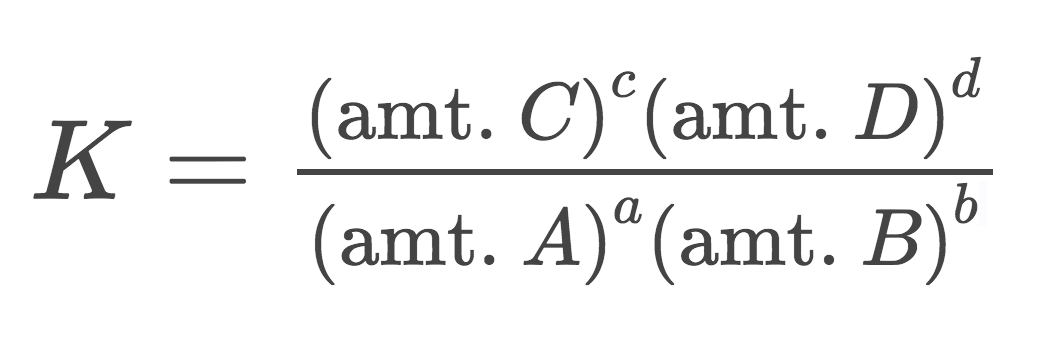
8
New cards
When the amts. are given in terms of \[\], the Kc equation is thus:
K(sub c(for concentration))=(((\[C\])^c)\*((\[D\])^d)))/(((\[A\])^a)\*((\[B\])^b)))
![K(sub c(for concentration))=(((\[C\])^c)\*((\[D\])^d)))/(((\[A\])^a)\*((\[B\])^b)))](https://knowt-user-attachments.s3.amazonaws.com/f1ec884d05ef4f5cb886c3b1b78399cf.jpeg)
9
New cards
When the amts. are given in terms of pressure (ONLY in a gaseous system), the Kp equation is thus:
K(sub p(for PARTIAL pressure of each compound))=(((P)^c)\*(((P))^d)))/(((P)^a)\*((P)^b)))
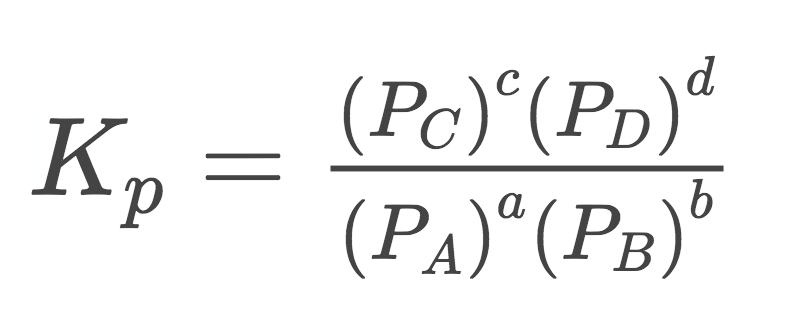
10
New cards
The Kp equation for a gaseous system, what is the Kp called? And what unit must the partial pressure be in?
1) The pressure-equilibrium constant
2) atmospheres
2) atmospheres
11
New cards
What equation represents the relationship between Kc and Kp? How do you find the Δn from the equation? What does R stand for? What does T stand for?
1) Kp=Kc((RT)^Δn)
2) Δn=ΣP(being the Σ of the coefficients)-ΣR(being the Σ of the coefficients)
3) The R stands for the universal gas constant: 0.08206 Latm/molK
4) The T stands for absolute temperature (k)
2) Δn=ΣP(being the Σ of the coefficients)-ΣR(being the Σ of the coefficients)
3) The R stands for the universal gas constant: 0.08206 Latm/molK
4) The T stands for absolute temperature (k)
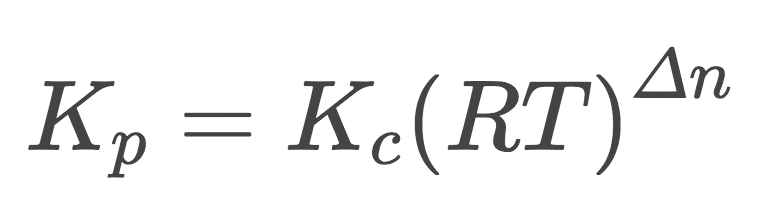
12
New cards
What does K depend on? The reactions stoichiometry or its mechanisms?
The mechanisms don’t matter, it depends on its stoichiometry
13
New cards
What is K independent of?
The initial \[\], partial pressure, or amount as its an EQUILIBRIUM-constant expression, thus depending on its final state at equilibrium
14
New cards
What varies K?
Temperature changes
15
New cards
If you add a new substance to an equation at equilibrium, what happens?
As long as the substance added doesn’t react with the R or P, it doesn’t change K
16
New cards
What are the units of K?
K uses no units
17
New cards
What do you NEVER include in an ECE equation?
You NEVER include pure liquids or solids, simply do not include them into the equation since they cannot be at equilibrium
18
New cards
If not given states, what do you assume of the environment?
You’re at STP (standard temperature and pressure)
19
New cards
If the magnitude of K>1, what does this imply about the relationship between R and P?
Products are favored, thus the equation is lopsided toward the right and must create more R to be at equilibrium
(when looking at the ECE equation, we see that having more P than R would mean it would need to be over 1)
(when looking at the ECE equation, we see that having more P than R would mean it would need to be over 1)
20
New cards
If the magnitude of K
Reactants are favored, thus the equation is lopsided toward the left and must create more P to be at equilibrium
(when looking at the ECE equation, we see that having more R than P would mean it would need to be under 1)
(when looking at the ECE equation, we see that having more R than P would mean it would need to be under 1)
21
New cards
When you going backward in a reaction (starting with the P, or B), are the two K equations the same?
No, as you must flip your R and P values since they’re reciprocals of each other
22
New cards
When reporting on K, what should you also specify?
The original equation and the temperature
23
New cards
If you only know the \[\] of only some substances at equilibrium, how do you solve for Kc? If you have a ratio of 2:1 for an equation, what must you do to the concentration for the substance in 2 quantities? What is this idea based on?
1) Make an ICE (initial, change, equilibrium) chart are use reaction stoichiometry to find the other \[\]s at equilibrium using algebra
2) Since this is stoichiometrically based, you must multiply the concentration by 2
2) This is based off the idea that depending on your
2) Since this is stoichiometrically based, you must multiply the concentration by 2
2) This is based off the idea that depending on your
24
New cards
By knowing/solving for your K value, you can:
* Predict the direction of a reaction (refer back to the magnitude of K)
* Calculate the amounts of R and P once equilibrium has been reached if we know the initial amounts of at least one substance and have the volume (use total volume when solving at equilibrium if the problem given accounts for additive volume changes)
* Calculate the amounts of R and P once equilibrium has been reached if we know the initial amounts of at least one substance and have the volume (use total volume when solving at equilibrium if the problem given accounts for additive volume changes)
25
New cards
Define the Reaction Quotient (Q):
What you get when you plug the R and P amts. at any given time into the ECE (the state of K at any given time between the initial concentrations and the equilibrium point (NOT THE EQUIVALENCE POINT))
26
New cards
What is the equation for Q? Can it change?
It’s simply Q(sub c)=(((\[C\])^c)\*((\[D\])^d)))/(((\[A\])^a)\*((\[B\])^b))), which can change depending on if you have a gaseous system or are going off of amts.
\*Simply put, its just Q=P/R at any time
\*Simply put, its just Q=P/R at any time
![It’s simply Q(sub c)=(((\[C\])^c)\*((\[D\])^d)))/(((\[A\])^a)\*((\[B\])^b))), which can change depending on if you have a gaseous system or are going off of amts.
\*Simply put, its just Q=P/R at any time](https://knowt-user-attachments.s3.amazonaws.com/9944ea6a546d4c4782755f0209f1b314.jpeg)
27
New cards
If Q>K, what does this mean for the equilibrium of the reaction? Is this the same case for Q
1) The system isn’t at equilibrium, which goes for Q
2) When Q=K, your system is at equilibrium
2) When Q=K, your system is at equilibrium
28
New cards
When you have Q>K, what do you need more of to reach equilibrium? In Q
1) You need more reactants
2) You need more products
2) You need more products
29
New cards
Define Le Chateliers Principle:
Systems at equilibrium disturbed by changes in temperature, pressure, or \[\] of a component will shift its equilibrium position to counteract the disturbances effect
30
New cards
![Describe the change to this system and what type it is (out of temp, pressure, and \[\]). What happens when we remove NH3? Why does N2 go down? When is H2 added, and when does it begin to reach equilibrium again?](https://knowt-user-attachments.s3.amazonaws.com/80663dfc428348b1a071276b72188c3c.jpeg)
Describe the change to this system and what type it is (out of temp, pressure, and \[\]). What happens when we remove NH3? Why does N2 go down? When is H2 added, and when does it begin to reach equilibrium again?
1) In this diagram, we added excess H2, disturbing the ratio of concentrations, forcing the reaction to shift RIGHT to a new equilibrium (making more P than R)
2) If NH3 is removed, the equation will still shift RIGHT to accommodate for the lack of NH3, as then we would still have a disproportionate amount of R over P
3) N2 goes down to accommodate for a new ratio of more NH3 being produced (almost like N2+4H2⇌3NH3)
4) H2 is added at the spike, and equilibrium has begun to establish as the elements levels plateau after the spike
2) If NH3 is removed, the equation will still shift RIGHT to accommodate for the lack of NH3, as then we would still have a disproportionate amount of R over P
3) N2 goes down to accommodate for a new ratio of more NH3 being produced (almost like N2+4H2⇌3NH3)
4) H2 is added at the spike, and equilibrium has begun to establish as the elements levels plateau after the spike
31
New cards
Increasing pressure make the system want to become what? How does this affect its shift?
It makes the system want to get smaller/compressed, thus this makes the equation shift to whatever side is larger (either given by volume on both the R and P side, or the amount of molecules on each side (which ever having the least molecules being larger and more voluminous, the one with the larger volume being larger and more accommodating to more molecules))
32
New cards
Decreasing pressure make the system want to become what? How does this affect its shift?
It makes the system want to get larger/expand, thus this makes the equation shift to whatever side is smaller (either given by volume on both the R and P side, or the amount of molecules on each side (which ever having the most molecules being smaller and less voluminous, the one with the smaller volume being smaller and more accommodating))
33
New cards
What does a change in pressures resulting shift depend on?
The number of molecules, where if you have equal number of molecules on either side, there should be no shift
34
New cards
If the partial pressures of the equation are affected, not just the total pressure, does this cause the equation to shift?
Yes
35
New cards
How do spectators affect the shift?
If you add a compound that doesn’t affect the already existing compounds and exists as a spectator, it will NOT create a shift
36
New cards
What must be changed to affect K by volume and pressure (P-V) changes?
Temperature
37
New cards
In exothermic reactions (∆H=-x):
1. As temperature rises:
2. As temperature decreases:
\
1. As temperature rises:
2. As temperature decreases:
\
1. Your equation shifts left and K decreases (think of heat as a product with exothermic reactions and when you have more P, you create more R, thus making a larger denominator and thus lowering K)
2. Your equation shifts right and K increases
38
New cards
In endothermic reactions (∆H=+x):
1. As temperature rises:
2. As temperature decreases:
1. As temperature rises:
2. As temperature decreases:
1. Your equation shifts right and K increases
2. Your equation shifts left and K decreases
39
New cards
When you add a catalyst, how does it affect the forward and reverse reactions and its shift? How to catalysts change a RXN?
1) The forward and reverse reaction rates increase PROPORTIONALLY, thus there is NO SHIFT
2) Catalysts really only change the reaction rate, but the final composition and K are unchanged
2) Catalysts really only change the reaction rate, but the final composition and K are unchanged
40
New cards
How does the Arrhenius theory on acids and bases define acids and bases as? What is it limited to?
1) Acids increase \[H+\] (protons) and bases increase \[OH-\] WHEN AQUEOUS IN WATER
2) They’re limited to aqueous solutions
2) They’re limited to aqueous solutions
41
New cards
How does the Bronsted-Lowry theory on acids and bases define acids and bases as? What compounds are typically used to represent the transfer of H+? What is it limited to?
1) Acids can transfer H+ to other substances (proton DONORS) and bases can accept H+ from other substances (proton acceptors)
2) H+ and H3O+ (H3O+ just being when H2O accepts an H+ ion)
3) It’s NOT limited to aqueous solutions
2) H+ and H3O+ (H3O+ just being when H2O accepts an H+ ion)
3) It’s NOT limited to aqueous solutions
42
New cards
Are Arrhenius and Bronsted-Lowry acids and bases exclusive?
No, they overlap somewhat, where an Arrhenius base can be a Bronsted-Lowry base as well
43
New cards
Can a BL acid use anything other than a BL base to donate its H+?
No, a BL acid can only donate its H+ to a BL acid
44
New cards
What must a base have to accept H+?
It must have a lone/nonbonding electron pair that can bind to the accepted H+
45
New cards
Define an amphoteric substance and give an example:
1) A substance that can act as either a base or acid depending on what its being reacted with and the RXN conditions
2) H2O, which if added to an acid can act as a weak base, and vice versa with its addition to a base where it acts as a weak acid
2) H2O, which if added to an acid can act as a weak base, and vice versa with its addition to a base where it acts as a weak acid
46
New cards
In acid-base equilibria, protons are donated in forward and reverse reactions to create on the P side:
Conjugate bases and acids
47
New cards
How do the two substances in a conjugate acid-base pair differ? Give an example compound and its conjugate acid or base:
1) They differ by the base losing a H+ (if its the first dissociation/ionization), and the acid has an extra H+
2) HNO2 (acid) turning into NO2- (conjugate base)
H2O (Amphoteric base) turning into H3O+ (conjugate acid)
2) HNO2 (acid) turning into NO2- (conjugate base)
H2O (Amphoteric base) turning into H3O+ (conjugate acid)
48
New cards
Do strong acids/bases struggle to donate/accept H+?
No, they have weak IMFs thus donate/accept H+ easily
49
New cards
Do weak acids/bases struggle to donate/accept H+?
Yes, as they have strong IMFs and are tightly bound to the H+, which is what creates this partial dissociation/ionization and makes equilibrium
50
New cards
Reacting strong acids/bases give you:
weak conjugate bases/acids (and vice versa if you began with a weak acid/base, it would create strong conjugate bases/acids)
51
New cards
When two water molecules react, what is this called, and what does it create?
1) The auto-ionization of water
2) H2O(l)+H2O(l)⇌H3O+(aq)+OH-(aq)
2) H2O(l)+H2O(l)⇌H3O+(aq)+OH-(aq)
52
New cards
Given the equation of the auto-ionization of water, what does this make its ECE/Kw(for water) equation? At what temperature does this occur? Why does it give both H3O+ and H+ as options?
1) Kw=\[((H3O)^+)\]\[((OH)^-)\]=\[((H)^+)\]\[((OH)^-)\]=(1.0\*10)^(-14)
2) 25°C
3) both H3O+ and H+ are interchangeable, so you can use either side
2) 25°C
3) both H3O+ and H+ are interchangeable, so you can use either side
![1) Kw=\[((H3O)^+)\]\[((OH)^-)\]=\[((H)^+)\]\[((OH)^-)\]=(1.0\*10)^(-14)
2) 25°C
3) both H3O+ and H+ are interchangeable, so you can use either side](https://knowt-user-attachments.s3.amazonaws.com/67b06d0930614105b60826e96a9d8a89.jpeg)
53
New cards
The Kw equation is valid in what situations? What must they be at?
Either with pure water and for dilute aqueous solutions that must be at 25°C
54
New cards
Considering the concentrations of \[H+\] and \[OH-\], when do you have an acid? A base? A neutral system?
1) You have an acid when \[H+\]>\[OH-\]
2) You have a base when \[H+\]
2) You have a base when \[H+\]
55
New cards
When you add a strong acid and a strong base, what does this result in?
A strong acid+strong base yields water+salt
56
New cards
How do you calculate for pH?
pH=-log(H3O+/H+)
57
New cards
As \[H+/H3O+\] increases, what happens to pH? What is the pH scale on?
1) pH decreases
2) The pH scale goes from 0-14, 0 being completely acidic and 14 being completely basic, 7 being neutral with equal concentrations of \[H+\] and \[OH-\]
2) The pH scale goes from 0-14, 0 being completely acidic and 14 being completely basic, 7 being neutral with equal concentrations of \[H+\] and \[OH-\]
58
New cards
Changing the pH by 1 unit requires you to change your \[H+\] or \[OH-\] by what factor?
By 10 (essentially, changing 1 pH level would mean a difference of 1, thus 10^1 is 10x more than the original concentration and thus 10x more basic if increasing)
59
New cards
When it comes to biochemical RXNS and their kinetics, the rate law is dependent not on the slowest reaction, but on what?
Its usually dependent on the \[H+\], meaning if you doubled the \[H+\] you would double the rate (if its a 1st order rate)
60
New cards
What is the sig fig rule for logs?
The number of sig figs in \[\] = the number of decimal places in the pH
61
New cards
If given \[OH-\] and you need to find pH, what are the steps to find pH?
1. Find your \[H+\] with the Kw equation
2. Plug the new \[H+\] into the pH log equation
62
New cards
What are the other equations to find pOH (the inverse of pH), \[H+\], \[OH-\], and pH and pOH added?
pOH=-log\[OH-\]
\[OH-\]=10^(-pOH)
\[H+\]=10^(-pH)
pH+pOH=14
\[OH-\]=10^(-pOH)
\[H+\]=10^(-pH)
pH+pOH=14
63
New cards
How does the pH relate to the \[H+\]?
They should be similar in number (not decimal place)
64
New cards
What are the strong bases?
HCl, HBr, HI, HClO3, HClO4, HNO3, H2SO4
65
New cards
What are the strong bases?
Anything with Li, Na, K, Rb, Cs, Ca, Sr, Ba, being most alkali and alkali earth metals, which are strong base cations
66
New cards
How do strong electrolytes behave in aqueous solutions? Can they reach equilibrium? How is this denoted?
They exist entirely as dissociated ions
2) Due to the fact they exist as dissociate ions, they can’t be at equilibrium
3) Since they can’t reach equilibrium, they’re always drawn with a one-sided arrow
2) Due to the fact they exist as dissociate ions, they can’t be at equilibrium
3) Since they can’t reach equilibrium, they’re always drawn with a one-sided arrow
67
New cards
What do oxides contain? Hydrides? Nitrides?
1) Oxides contain O^(2-)
2) Hydrides contain H^-
3) Nitrides contain N^(3-)
2) Hydrides contain H^-
3) Nitrides contain N^(3-)
68
New cards
When you add metal oxides, hydrides, or nitrides to water, what do they react to produce?
They react to produce strong basic solutions
69
New cards
Most acids are what type? What does this mean in terms of its ionization and IMFs?
1) Most acids are weak acids
2) This essentially means they only PARTIALLY IONIZE, making equilibrium, and are difficult to separate, meaning their IMFs are strong
2) This essentially means they only PARTIALLY IONIZE, making equilibrium, and are difficult to separate, meaning their IMFs are strong
70
New cards
How are weak acid decompositions typically written as?
HX(aq)⇌H^(+)(aq)+X^(-)(aq)
71
New cards
What is the equation for the acid-dissociation constant?
K(sub a(for acid))=(\[H^(+)\]\*\[X^(+)\])/\[HX\]
![K(sub a(for acid))=(\[H^(+)\]\*\[X^(+)\])/\[HX\]](https://knowt-user-attachments.s3.amazonaws.com/bab880e1089445fd9aca4309fadad505.jpeg)
72
New cards
A large Ka indicates what?
It indicates a “strong” weak acid, meaning the acid dissociates easier (while still not fully), thus theres more P and more in the numerator, making the Ka>1
73
New cards
A small Ka indicates what?
It indicates a “weak” weak acid, meaning the acid is much harder to dissociate, thus meaning theres more R and more in the denominator, making Ka
74
New cards
What is the % ionization equation?
% ionization=((\[H^(+)\] at equilibrium)/(\[acid\] originally))\*100
![% ionization=((\[H^(+)\] at equilibrium)/(\[acid\] originally))\*100](https://knowt-user-attachments.s3.amazonaws.com/aaac28b7480643b6806be14fe22423d0.jpeg)
75
New cards
Where does the donated H attach to in organic acids?
They attach to the O, NOT the H (as organic acids are only made of C, H, and O)
76
New cards
% ionization increases as…. (and why does this occur?)
1) your acid \[\] decreases (and vice versa)
2) The more your acid ionizes, the more P you produce, thus taking away from your original acids \[\]
2) The more your acid ionizes, the more P you produce, thus taking away from your original acids \[\]
77
New cards
SOMETIMES, the less concentrated a substance is….
the more “active” it might be, as it results in more P
78
New cards
According to the last 2 notes, the LESS concentrated the weak acid….
The greater the % ionization will be
79
New cards
Define polyprotic acids and give an example:
1) They have more than one ionizable H+
2) Sulfurous acid, H2SO3
2) Sulfurous acid, H2SO3
80
New cards
Ka decreases as….
H+ is removed
81
New cards
Why does the Ka decrease because of this removal?
It is both harder to remove additional H+ because your acid in its second ionization holds onto its H+ more and thus produce less P in proportion to its \[\], causing a larger denominator and thus a Ka much smaller, and also since once this H+ is removed theres less repulsion, shielding, competing electronegativities with more attraction, thus increasing the acids hold onto the leftover H+
82
New cards
Why can you typically ignore Ka2 when calculating for Ka?
Ka2 is at least 1000x smaller than Ka, thus being relatively negligible
83
New cards
What is the equation for the base-dissociation constant? What is this used for?
1) K(sub b(for base))=(\[conj. acid\]\*\[OH^(-)\])/\[weak base\]
2) To find the Kb for WEAK bases
2) To find the Kb for WEAK bases
84
New cards
How are weak bases typically written?
weak base(aq)+H2O(l)⇌conj. acid (aq)+OH^(-)(aq)
85
New cards
What are weak bases often composed with?
Nitrogen, these nitrogen-containing called amines
86
New cards
Whats the similarity between Ka and Kb with Kw?
When you have acids or a conj. base, Kw and (Ka)•(Kb) both equal 1.0•10^(-14)
87
New cards
This similarity between KaKb with Kw would mean what for pH? Whats its new equation?
1) You can replace the “H” in pH with pKa or pKb and solve as you would for pH or pOH
2) pKa=-logKa or pKb=-logKb and pKa+pKb=14 (at 25ºC)
2) pKa=-logKa or pKb=-logKb and pKa+pKb=14 (at 25ºC)
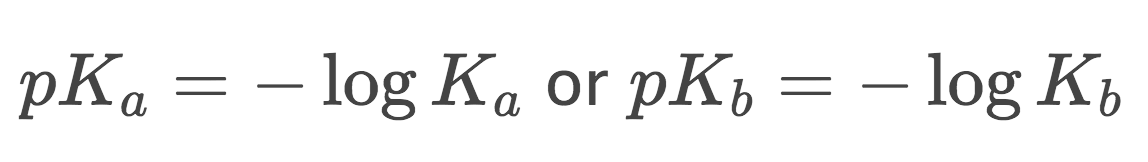
88
New cards
Why can salt solutions be acidic or basic?
Salt solutions can exhibit acidic or basic properties, acting as either proton donors or acceptors
89
New cards
Define why hydrolysis occurs and give its common equation:
1) Hydrolysis occurs when dissociated salt ions split the H2O in solution into H+ and OH-, or more specifically when the ACETATE ion (derived from SODIUM ACETATE) reacts with H2O to produce ACETIC ACID and the HYDROXIDE ION
2) OAc^(-)+H2O⇌HOAc+OH^(-)
2) OAc^(-)+H2O⇌HOAc+OH^(-)
90
New cards
How are conjugate bases basic? Why?
1) The anion of a weak acid (or its conjugate base) reacts with H2O as a base to form OH- and go back to its acidic state, thus making a basic solution (or the anion, X- acts as the proton ACCEPTOR thus acting as a base)
2) Since the acid is weak, it has strong enough IMFs to attract the H+ from the H2O back and become an acid again
2) Since the acid is weak, it has strong enough IMFs to attract the H+ from the H2O back and become an acid again
91
New cards
Do anions of STRONG acids affect the pH?
No, since the IMFs aren’t strong enough to attract the H+ back
92
New cards
With amphoteric substances, how do you determine pH?
You must compare the Ka and Kb to see which is larger and thus if its more acidic or basic
93
New cards
When an amphoteric substance has a larger Ka>Kb….
The pH is more acidic and thus the P is favored
94
New cards
When an amphoteric substance has a larger Kb>Ka….
The pH is more basic and thus the R is favored
95
New cards
If given a multistep equation for an amphoteric substance to solve for its pH, how do you approach the problem?
Take one step of the problem, reverse it to be either its basic or acidic equation by conjugatizing its Ka and compare it to an adjacent step to find which is larger out of the Ka or Kb
96
New cards
Most cations do what to their H+?
They usually free them
97
New cards
All cations (except those in strong bases) act as what?
They all act as weak acids in aqueous solutions
98
New cards
What is the ph when you combine a strong acid and a strong base?
It should have a neutral pH of 7 (in a neutralization reaction)
99
New cards
What is the ph when you combine a weak acid and a strong base?
You have more OH-, thus Kb>Ka and it is more basic, making the pH>7
100
New cards
What is the ph when you combine a strong acid and a weak base?
You have more H+, thus Kb