the Maxwell Boltzmann distribution 5.2
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
Last updated 5:57 PM on 11/29/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
1
New cards
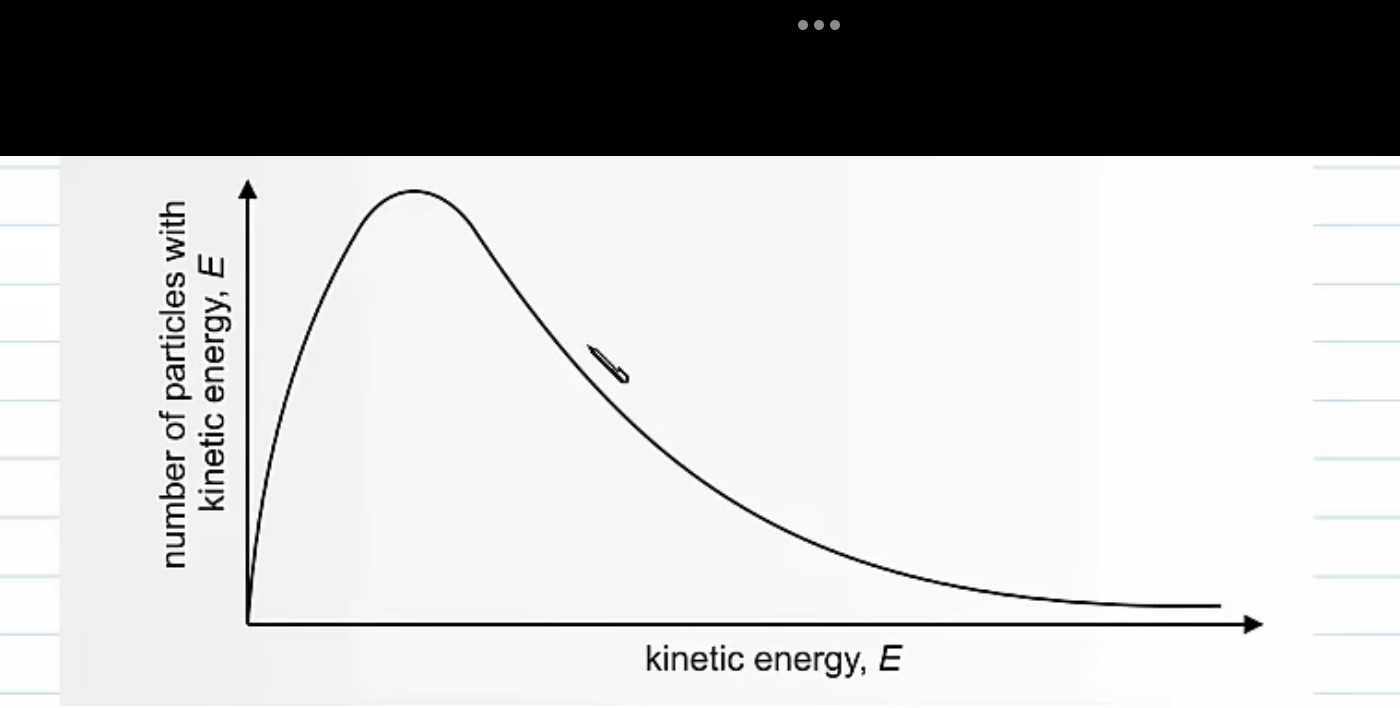
What does the maxwell Boltzmann distribution tell us?
it tells us about the distribution of energy amongst the particles
-no particles have 0 energy
-most particles have intermediate energies-around the peak of the curve
-a few have very high energies
-Average energy is not the same as the most probable energy.
-no particles have 0 energy
-most particles have intermediate energies-around the peak of the curve
-a few have very high energies
-Average energy is not the same as the most probable energy.
2
New cards
What are the features of the curve ?
The sample under the curve is equal to the total number of molecules in a sample.
The curve starts at the origin =there are no molecules in the system with zero energy .
Only molecules with and energy greater than the activation energy EA can react
The curve starts at the origin =there are no molecules in the system with zero energy .
Only molecules with and energy greater than the activation energy EA can react
3
New cards
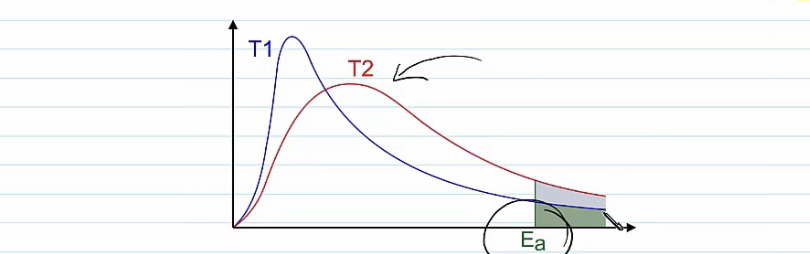
Botlzman curve ,temperature
At higher temperature the kinetic energy of the molecules increases
The peak moves
To the right
Lower peak
The area under the curve remains the same time
The number of Molecules in the system remain the same .
area under curve beyond activation energy increases
The peak moves
To the right
Lower peak
The area under the curve remains the same time
The number of Molecules in the system remain the same .
area under curve beyond activation energy increases
4
New cards
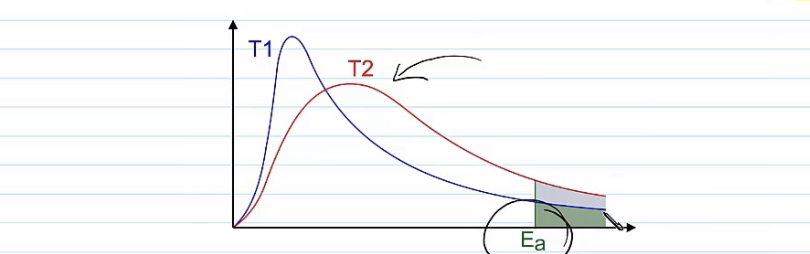
What happens ?
The molecules are moving faster as they have more kinetic energy .
A greater proportion of molecules have an energy that is greater than the activation energy
More successful collisions occur in a given length of time
The rate of reaction will increase
A greater proportion of molecules have an energy that is greater than the activation energy
More successful collisions occur in a given length of time
The rate of reaction will increase
5
New cards
What happens when we decrease temperature ?
Peak moves to the left
peak is higher
area under curve remains same
area under curve beyond activation energy decreases
a smaller proportion of the molecules will have energy greater than the activation energy
peak is higher
area under curve remains same
area under curve beyond activation energy decreases
a smaller proportion of the molecules will have energy greater than the activation energy