Chap 2: Biological molecules
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
benedict’s test for reducing sugars
add benedict’s reagent (contains copper (II) sulfate ions)
heat the sample using water bath at 80ºC
Result: blue → green → yellow → orange → red (→ increasing conc. of reducing sugar)
iodine test for starch
present = blue-black
not present = orange/brown/no change
emulsion test for lipids
add ethanol → shake
present = milky emulsion, solution cloudy
not present = no change/ clear
biuret test for lipids
add biuret reagent (CuSO4 + hydroxide)
present = lilac/purple
not present = no colour change/blue
acid hydrolysis & Benedict’s test for non-reducing sugars
hydrolyse with HCl and heat
neutralise with alkali (NaOH)
Result: blue → green → yellow → orange → red (→ increasing conc. of non-reducing sugar)
monomer
one of many small molecules that combine together to form a polymer
polymer
a giant molecule made from many similar repeating subunits joined together in a chain
macromolecule
a large molecule formed by condensation reactions between smaller molecules
→ Polymers are a type of macromolecule , but not all macromolecules are formed from repeating units to be polymer
monosaccharide
a molecule consisting of a singular sugar unit with the general formula (CH2O)n
Main types: trioses(3C), pentoses(5C-ribose, deoxyribose), hexoses (6C-glucose, fructose, galactose)
disaccharide
a sugar molecule consisting of two monosaccharides joined together by a glycosidic bond
polysaccharide
a polymer whose sub-units are monosaccharides joined together by glycosidic bonds
isomer
organic molecules that have the same molecular formula but different structures which result in different properties
glycosidic bond
a H-O-H link between two sugar molecules ; formed by condensation reaction ; it is a covalent bond
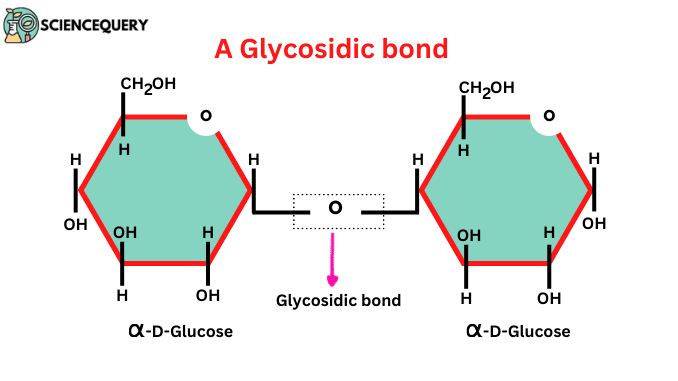
maltose
1,4 linked alpha-glucose + alpha-glucose
sucrose
reducing sugar
1,2 linked alpha-glucose + beta-fructose → sucrose + H2O
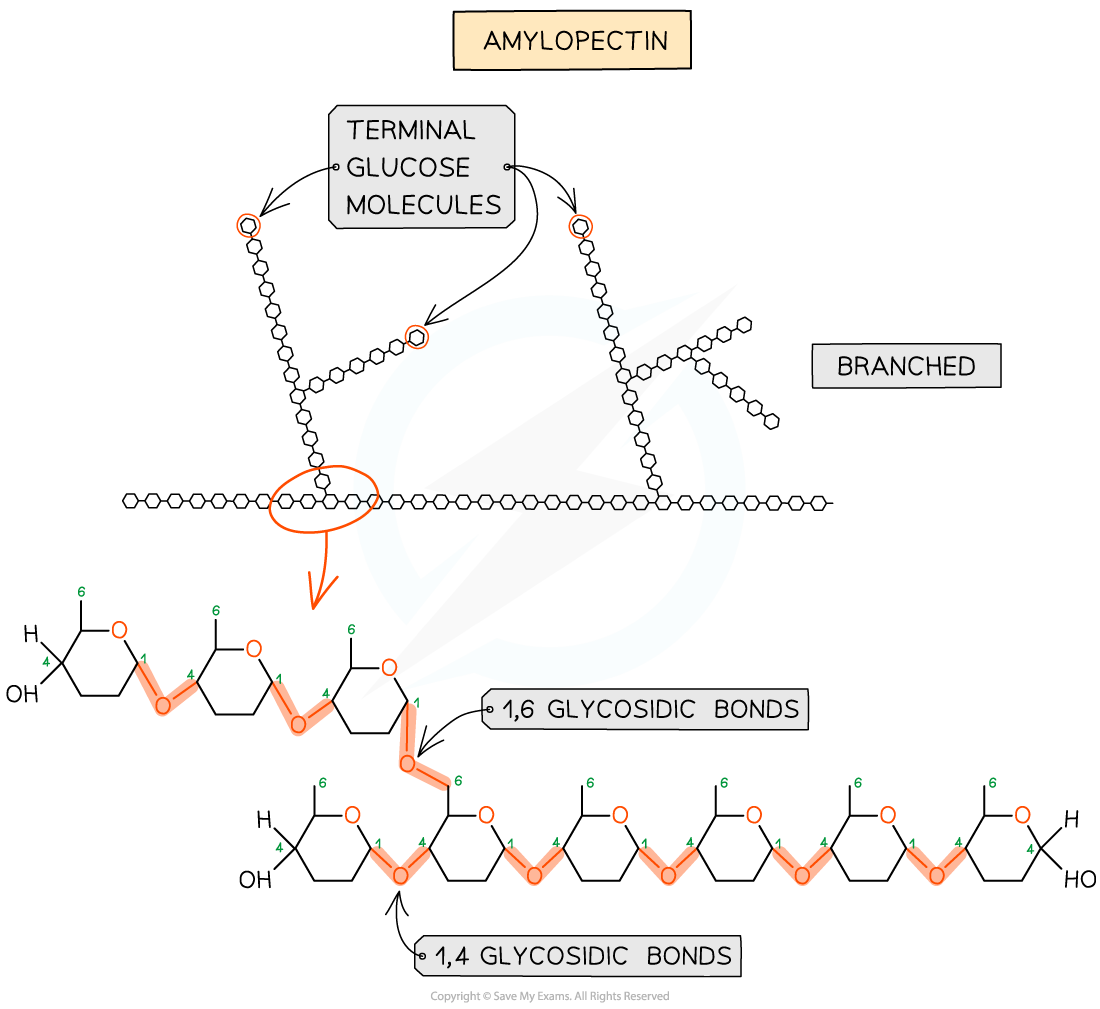
amylopectin
made of 1,4 glycosidic bonds linked α-glucose
branches are formed by 1-6 linkages
the branches result in many terminal glucose molecules that can be easily hydrolysed for use during cellular respiration or added to storage
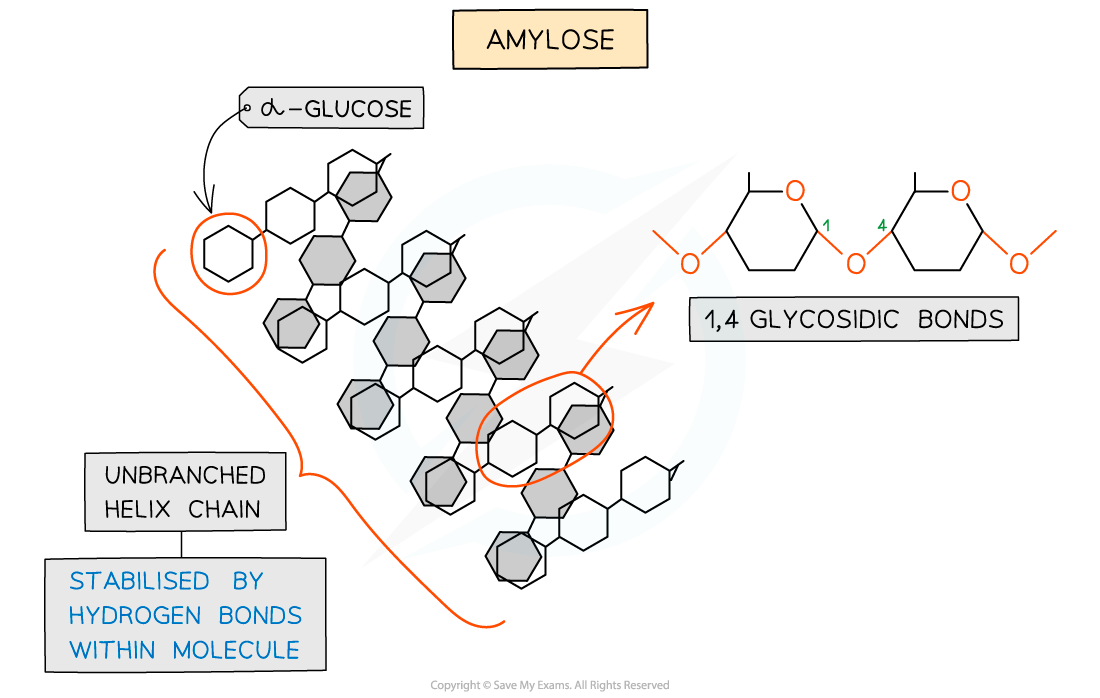
amylose
Unbranched helix-shaped chain with 1,4 glycosidic bonds between α-glucose molecules
The helix shape enables it to be more compact and thus it is more resistant to digestion
Hydrogen bonds within molecules stabilise the unbranched helix shape
starch
made up of amylose and amylopectin
glycogen
storage polysaccharide of animals & fungi
made of chains of 1,4 linked α-glucose with 1,6 linkages forming branches
→ similar structure to amylopectin but more branched
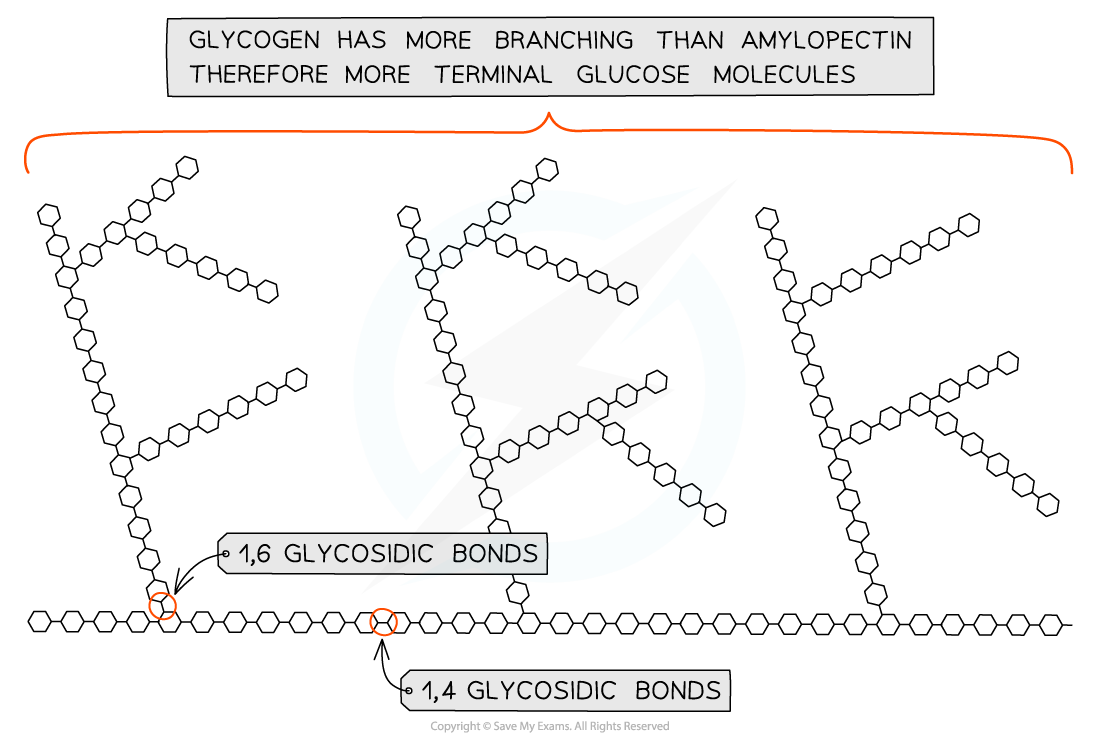
How molecular structure of glycogen makes it suitable for storage
highly branched and not coiled
more branching than amylopectin → more compact → animals store more
more branched = more free ends where glucose molecules can be added or removed, allowing condensation and hydrolysis reactions to occur more rapidly
storage or release of glucose can suit the demands of the cell
functions of amylose, amylopectin and glycogen
Compact :many molecules fit into small space, so large volumes can be stored
Insoluble : don't dissolve in the cell cytoplasm → no osmotic effect
cellulose
a polysaccharide made from B-glucose subunits ; used as a strengthening
material in plant cell walls
structure of cellulose
unbranched
long chains of beta-glucose joined by 1,4 glycosidic bonds
to form 1,4 glycosidic bonds, the beta-glucose will be inverted
due to the inversion, many hydrogen bonds are formed → more strength

functions of cellulose
Cellulose gives strength to plant cell walls through many hydrogen bonds in parallel microfibrils, allowing walls to withstand turgor pressure.
Cell walls are supportive and permeable, as the cellulose–lignin matrix provides structural support while still allowing water and solutes to pass through.
Cellulose is indigestible to most organisms, due to the lack of cellulase, making it an important source of dietary fibre.
cellulose fibres
hydrogen bonds result in a strong molecule
cellulose molecules become tightly cross linked to form bundles called microfibrils
microfibrils are held together in bundles called fibres by hydrogen bonding
cellulose fibres have very high tensile strength - makes it possible for a cell to withstand high turgopressures
polar molecules
1) have groups with dipoles
2) they're attracted to H2O molecules as they also have dipoles therefore are hydrophilic
3) soluble in water
Eg- amino acids, glucose, NaCl
non-polar molecules
1) do not have dipoles
2) not attracted to water and hydrophobic
3) insoluble in water
Eg- oils, cholesterol
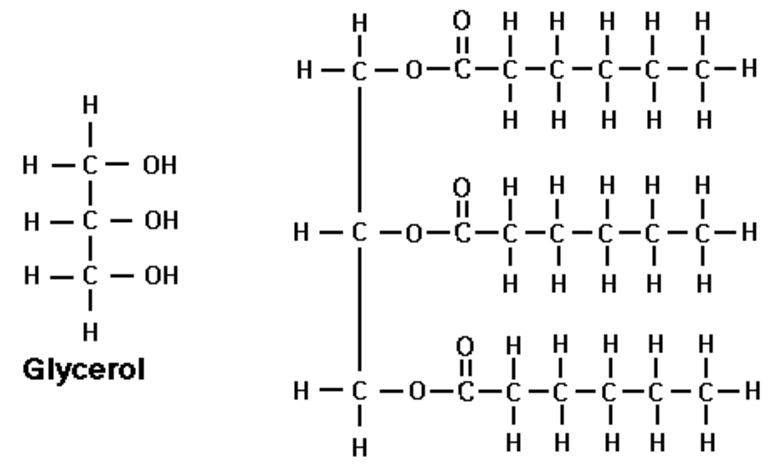
triglycerides
non-polar, hydrophobic
monomers:
glycerol: an alcohol
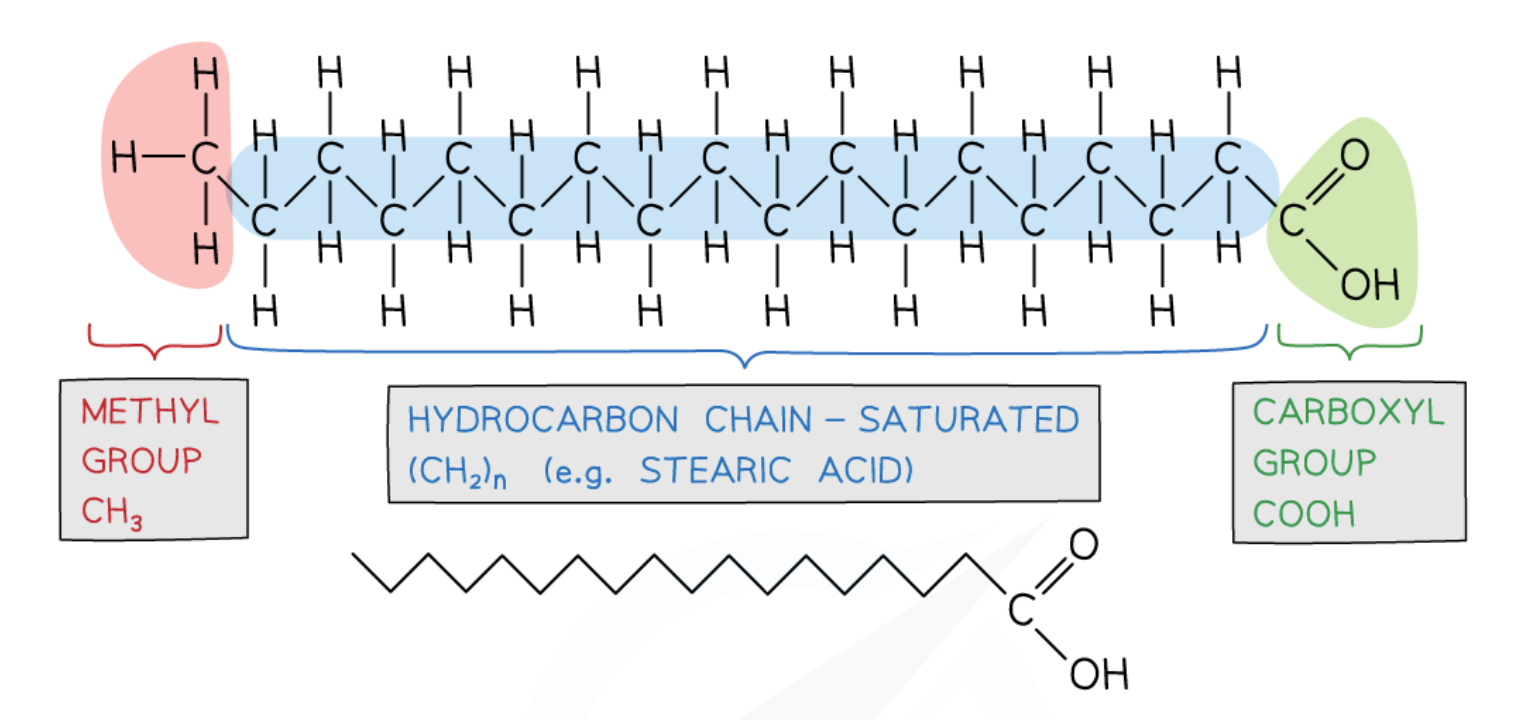
fatty acid: contain a methyl group at one end of the a hydrocarbon chain
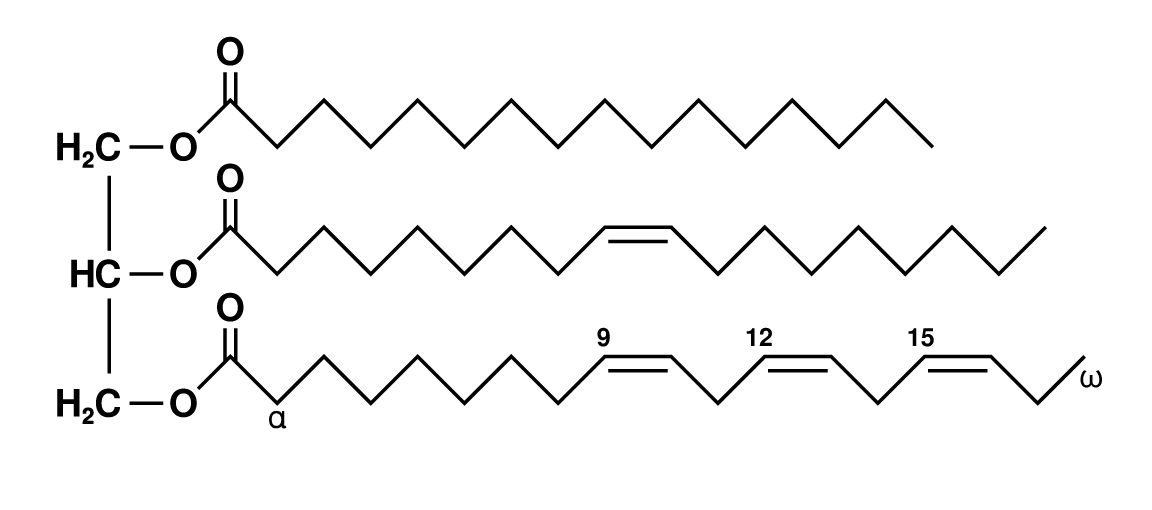
saturated fatty acids
no C=C double bonds in the hydrocarbon chain
mainly in animal fat
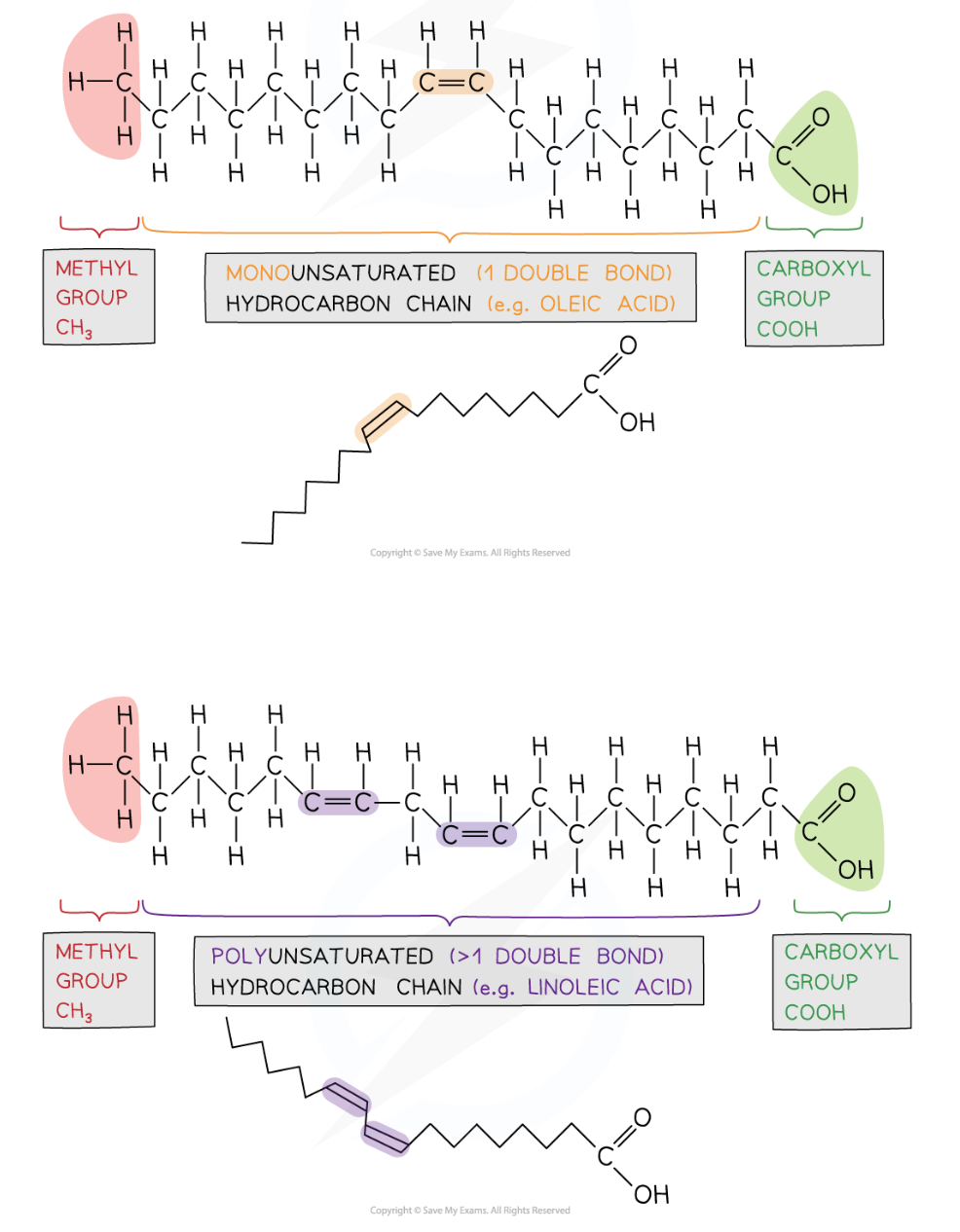
unsaturated fatty acids
monounsaturated → 1 C=C
polyunsaturated → >1 C=C
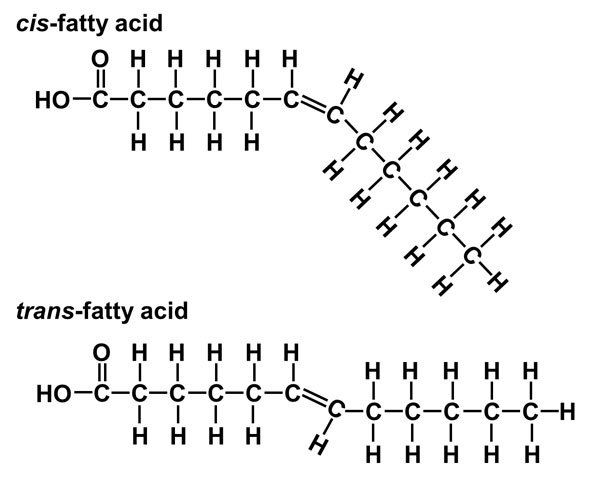
trans-fatty acid and cis-fatty acid
trans:
H on opposite sides of C=C
can’t be metabolised because it cannot forms enzyme-substrate complex
cis:
H on the same side of C=C
can be metabolised by enzymes
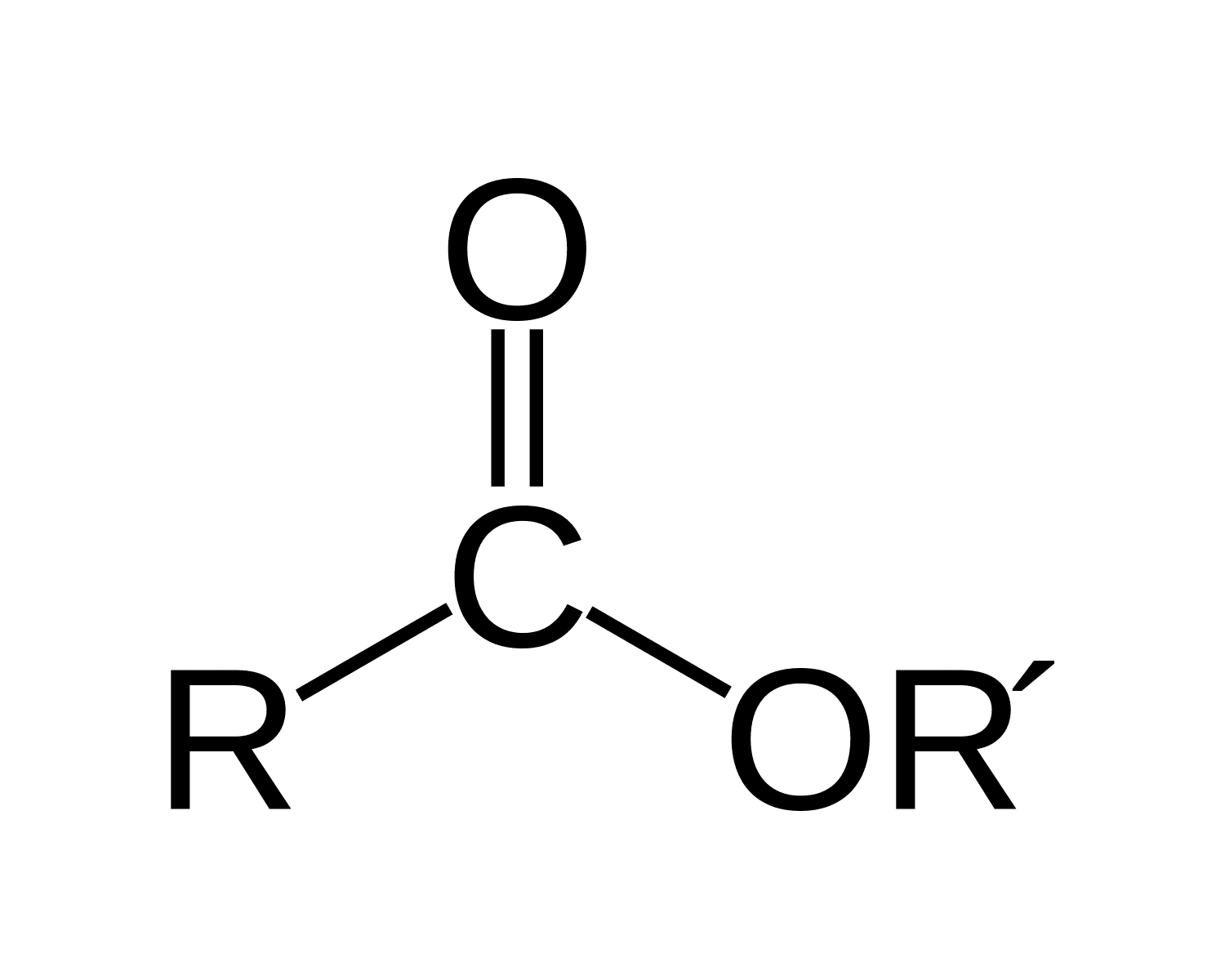
ester bond
An ester bond (-COO-) forms when the hydroxyl group (-OH) of the glycerol bonds with the carboxyl group (-COOH) of the fatty acid
For each ester bond formed a water molecule is released
Therefore, for one triglyceride to form three water molecules are released
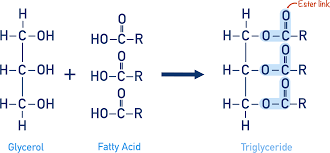
formation of triglycerides
1 glycerol + 3 fatty acid → triglyceride + 3H2O
functions of triglycerides
energy storage
insulation
ability to float
protection
structure of phospholipids
2 fatty acids + 1 glycerol + 1 phosphate ion (PO43-) → 1 phospholipids
phosphate → polar → hydrophilic
fatty acid → non-polar → hydrophobic
=> amphipathic (they have both hydrophobic and hydrophilic parts) so they form monolayers or bilayers in water
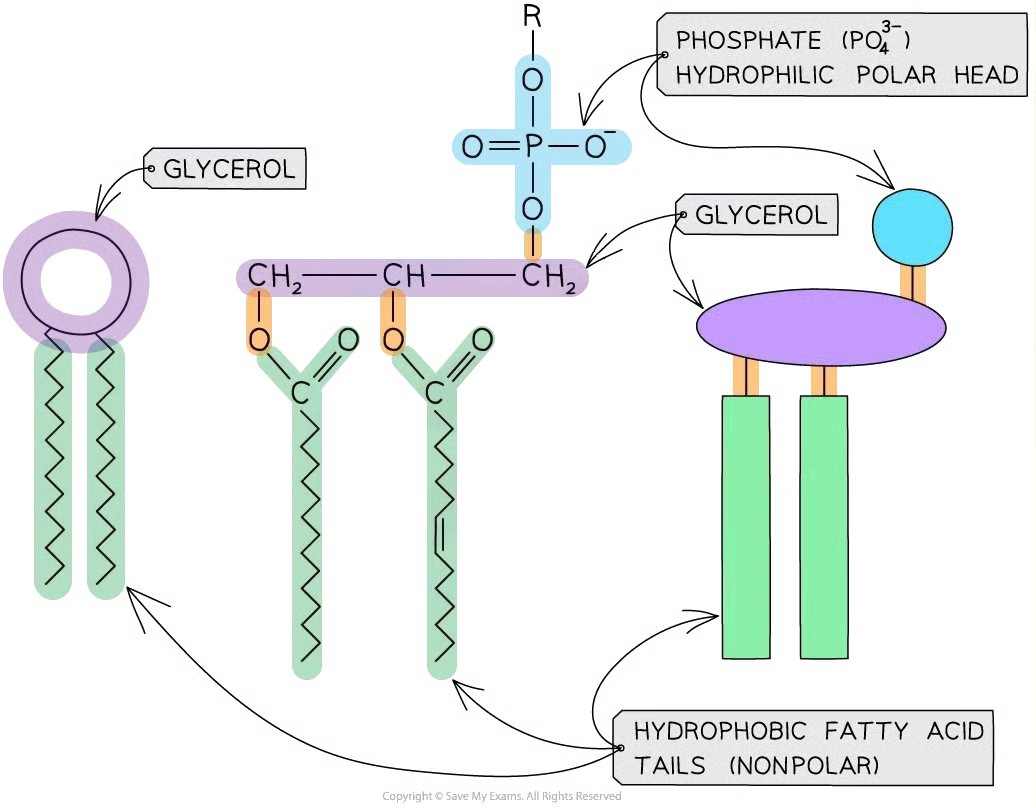
role of phospholipids
Main component of cell membrane
Structure & Function: Hydrophobic tails form a core that blocks water-soluble substances; hydrophilic heads interact with water to create compartments for organelles.
Fluidity: More saturated tails → less fluid; more unsaturated tails → more fluid.
Protein Positioning: Hydrophobic interactions with phospholipids keep membrane proteins in place while allowing lateral movement.
general structure of amino acid
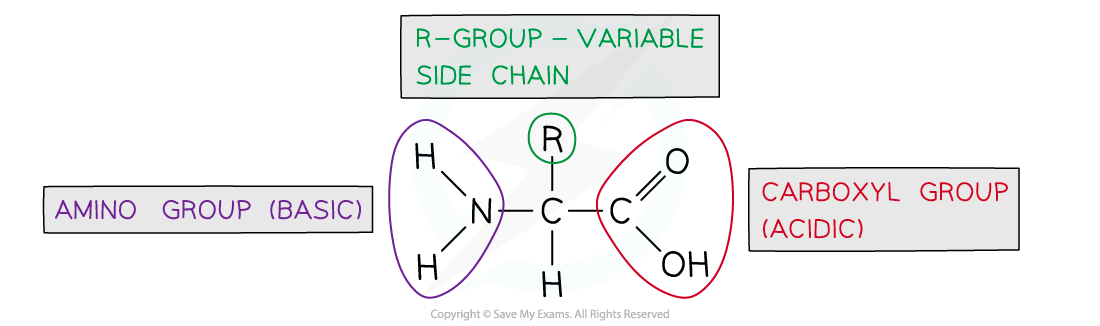
peptide bond
The chemical bond that forms between the carboxyl group of one amino acid and the amino group of another amino acid

primary structure
Sequence of amino acids in a polypeptide or protein
secondary structure
the structure of a protein molecule resulting from the regular coiling or folding of the chain of amino acids (an α-helix or β-pleated sheet)
α-helix
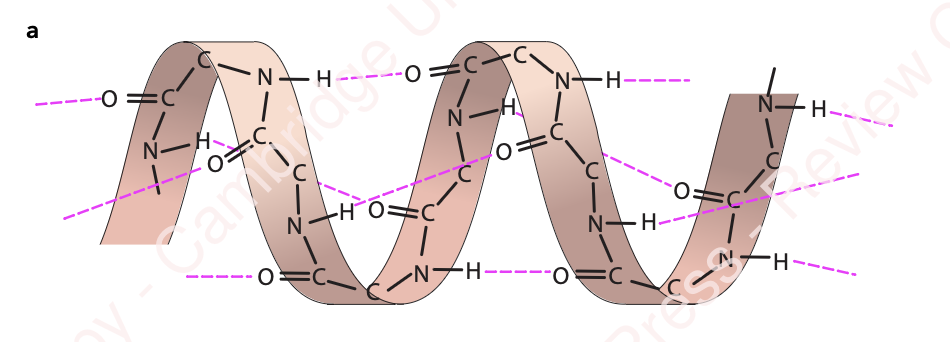
occurs when the hydrogen bonds form between every fourth peptide bond
β-pleated sheet
forms when the protein folds so that two parts of the polypeptide chain are parallel to each other, enabling hydrogen bonds to form between parallel peptide bonds

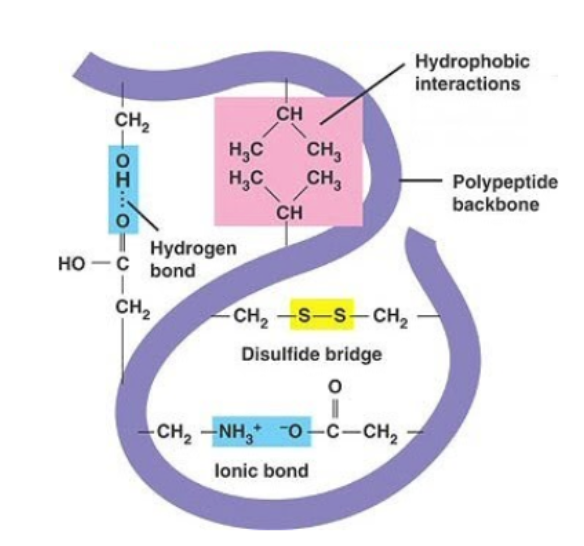
tertiary structure
=> the compact structure of a protein molecule resulting from the 3D coiling of the chain of amino acids
1) hydrogen bonds (form between strongly polar groups (NH2, CO, OH)
2) disulphide bonds (form between cysteine molecules, strongest)
3) ionic bonds (between ionised amine (NH3+) and carboxylic acid groups (COOH-). Broken by pH changes.
4) hydrophobic interactions (between non polar R groups)
quaternary structure
the 3D arrangement of two or more polypeptides, or of a polypeptide & non-protein component
hydrophobic interactions
form between the non-polar (hydrophobic) R groups within the interior of proteins
→ weak
ionic bonds
forms between cations and anions
stronger than hydrogen bonds
can be broken by pH
hydrogen bonds
weakest bond
dp-dp forces due to the polarity of R groups
disulfide bonds
strong covalent bonds that form between two cysteine R groups
can be broken by reduction
globular proteins
Roughly spherical
Irregular w wide range of R groups
Functional
E.g: Haemoglobin, insulin
Generally soluble in (water hydrophobic R groups inside, hydrophilic R groups outside)
fibrous proteins
Long strands
Little to no tertiary structure
Repetitive w a limited range of R groups
Structural
E.g: Collagen, keratin
Generally insoluble in water
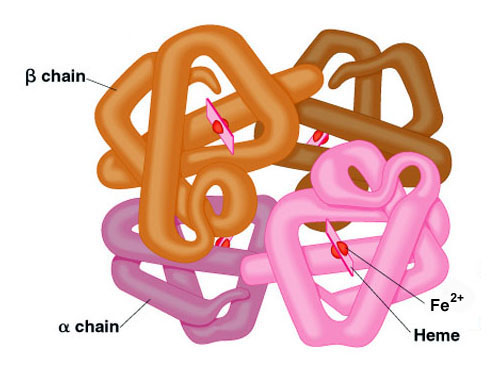
haemoglobin
1) made of 4 polypeptide chains
2) 2 chains are alpha goblin, other 2 are β goblin
3) each chain has a haem group (contains Fe2+) attached
4) held tgt by disulphide bonds
5) globular protein
haemoglobin functions
Haemoglobin binds and transports oxygen because O₂ is poorly soluble in water.
The haem group with Fe²⁺ allows reversible binding of oxygen.
Binding of each O₂ molecule changes haemoglobin’s shape, increasing its affinity for the next O₂
Amino acids alone cannot bind oxygen effectively → needs haem
collagen
fibrous protein
consists of 3 polypeptide chains each in a helical shape
3 polypeptides are wound together creating a triple helix
strands are held together by H and console by bonds
every 3rd amino acid is glycine
each 3 stranded molecule interacts with other collagen molecules running parallel to it
covalent bonds form between R groups of amino acids forming fibrils
many fibrils lie alongside each other forming strong bonds called fibre
flexible but has high tensile strength
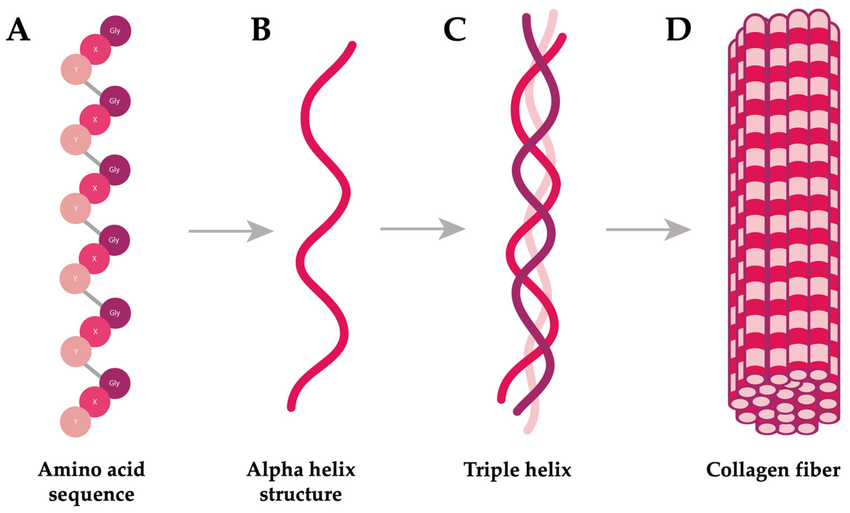
collagen functions
Flexible structural protein found in connective tissues.
Triple helix with many hydrogen bonds → very high tensile strength.
Staggered molecule arrangement in fibrils adds extra strength.
High proline and hydroxyproline content increases stability.
Long molecules dissolve slowly → collagen is insoluble in water.
properties of water to its roles in
living organisms
solvent action
→ polar so both ionic and covalent compounds will dissolve
high specific heat capacity
→ due to the many hydrogen bonds , it takes a lot of thermal energy to raise 1kg of water by 1ºC
high latent heat of vaporisation
→ Only a little water is required to evaporate from the surface of the organism in order to lose a great amount of thermal energy
glycerolycerol